Identification of Novel Parishin Compounds from the Twig of Maclura tricuspidata and Comparative Analysis of Parishin Derivatives in Different Parts
Abstract
1. Introduction
2. Results and Discussions
2.1. HPLC-QTOF-MS Analysis of MT Twig Extract
2.2. Isolation and Structure Elucidation of Parishin-Related Compounds
2.3. Structural Elucidation of Novel Parishin Compounds
2.4. Comparison of Parishin Compounds in Different Parts of MT
3. Materials and Methods
3.1. Plant Materials
3.2. Reagents and Chemicals
3.3. Isolation of Parishin Compounds in MT Twig
3.4. Sample Extraction, HPLC and HPLC-QTOF-MS Analysis
3.5. General Experimental Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Song, S.-H.; Ki, S.H.; Park, D.-H.; Moon, H.-S.; Lee, C.-D.; Yoon, I.-S.; Cho, S.-S. Quantitative Analysis, Extraction Optimization, and Biological Evaluation of Cudrania tricuspidata Leaf and Fruit Extracts. Molecules 2017, 22, 1489. [Google Scholar] [CrossRef]
- Xin, L.-T.; Yue, S.-J.; Fan, Y.-C.; Wu, J.-S.; Yan, D.; Guan, H.-S.; Wang, C.-Y. Cudrania tricuspidata: An updated review on ethnomedicine, phytochemistry and pharmacology. RSC Adv. 2017, 7, 31807–31832. [Google Scholar] [CrossRef]
- Seo, W.-G.; Pae, H.-O.; Oh, G.-S.; Chai, K.-Y.; Yun, Y.-G.; Chung, H.-T.; Jang, K.K.; Kwon, T.-O. Ethyl Acetate Extract of the Stem Bark of Cudrania tricuspidata Induces Apoptosis in Human Leukemia HL-60 Cells. Am. J. Chin. Med. 2001, 29, 313–320. [Google Scholar] [CrossRef]
- Kwon, S.-B.; Kim, M.-J.; Yang, J.M.; Lee, H.-P.; Hong, J.T.; Jeong, H.-S.; Kim, E.S.; Yoon, Y. Cudrania tricuspidata Stem Extract Induces Apoptosis via the Extrinsic Pathway in SiHa Cervical Cancer Cells. PLoS ONE 2016, 11, e0150235. [Google Scholar] [CrossRef]
- Chang, S.H.; Jung, E.J.; Lim, D.G.; Oyungerel, B.; Lim, K.I.; Her, E.; Choi, W.S.; Jun, M.H.; Choi, K.D.; Han, D.J.; et al. Anti-inflammatory action of Cudrania tricuspidata on spleen cell and T lymphocyte proliferation. J. Pharm. Pharmacol. 2008, 60, 1221–1226. [Google Scholar] [CrossRef]
- Jeong, C.-H.; Choi, G.-N.; Kim, J.-H.; Kwak, J.-H.; Heo, H.-J.; Shim, K.-H.; Cho, B.-R.; Bae, Y.-I.; Choi, J.-S. In vitro Antioxidative Activities and Phenolic Composition of Hot Water Extract from Different Parts of Cudrania tricuspidata. Prev. Nutr. Food Sci. 2009, 14, 283–289. [Google Scholar] [CrossRef]
- Kang, D.-H.; Kim, J.-W.; Youn, K.-S. Antioxidant Activities of Extracts from Fermented Mulberry (Cudrania tricuspidata) Fruit. and Inhibitory Actions on Elastase and Tyrosinase. Korean J. Food Preserv. 2011, 18, 236–243. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, S.; Chung, Y.W.; Kim, B.M.; Kim, H.; Kim, K.; Yang, K.M. Antiobesity and Antidiabetes Effects of a Cudrania tricuspidata Hydrophilic Extract Presenting PTP1B Inhibitory Potential. BioMed Res. Int. 2016, 2016, 8432759. [Google Scholar] [CrossRef]
- Han, X.H.; Hong, S.S.; Jin, Q.; Li, D.; Kim, H.-K.; Lee, J.; Kwon, S.H.; Lee, D.; Lee, C.-K.; Lee, M.K.; et al. Prenylated and Benzylated Flavonoids from the Fruits of Cudrania tricuspidata. J. Nat. Prod. 2008, 72, 164–167. [Google Scholar] [CrossRef]
- Hwang, J.H.; Hong, S.S.; Han, X.H.; Lee, D.; Lee, H.; Yun, Y.P.; Kim, Y.; Ro, A.J.S.; Hwang, B.Y. Prenylated Xanthones from the Root Bark of Cudrania tricuspidata. J. Nat. Prod. 2007, 70, 1207–1209. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mukwaya, E.; Wong, M.S.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2013, 52, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W.; Gal, S.W.; Park, K.-M. Cytotoxic Xanthones from Cudrania tricuspidata. J. Nat. Prod. 2005, 68, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.-R.; Lee, H.-J.; Kang, S.-K.; Auh, Q.-S.; Lee, Y.-M.; Kim, Y.-C.; Kim, E.-C. Isocudraxanthone K Induces Growth Inhibition and Apoptosis in Oral Cancer Cells via Hypoxia Inducible Factor-1α. BioMed Res. Int. 2014, 2014, 934691. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W.; Lee, J.H.; Lee, S.-T.; Lee, H.S.; Lee, W.S.; Jeong, T.-S.; Park, K.H. Antioxidant and cytotoxic activities of xanthones from Cudrania tricuspidata. Bioorganic Med. Chem. Lett. 2005, 15, 5548–5552. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W.; Lee, J.H.; Gal, S.W.; Moon, Y.H.; Park, K.H. Selective ABTS Radical-Scavenging Activity of Prenylated Flavonoids from Cudrania tricuspidata. Biosci. Biotechnol. Biochem. 2006, 70, 427–432. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, S.; Lee, S.J.; Ham, I.; Whang, W.K. Antioxidant activities of new flavonoids from Cudrania tricuspidata root bark. Arch. Pharm. Res. 2009, 32, 195–200. [Google Scholar] [CrossRef]
- Hiep, N.T.; Kwon, J.; Kim, D.-W.; Hwang, B.Y.; Lee, H.-J.; Mar, W.; Lee, D. Isoflavones with neuroprotective activities from fruits of Cudrania tricuspidata. Phytochemistry 2015, 111, 141–148. [Google Scholar] [CrossRef]
- Lee, I.-K.; Kim, C.-J.; Song, K.-S.; Kim, H.-M.; Koshino, H.; Uramoto, M.; Yoo, I.-D. Cytotoxic benzyl dihydroflavonols from Cudrania tricuspidata. Phytochemistry 1996, 41, 213–216. [Google Scholar] [CrossRef]
- Zou, Y.-S.; Hou, A.-J.; Zhu, G.-F.; Chen, Y.-F.; Sun, H.-D.; Zhao, Q.-S. Cytotoxic isoprenylated xanthones from Cudrania tricuspidata. Bioorganic Med. Chem. 2004, 12, 1947–1953. [Google Scholar] [CrossRef]
- Kim, O.-K.; Nam, D.-E.; Jun, W.; Lee, J. Anti-Inflammatory and Gastroprotective Activities of Cudrania tricuspidata Leaf Extract Against Acute HCl/Ethanol-Induced Gastric Mucosal Injury in Sprague-Dawley Rats. J. Food Biochem. 2015, 39, 508–516. [Google Scholar] [CrossRef]
- Lim, E.-J.; Jung, H.-J.; Park, E.-H.; Kang, H.-J. Anti-angiogenic, anti-inflammatory and anti-nociceptive activity of 4-hydroxybenzyl alcohol. J. Pharm. Pharmacol. 2007, 59, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.-H.; Kim, H.-C.; Cui, J.-M. Hepatoprotective constituents of Cudrania tricuspidata. Arch. Pharm. Res. 2005, 28, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.J.; Curtis-Long, M.J.; Lee, B.W.; Kim, H.Y.; Ryu, Y.B.; Jeong, T.-S.; Lee, W.S.; Park, K.H. Xanthones from Cudrania tricuspidata displaying potent α-glucosidase inhibition. Bioorganic Med. Chem. Lett. 2007, 17, 6421–6424. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-W.; Lee, W.-J.; Gebru, Y.A.; Choi, H.-S.; Yeo, S.-H.; Jeong, Y.-J.; Kim, S.; Kim, Y.-H.; Kim, M.-K. Comparison of Bioactive Compounds and Antioxidant Activities of Maclura tricuspidata Fruit Extracts at Different Maturity Stages. Molecules 2019, 24, 567. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-J.; Sheen, L.-Y. Gastrodiae Rhizoma (tiān má): A review of biological activity and antidepressant mechanisms. J. Tradit. Complement. Med. 2011, 1, 31–40. [Google Scholar] [CrossRef]
- Matias, M.; Silvestre, S.; Falcão, A.; Alves, G. Gastrodia elata and epilepsy: Rationale and therapeutic potential. Phytomedicine 2016, 23, 1511–1526. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Peng, M.; Meng, H.; Ma, H.; Cai, P.; Xu, Y.; Zhao, Q.; Si, G. A Review on Central Nervous System Effects of Gastrodin. Front. Pharmacol. 2018, 9, 24. [Google Scholar] [CrossRef]
- Nepal, M.R.; Jeong, K.S.; Kim, G.H.; Cha, D.H.; Kang, M.J.; Kim, J.S.; Kim, J.-H.; Jeong, T.C. Role of Intestinal Microbiota in Metabolism of Gastrodin In Vitro and In Vivo. Metabolites 2019, 9, 69. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, J.; Wang, X.; Lei, S.; Wu, X.; Chen, Y.; Wu, J.; Zhao, Y. 4-Hydroxybenzyl Alcohol Confers Neuroprotection Through Up-Regulation of Antioxidant Protein Expression. Neurochem. Res. 2013, 38, 1501–1516. [Google Scholar] [CrossRef]
- Zhang, A.; Wan, L.; Wu, C.; Fang, Y.; Han, G.; Li, H.; Zhang, Z.; Wang, H. Simultaneous Determination of 14 Phenolic Compounds in Grape Canes by HPLC-DAD-UV Using Wavelength Switching Detection. Molecules 2013, 18, 14241–14257. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, N.; Lun, X.; Chen, X.; Hou, X. LC-QTOF-MS method for the analysis of residual pharmaceuticals in wastewater: Application to a comparative multiresidue trace analysis between spring and winter water. Anal. Methods 2014, 6, 6956–6962. [Google Scholar] [CrossRef]
- Zheng, Z.-P.; Liang, J.; Hu, L.-H. Water-Soluble Constituents of Cudrania tricuspidata (Carr.) Bur. J. Integr. Plant Biol. 2006, 48, 996–1000. [Google Scholar] [CrossRef]
- Shin, G.R.; Lee, S.; Lee, S.; Do, S.-G.; Shin, E.; Lee, C.H. Maturity stage-specific metabolite profiling of Cudrania tricuspidata and its correlation with antioxidant activity. Ind. Crops Prod. 2015, 70, 322–331. [Google Scholar] [CrossRef]
- Li, Z.F.; Wang, Y.W.; Ouyang, H.; Lu, Y.; Qiu, Y.; Feng, Y.L.; Jiang, H.L.; Zhou, X.; Yang, S.L. A novel dereplication strategy for the identification of two new trace compounds in the extract of Gastrodia elata using UHPLC/Q-TOF-MS/MS. J. Chromatogr. B 2015, 988, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, Y.; Zhao, J.; Wang, M.; Avula, B.; Peng, Q.; Ouyang, H.; Lingyun, Z.; Zhang, J.; Khan, I.A. Identification and Characterization of Key Chemical Constituents in Processed Gastrodia elata Using UHPLC-MS/MS and Chemometric Methods. J. Anal. Methods Chem. 2019, 2019, 4396201. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H. Purification by solvent extraction using partition coefficient. In Natural Products Isolation, 2nd ed.; Sarker, S.D., Latif, Z., Gray, A.I., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2006. [Google Scholar]
- Shimizu, Y.; Li, B. Purification of water soluble natural products. In Natural Products Isolation, 2nd ed.; Sarker, S.D., Latif, Z., Gray, A.I., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2006; pp. 415–438. [Google Scholar]
- Taguchi, H.; Yosioka, I.; Yamasaki, K.; Kim, I. Studies on the constituents of Gastrodia elata Blume. Chem. Pharm. Bull. 1981, 29, 55–62. [Google Scholar] [CrossRef]
- Baek, N.-I.; Choi, S.Y.; Park, J.K.; Cho, S.-W.; Ahn, E.-M.; Jeon, S.G.; Lee, B.R.; Bahn, J.H.; Kim, Y.K.; Shon, I.H. Isolation and identification of succinic semialdehyde dehydrogenase inhibitory compound from the rhizome of Gastrodia elata blume. Arch. Pharm. Res. 1999, 22, 219–224. [Google Scholar] [CrossRef]
- Chae, S.-W.; Lee, A.-Y.; Lee, H.-W.; Yoon, T.-S.; Moon, B.-C.; Choo, B.-K.; Kim, H.-K. Three Phenolic Glycosides from Gastrodia elata. J. Appl. Biol. Chem. 2008, 51, 61–65. [Google Scholar] [CrossRef]
- Lin, J.-H.; Liu, Y.-C.; Hau, J.-P.; Wen, K.-C. Parishins B and C from rhizomes of Gastrodia elata. Phytochemistry 1996, 42, 549–551. [Google Scholar] [CrossRef]
- Jeon, J.-S.; Kim, J.; Park, S.; Ryou, C.; Kim, C.Y. Preparative purification of plasmin activity stimulating phenolic derivatives from Gastrodia elata using centrifugal partition chromatography. Biomed. Chromatogr. 2015, 30, 976–982. [Google Scholar] [CrossRef]
- Dahmén, J.; Leander, K. The structure of parishin, a glucoside from Vanda parishii. Phytochemistry 1976, 15, 1986–1987. [Google Scholar] [CrossRef]
- Yang, X.-D.; Zhu, J.; Yang, R.; Liu, J.-P.; Li, L.; Zhang, H.-B. Phenolic constituents from the rhizomes of Gastrodia elata. Nat. Prod. Res. 2007, 21, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Yao, X.; Liu, D.; Zhou, T.; Wu, G.; Zheng, X.; Wang, X. Effect of inorganic salt on partition of high-polarity parishins in two-phase solvent systems and separation by high-speed counter-current chromatography from Gastrodia elata Blume. J. Sep. Sci. 2018, 42, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.Y.; Do, J.C.; Son, K.H. Kaempferol 3-O-[6″-O-(3-hydroxy-3-methylglutaroyl) glucoside] from leaves of Polygala japonica. Phytochemistry 1993, 34, 1196–1197. [Google Scholar] [CrossRef]
- Porter, E.A.; van den Bos, A.A.; Kite, G.C.; Veitch, N.C.; Simmonds, M.S. Flavonol glycosides acylated with 3-hydroxy-3-methylglutaric acid as systematic characters in Rosa. Phytochemistry 2012, 81, 90–96. [Google Scholar] [CrossRef]
- Tang, C.; Wang, L.; Liu, X.; Cheng, M.; Qu, Y.; Xiao, H. Comparative pharmacokinetics of gastrodin in rats after intragastric administration of free gastrodin, parishin and Gastrodia elata extract. J. Ethnopharmacol. 2015, 176, 49–54. [Google Scholar] [CrossRef]
- Tang, C.; Wang, L.; Liu, X.; Cheng, M.; Xiao, H. Pharmacokinetic study of Gastrodia elata in rats. Anal. Bioanal. Chem. 2015, 407, 8903–8910. [Google Scholar] [CrossRef]
- Zhan, H.-D.; Zhou, H.-Y.; Sui, Y.-P.; Du, X.-L.; Wang, W.-H.; Dai, L.; Sui, F.; Huo, H.-R.; Jiang, T.-L. The rhizome of Gastrodia elata Blume—An ethnopharmacological review. J. Ethnopharmacol. 2016, 189, 361–385. [Google Scholar] [CrossRef]
- Beg, Z.; Lupien, P. In vitro and in vivo inhibition of hepatic cholesterol synthesis by 3-hydroxy-3-methylglutaric acid. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1972, 260, 439–448. [Google Scholar] [CrossRef]
- Di Padova, C.; Bosisio, E.; Cighetti, G.M.; Rovagnati, P.; Mazzocchi, M.; Colombo, C.; Tritapepe, R. 3-hydroxy-3-methylglutaric acid (HMGA) reduces dietary cholesterol induction of saturated bile in hamster. Life Sci. 1982, 30, 1907–1914. [Google Scholar] [CrossRef]
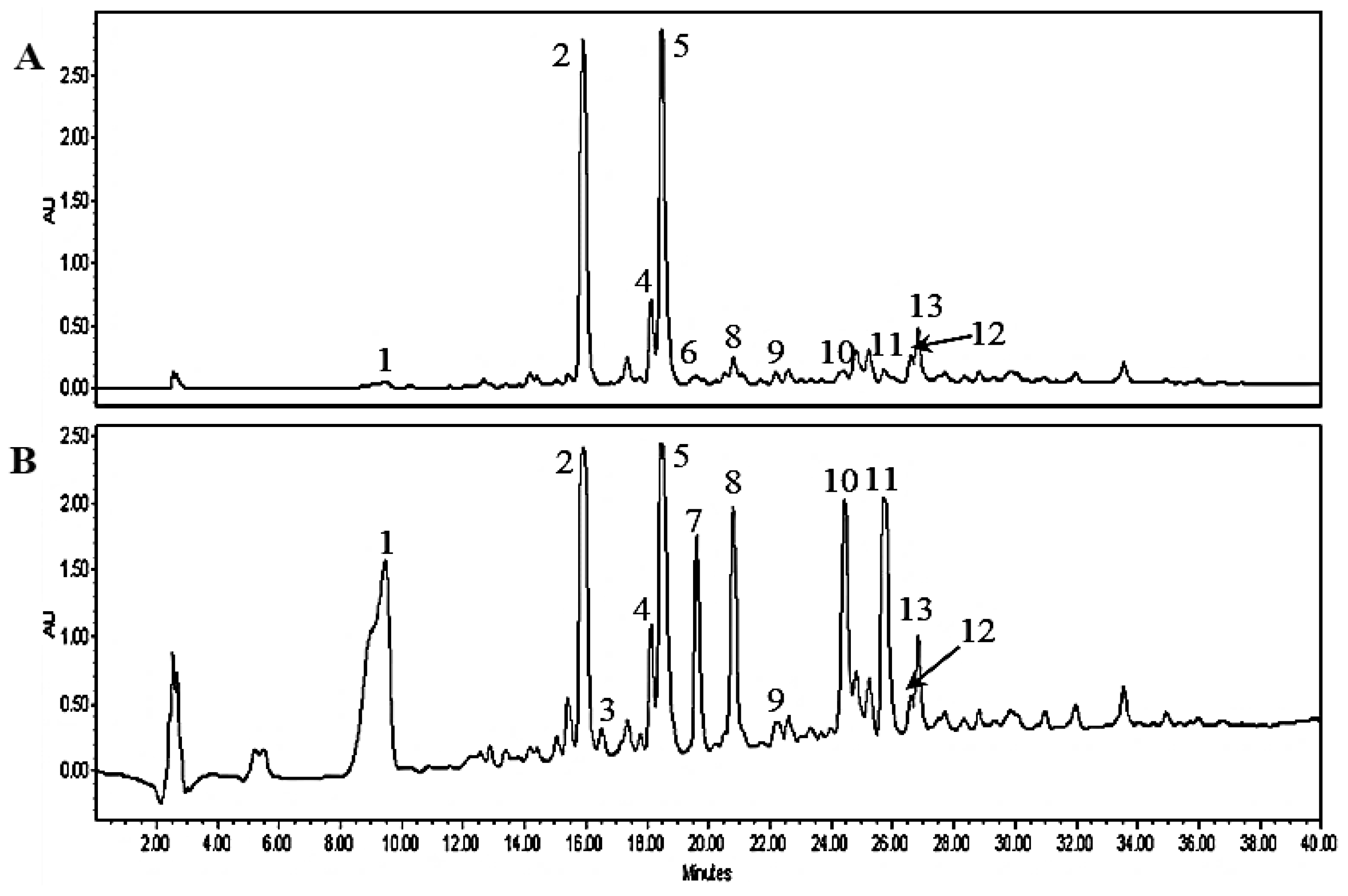

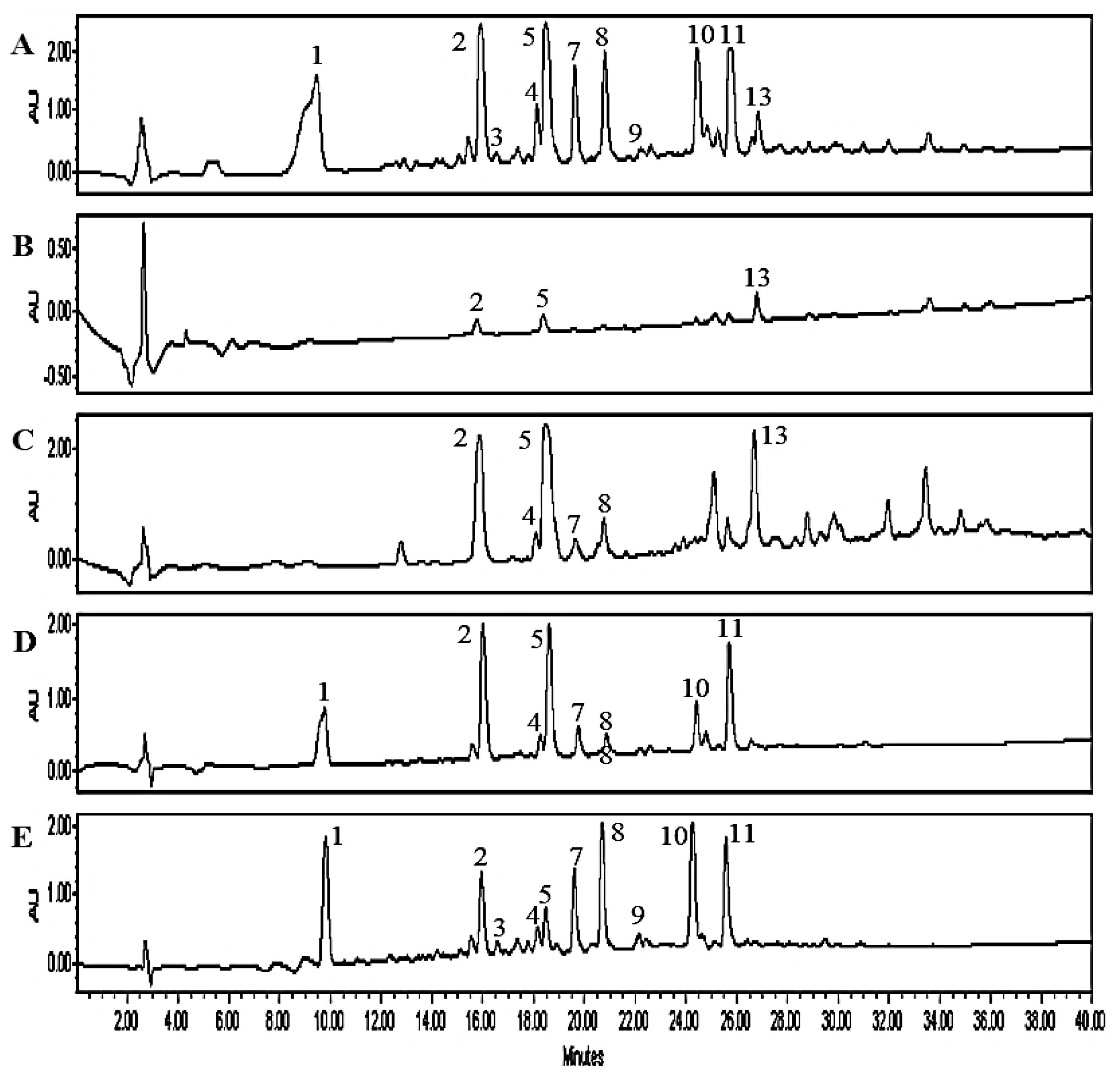
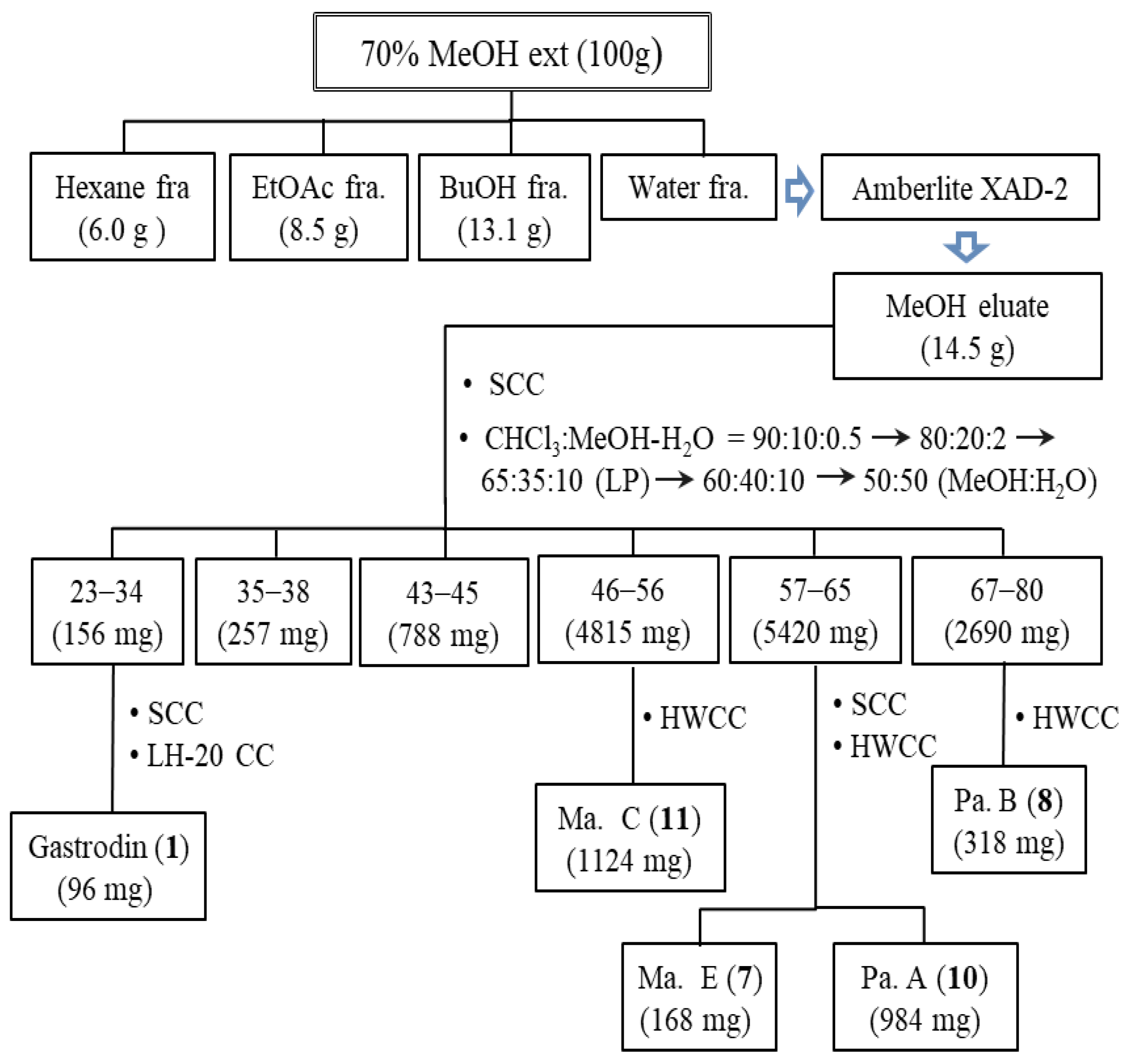
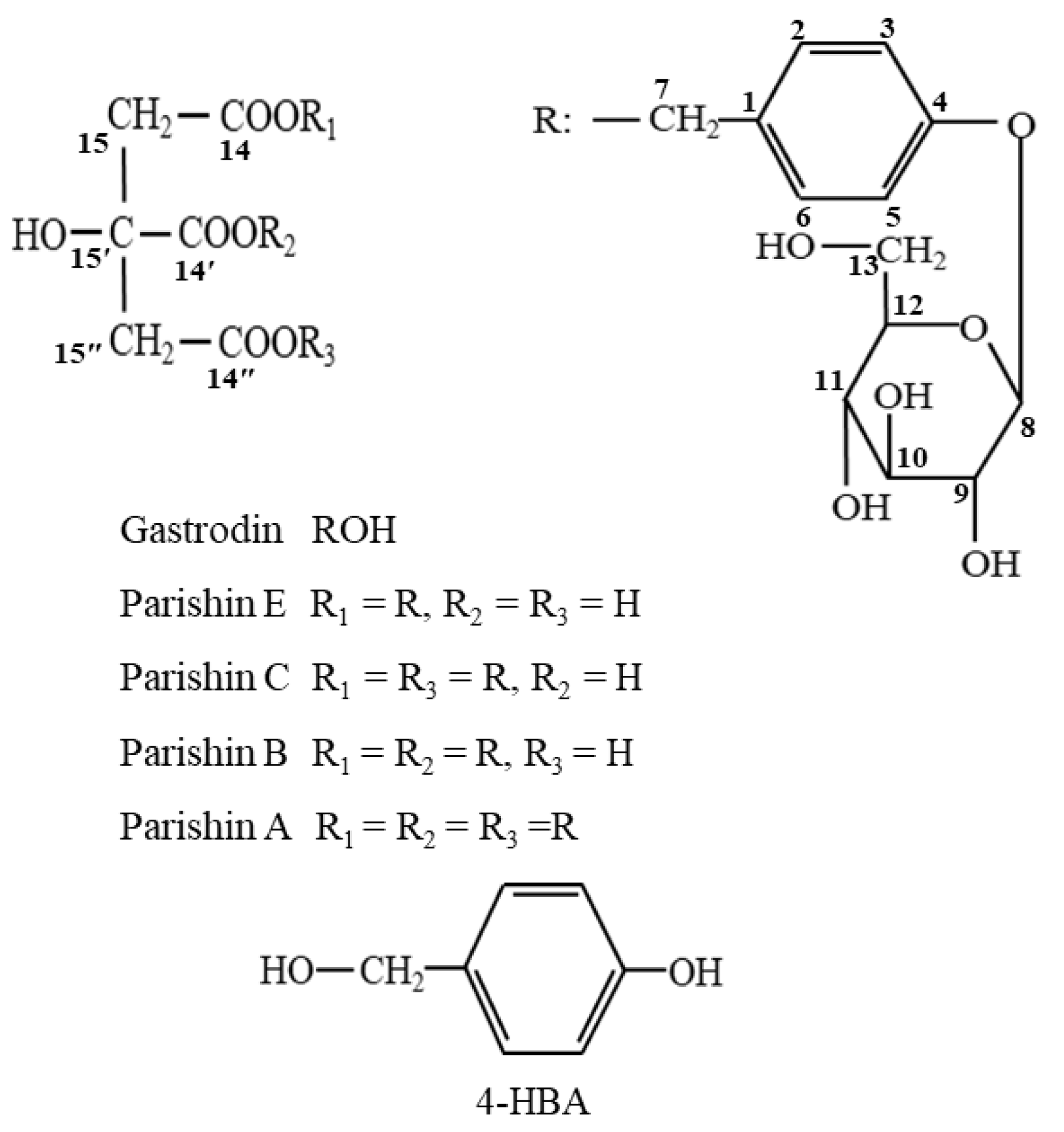

| Peak no. | RT (min) | [M − H]− or [M + HCOO]− (m/z) | Molecular Formula | Error (ppm) | MW (m/z) | Identification |
|---|---|---|---|---|---|---|
| 1 | 9.20 | 331.1031 | C13H18O7 | 1.02 | 332.11 | Gastrodin |
| 2 | 17.77 | 465.1031 | C21H22O12 | 0.73 | 466.11 | Taxifolin-7-β-d-glucoside |
| 3 | 18.46 | 459.1138 | C19H24O13 | 0.68 | 460.12 | Parishin E |
| 4 | 19.35 | 353.0872 | C16H18O9 | 1.55 | 354.09 | Chlorogenic acid |
| 5 | 19.96 | 449.1082 | C21H22O11 | 0.14 | 450.12 | Dihydrokaempferol-7-β-d- glucoside |
| 6 | 20.49 | 625.1405 | C27H30O17 | 0.89 | 626.15 | Quercetin-3-β-d-sophoroside |
| 7 | 21.16 | 429.1396 | C19H26O11 | 0.81 | 430.15 | Unknown-1 |
| 8 | 21.54 | 727.2085 | C32H40O19 | 0.72 | 728.22 | Parishin B |
| 9 | 22.46 | 727.2086 | C32H40O19 | 0.59 | 728.22 | Parishin C |
| 10 | 23.71 | 995.3025 | C45H56O25 | 1.16 | 996.31 | Parishin A |
| 11 | 24.83 | 743.2399 | C33H44O19 | 0.61 | 744.25 | Unknown-2 |
| 12 | 27.06 | 287.0558 | C15H12O6 | 0.71 | 288.06 | Dihydrokaempferol |
| 13 | 27.53 | 447.0927 | C21H20O11 | 1.37 | 448.10 | Kaempferol-3-β-d-glucoside |
| No. | Macluraparishin E (7) | Macluraparishin C (11) | ||
|---|---|---|---|---|
| δC | δH (JHz) | δC | δH (JHz) | |
| 1 | 172.7 | - | 172.4 | |
| 2 | 46.9 | 2.64 (2H, s) | 46.3 | 2.67 (2H, d, 6.0) |
| 3 | 70.9 | - | 70.9 | - |
| 4 | 46.7 | 2.56 (1H, d, 15.0) 2.48 (1H, d, 15.0) | 46.3 | 2.67 (2H, d, 6.0) |
| 5 | 177.5 | - | 172.4 | - |
| 6 | 27.7 | 1.31 (3H, s) | 28.0 | 1.32 (3H, s) |
| 1′ | 131.4 | - | 131.4 | - |
| 2′/6′ | 130.9 | 7.30 (2H, d, 8.4) | 131.0 | 7.28 (2H, d, 8.4) |
| 3′/5′ | 117.7 | 7.08 (2H, d, 8.4) | 117.7 | 7.07 (2H, d, 8.4) |
| 4′ | 159.0 | - | 159.1 | - |
| 7′ | 66.9 | 5.05 (2H, s) | 67.0 | 5.03 (2H, s) |
| 1′′ | 102.2 | 4.91 (1H, d, 7.8) | 102.2 | 4.90 (2H, d, 7.8) |
| 2′′ | 74.9 | 3.47 (1H, dd, 7.8, 6.6) | 74.9 | 3.44 (2H, dd, 7.8, 6.6) |
| 3′′ | 77.9 | 3.54 (1H, dd, 6.6, 6.6) | 78.0 | 3.46 (2H, dd, 6.6, 6.6) |
| 4′′ | 71.3 | 3.42 (1H, dd, 8.4, 6.6) | 71.4 | 3.40 (2H, dd, 8.4, 6.6) |
| 5′′ | 78.1 | 3.44 (1H, ddd, 8.4, 4.8, 2.4) | 78.2 | 3.43 (2H, ddd, 8.4, 5.4, 2.4) |
| 6′′ | 62.5 | 3.88 (1H, dd, 12.6, 2.4) 3.70 (1H, dd, 12.6, 4.8) | 62.5 | 3.88 (2H, dd, 12.6, 2.4) 3.69 (2H, dd, 12.6, 5.4) |
| 1′′′ | 131.4 | - | ||
| 2′′′/6′′′ | 131.0 | 7.28 (4H, d, 8.4) | ||
| 3′′′/5′′′ | 117.7 | 7.07 (4H, d, 8.4) | ||
| 4′′′ | 159.1 | - | ||
| 7′′′ | 67.0 | 5.03 (2H, s) | ||
| 1′′′′ | 102.2 | 4.90 (2H, d, 7.8) | ||
| 2′′′′ | 74.9 | 3.44 (2H, dd, 7.8, 6.6) | ||
| 3′′′′ | 78.0 | 3.46 (2H, dd, 6.6, 6.6) | ||
| 4′′′′ | 71.4 | 3.40 (2H, dd, 8.4, 6.6) | ||
| 5′′′′ | 78.2 | 3.43 (2H, ddd, 8.4, 5.4, 2.4) | ||
| 6′′′′ | 62.5 | 3.88 (2H, dd, 12.6, 2.4) 3.69 (2H, dd, 12.6, 5.4) | ||
| Peak No. | Compound | Twig | Bark | Root | Leaves | Xylem | Fruit |
|---|---|---|---|---|---|---|---|
| 1 | Gastrodin | 7420.0 ± 150.8 | 2442.2 ± 25.5 | 2470.4 ± 10.6 | 1758.4 ± 14.3 | 1397.1 ± 104.2 | 855.9 ± 5.4 |
| 3 | Parishin E | 330.9 ± 9.9 | 466.4 ± 28.6 | 283.0 ± 11.2 | - | 42.3 ± 10.5 | 26.1 ± 7.2 |
| 7 | Macluraparishin E | 2567.4 ± 75.5 | 4341.4 ± 24.4 | 3558.1 ± 21.5 | - | 289.1 ± 66.3 | 121.7 ± 9.6 |
| 8 | Parishin B | 3744.8 ± 63.5 | 3555.0 ± 87.0 | 1291.8 ± 25.7 | - | 331.9 ± 23.5 | - |
| 9 | Parishin C | - | 381.3 ± 8.6 | 148.3 ± 40.4 | - | 14.5 ± 1.6 | 76.8 ± 1.5 |
| 10 | Parishin A | 2498.1 ± 90.0 | 3234.8 ± 78.6 | 1490.2 ± 46.1 | - | 461.8 ± 7.9 | 1954.3 ± 10.1 |
| 11 | Macluraparishin C | 2639.1 ± 63.1 | 3104.3 ± 29.2 | 3151.1 ± 17.5 | - | 202.0 ± 53.4 | 84.2 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-W.; Kim, J.-K.; Gebru, Y.A.; Kim, Y.-H.; Choi, H.-S.; Kim, M.-K. Identification of Novel Parishin Compounds from the Twig of Maclura tricuspidata and Comparative Analysis of Parishin Derivatives in Different Parts. Molecules 2023, 28, 7. https://doi.org/10.3390/molecules28010007
Kim D-W, Kim J-K, Gebru YA, Kim Y-H, Choi H-S, Kim M-K. Identification of Novel Parishin Compounds from the Twig of Maclura tricuspidata and Comparative Analysis of Parishin Derivatives in Different Parts. Molecules. 2023; 28(1):7. https://doi.org/10.3390/molecules28010007
Chicago/Turabian StyleKim, Dae-Woon, Jong-Kuk Kim, Yoseph Asmelash Gebru, Young-Hoi Kim, Han-Seok Choi, and Myung-Kon Kim. 2023. "Identification of Novel Parishin Compounds from the Twig of Maclura tricuspidata and Comparative Analysis of Parishin Derivatives in Different Parts" Molecules 28, no. 1: 7. https://doi.org/10.3390/molecules28010007
APA StyleKim, D.-W., Kim, J.-K., Gebru, Y. A., Kim, Y.-H., Choi, H.-S., & Kim, M.-K. (2023). Identification of Novel Parishin Compounds from the Twig of Maclura tricuspidata and Comparative Analysis of Parishin Derivatives in Different Parts. Molecules, 28(1), 7. https://doi.org/10.3390/molecules28010007







