Synthesis and In Vitro Comparison of DOTA, NODAGA and 15-5 Macrocycles as Chelators for the 64Cu-Labelling of Immunoconjugates
Abstract
1. Introduction
2. Results and Discussion
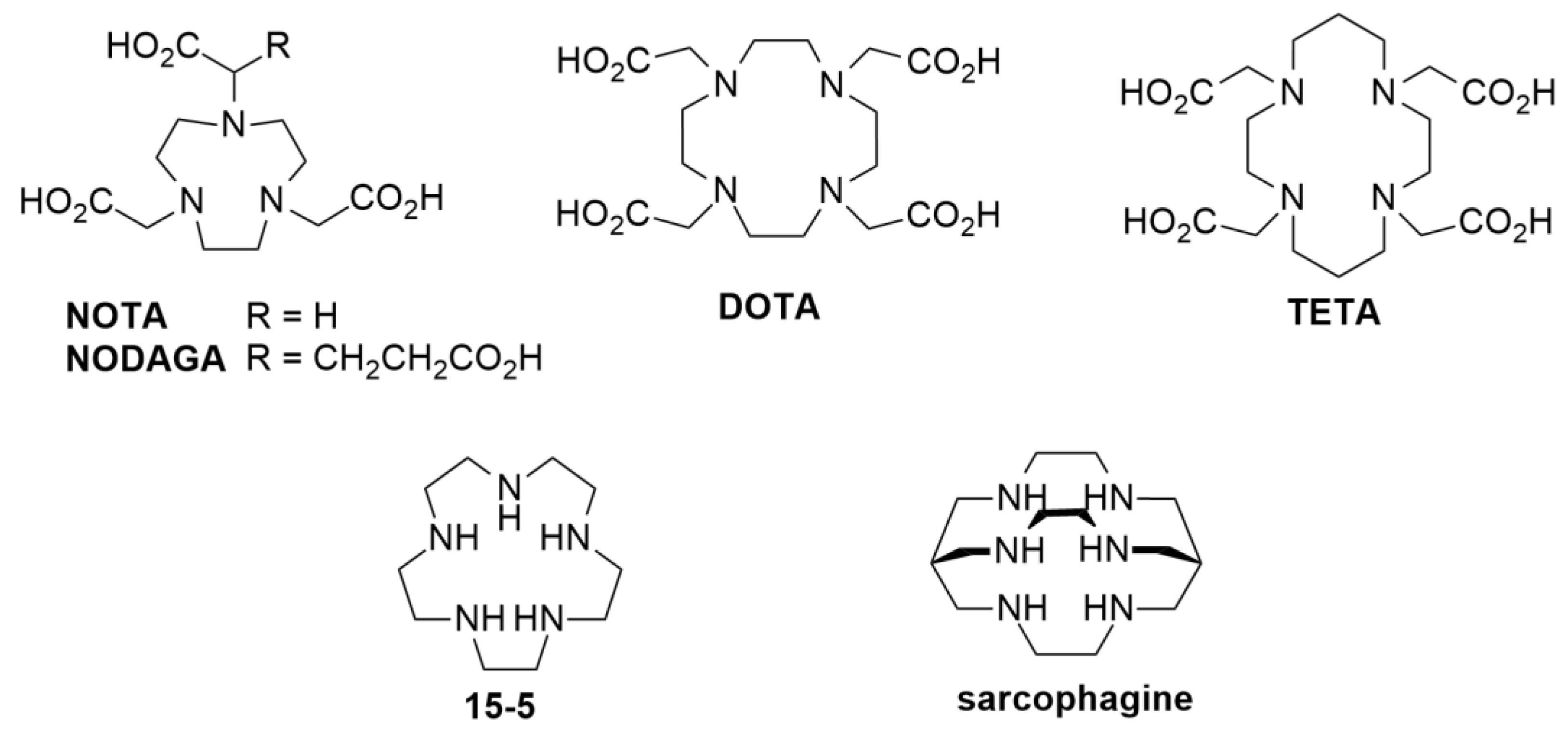
2.1. Organic synthesis
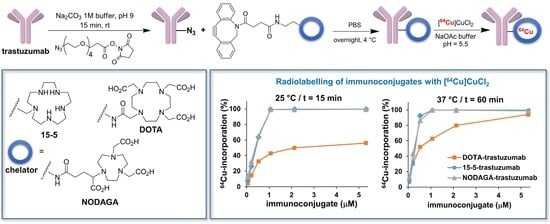
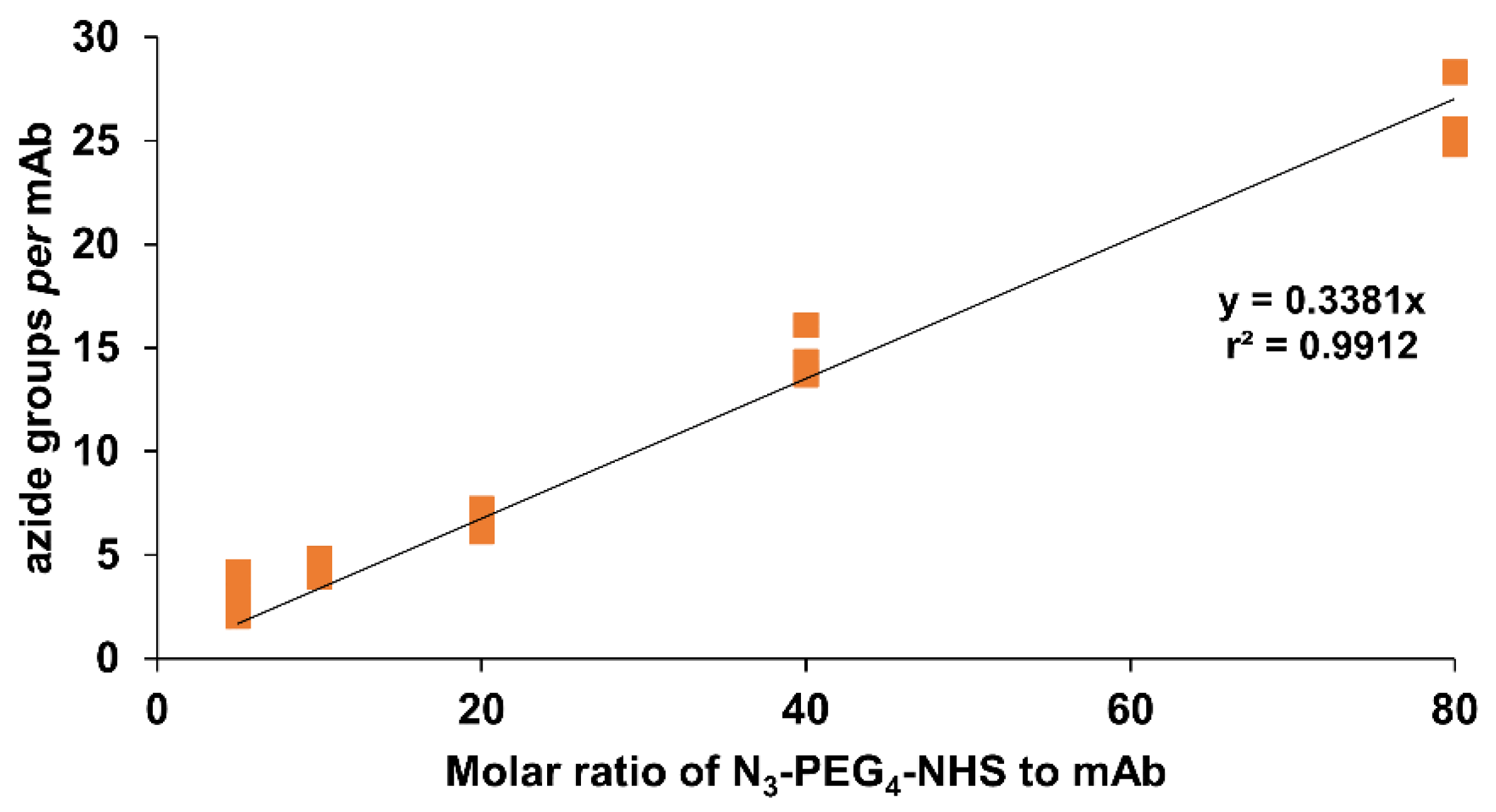
2.2. Immunoconjugation
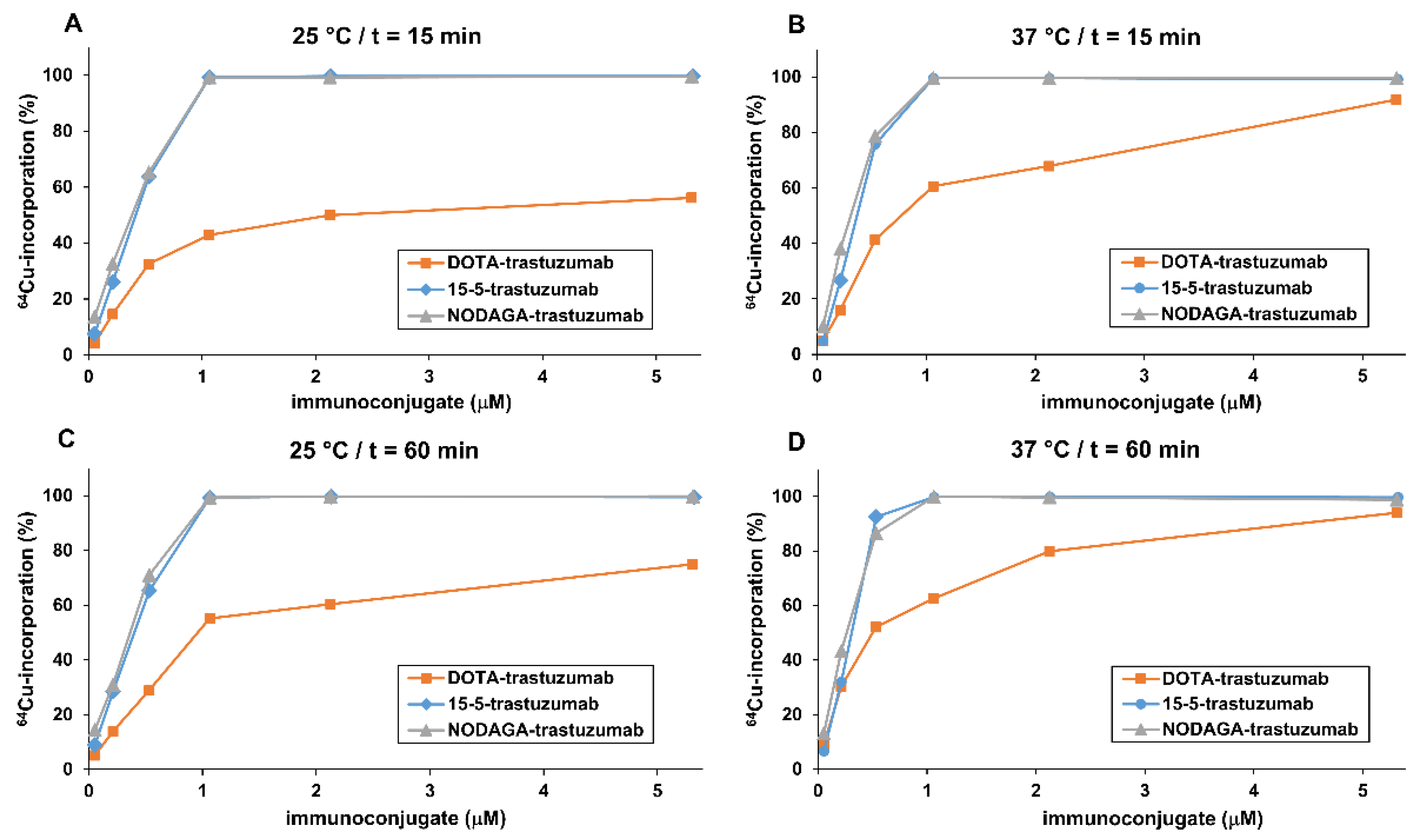
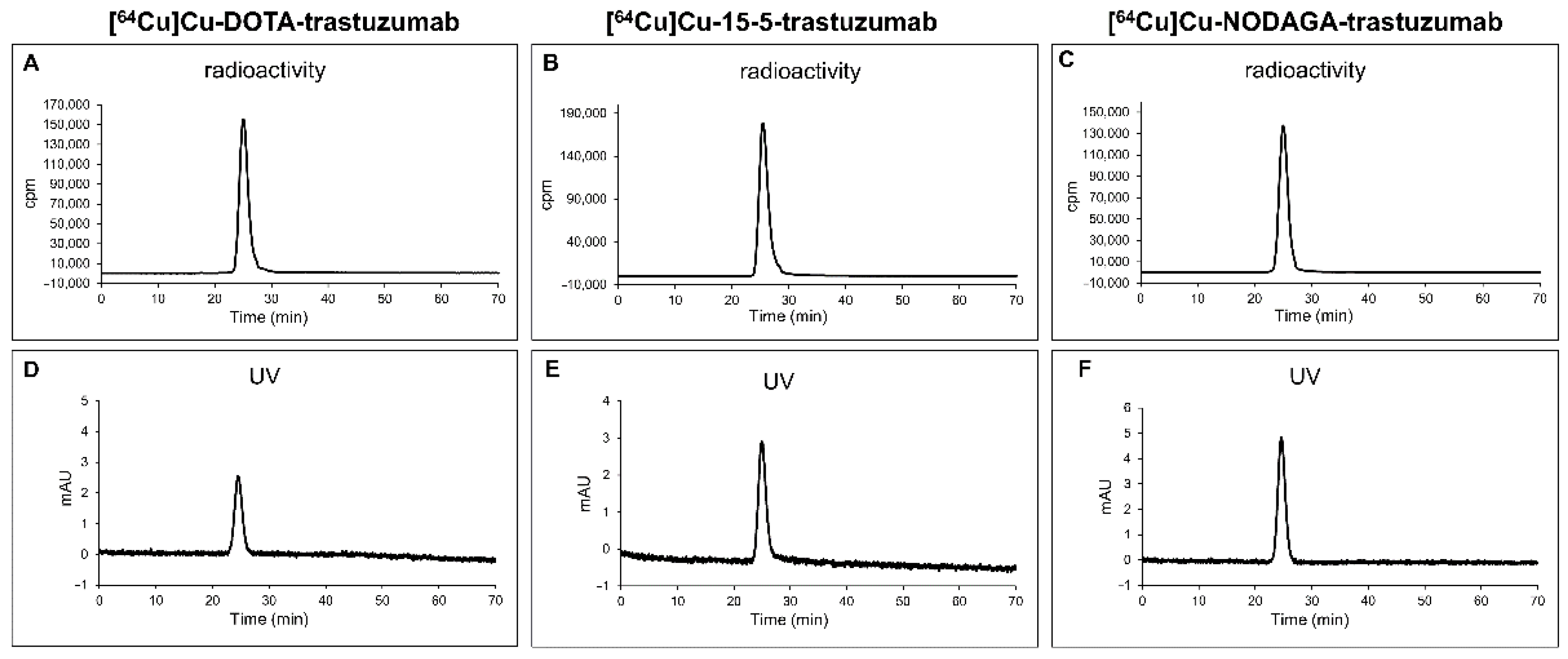
2.3. 64Cu-Labelling of Chelator-Trastuzumab Conjugates
2.4. In Vitro Stability of 64Cu-Labelled Immunoconjugates
2.5. In Vitro Receptor Binding Assays
3. Materials and Methods
3.1. General
3.2. Organic Synthesis
3.2.1. 1,4,7,10,13-Pentaazacyclopentadecane, pentahydrochloride Salt (2)
3.2.2. 1,4,7,10,13-Pentaazacyclopentadecane (3)
3.2.3. Benzyl (3-(1,4,7,10,13-Pentaazacyclopentadecan-1-yl)propyl)carbamate (4)
3.2.4. Tetra-tert-butyl 13-(3-(((benzyloxy)carbonyl)amino)propyl)-1,4,7,10,13-pentaazacyclopentadecane-1,4,7,10-tetracarboxylate (5)
3.2.5. Tetra-tert-butyl 13-(3-aminopropyl)-1,4,7,10,13-pentaazacyclopentadecane-1,4,7,10-tetracarboxylate (6)
3.2.6. 4-(11,12-Didehydrodibenzo[b,f]azocin-5(6H)-yl)-4-oxo-N-(3-(4,7,10,13-tetrakis(2-(tert-butoxy)-2-oxoethyl)-1,4,7,10,13-pentaazacyclopentadecan-1-yl)propyl)butanamide (7)
3.2.7. 4-(11,12-Didehydrodibenzo[b,f]azocin-5(6H)-yl)-4-oxo-N-(3-(1,4,7,10,13-pentaazacyclopentadecan-1-yl)propyl)butanamide (8 or 15-5-ADIBO)
3.2.8. 2,2′,2″-(10-(2-oxo-2-((2-(4-(11,12-didehydrodibenzo[b,f]azocin-5(6H)-yl)-4-oxobutanamido)ethyl)amino)ethyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetic acid, triammonium salt (11 or DOTA-ADIBO)
3.2.9. 2,2′-(7-(1-carboxy-4-oxo-4-((2-(4-(11,12-didehydrodibenzo[b,f]azocin-5(6H)-yl)-4-oxobutanamido)ethyl)amino)butyl)-1,4,7-triazonane-1,4-diyl)diacetic acid, triammonium salt (14 or NODAGA-ADIBO)
3.3. Immunoconjugation
3.3.1. Azide Functionalisation
3.3.2. SPAAC Functionalisation
3.3.3. Determination of the Average Number of Azide and BFC Groups per mAb
3.4. Radiochemistry
3.4.1. Effects of Reaction Time, Temperature and Concentration of Immunoconjugates on RCC
3.4.2. Synthesis of [64Cu]Cu-15-5-Trastuzumab, [64Cu]Cu-DOTA-Trastuzumab and [64Cu]Cu-NODAGA-Trastuzumab for Stability and Binding Studies
3.4.3. In Vitro Stability of 64Cu-Labelled Conjugates
3.4.4. EDTA Challenge Assays
3.5. Cell line and Culture
3.6. Determination of the Immunoreactive Fraction
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Freise, A.C.; Wu, A.M. In vivo imaging with antibodies and engineered fragments. Mol. Immunol. 2015, 67, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Karczmarczyk, U.; Sawicka, A.; Garnuszek, P.; Maurin, M.; Wojdowska, W. Does the number of bifunctional chelators conjugated to a mAb affect the biological activity of its radio-labeled counterpart? Discussion using the example of mAb against CD-20 labeled with 90Y or 177Lu. J. Med. Chem. 2022, 65, 6419–6430. [Google Scholar] [CrossRef] [PubMed]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating radiometals of copper, gallium, indium, yttrium and zirconium for PET and SPECT imaging of disease. Chem. Rev. 2010, 110, 2858–2902. [Google Scholar] [CrossRef]
- Cai, Z.; Anderson, C.J. Chelators for copper radionuclides in positron emission tomography radiopharmaceuticals. J. Label. Comp. Radiopharm. 2014, 57, 224–230. [Google Scholar] [CrossRef] [PubMed]
- McInnes, L.E.; Rudd, S.E.; Donnelly, P.S. Copper, gallium and zirconium positron emission tomography imaging agents: The importance of metal ion speciation. Coord. Chem. Rev. 2017, 352, 499–516. [Google Scholar] [CrossRef]
- Gutfilen, B.; Al Souza, S.A.; Valentini, G. Copper-64: A real theranostic agents. Drug Des. Devel. Ther. 2018, 12, 3235–3245. [Google Scholar] [CrossRef] [PubMed]
- Keinänen, O.; Fung, K.; Brennan, J.M.; Zia, N.; Harris, M.; van Dam, E.; Biggin, C.; Hedt, A.; Stoner, J.; Donnelly, P.S.; et al. Harnessing 64Cu/67Cu for a theranostic approach to pretargeted radioimmunotherapy. Proc. Natl. Acad. Sci. USA 2020, 117, 28316–28327. [Google Scholar] [CrossRef]
- Cabbiness, D.K.; Margerum, D.W. Macrocyclic effect of the stability of copper(II) tetramine complexes. J. Am. Chem. Soc. 1969, 91, 6540–6541. [Google Scholar] [CrossRef]
- Maheshwari, V.; Dearling, J.L.J.; Treves, S.T.; Packard, A.B. Measurement of the rate of copper(II) exchange for 64Cu complexes of bifunctional chelators. Inorg. Chim. Acta 2012, 393, 318–323. [Google Scholar] [CrossRef]
- Ghosh, S.C.; Pinkston, K.L.; Robinson, H.; Harvey, B.R.; Wilganowski, N.; Gore, K.; Sevick-Muraca, E.M.; Azhdarinia, A. Comparison of DOTA and NODAGA as chelators for 64Cu-labeled immunoconjugates. Nucl. Med. Biol. 2015, 42, 177–183. [Google Scholar] [CrossRef]
- Carrasquillo, J.A.; Morris, P.G.; Humm, J.L.; Smith-Jones, P.M.; Beylergil, V.; Akhurst, T.; O’donoghue, J.A.; Ruan, S.; Modi, S.; Hudis, C.A.; et al. Copper-64 trastuzumab PET imaging: A reproducibility study. Q. J. Nucl. Med. Mol. Imaging 2019, 63, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Adhikarla, V.; Poku, E.K.; Palmer, J.; Chaudhry, A.; Biglang-Awa, V.E.; Bowles, N.; Nathwani, N.; Rosenzweig, M.; Sahebi, F.; et al. Identifying CD38+ cells in patients with multiple myeloma: First-in-human imaging using copper-64-labeled daratumumab. Blood Adv. 2020, 4, 5194–5202. [Google Scholar] [CrossRef] [PubMed]
- Denis, C.; Gaumet, V.; Vidal, A.; Auzeloux, P.; Madelmont, J.C.; Miot-Noirault, E.; Chezal, J.M. Macrocyclic Complexes, Their Process of Preparation and Use as PET Imaging Agents. Patent WO2016016272, 4 February 2016. [Google Scholar]
- Cousins, R.J. Absorption, transport, and hepatic metabolism of copper and zinc: Special reference to metallothionein and ceruloplasmin. Physiol. Rev. 1985, 65, 238–309. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, K.; Sandahl, T.D.; Frisch, K.; Vase, K.H.; Keiding, S.; Vilstrup, H.; Ott, P.; Gormsen, L.C.; Munk, O.L. Intravenous and oral copper kinetics, biodistribution and dosimetry in healthy humans studied by [64Cu]copper PET/CT. EJNMMI Radiopharm. Chem. 2020, 5, 15. [Google Scholar] [CrossRef]
- Zeng, D.; Guo, Y.; White, A.G.; Cai, Z.; Modi, J.; Ferdani, R.; Anderson, C.J. Comparison of conjugation strategies of cross-bridged macrocyclic chelators with cetuximab for copper-64 radiolabeling and PET imaging of EGFR in colorectal tumor-bearing mice. Mol. Pharm. 2014, 11, 3980–3987. [Google Scholar] [CrossRef]
- Navarro, A.S.; Le Bihan, T.; Le Saëc, P.; Le Bris, N.; Bailly, C.; Saï-Maurel, C.; Bourgeois, M.; Chérel, M.; Tripier, R.; Faivre-Chauvet, A. TE1PA as innovating chelator for 64Cu immuno-TEP imaging: A comparative in vivo study with DOTA/NOTA by conjugation on 9E7.4 mAb in a syngeneic multiple myeloma model. Bioconjug. Chem. 2019, 30, 2393–2403. [Google Scholar] [CrossRef]
- Klasen, B.; Moon, E.S.; Rösch, F. AAZTA5-squaramide ester competing with DOTA-, DTPA- and CHX-A’’-DTPA-analogues: Promising tool for 177Lu-labeling of monoclonal antibodies under mild conditions. Nucl. Med. Biol. 2021, 96–97, 80–93. [Google Scholar] [CrossRef]
- Kovacs, Z.; Archer, E.A.; Russell, M.K.; Sherry, A.D. A convenient synthesis of 1,4,7,10,13-pentaazacyclopentadecane. Synth. Commun. 1999, 29, 2817–2822. [Google Scholar] [CrossRef]
- Massue, J.; Plush, S.E.; Bonnet, C.S.; Moore, D.A.; Gunnlaugsson, T. Selective mono N-alkylations of cyclen in one step synthesis. Tetrahedron Lett. 2007, 48, 8052–8055. [Google Scholar] [CrossRef]
- La-Venia, A.; Dzijak, R.; Rampmaier, R.; Vrabel, M. An optimized protocol for the synthesis of peptides containing trans-cyclooctene and bicyclononyne dienophiles as useful multifunctional bioorthogonal probes. Chem. Eur. J. 2021, 27, 13632–13641. [Google Scholar] [CrossRef]
- Debets, M.F.; van Berkel, S.S.; Schoffelen, S.; Rutjes, F.P.J.T.; van Hest, J.C.M.; van Delft, F.L. Azadibenzocyclooctynes for fast and efficient enzyme PEGylation via copper-free (3 + 2) cycloaddition. Chem. Commun. 2010, 46, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Academic Press, Elsevier: Boston, MA, USA, 2013. [Google Scholar]
- Mortimer, J.E.; Bading, J.R.; Park, J.M.; Frankel, P.H.; Carroll, M.I.; Tran, T.T.; Poku, E.K.; Rockne, R.C.; Raubitschek, A.A.; Shively, J.E.; et al. Tumor uptake of 64Cu-DOTA-trastuzumab in patients with metastatic breast cancer. J. Nucl. Med. 2018, 59, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Lindmo, T.; Boven, E.; Cuttitta, F.; Fedorko, J.; Bunn, P.A. Determination of the immunoreactive function of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J. Immunol. Methods 1984, 72, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Mattes, J. Limitations of the lindmo method in determining antibody immunoreactivity. Int. J. Cancer 1995, 61, 286–288. [Google Scholar] [CrossRef]
- Konishi, S.; Hamacher, K.; Vallabhajosula, S.; Kothari, P.; Bastidas, D.; Bander, N.; Goldsmith, S. Determination of immunoreactive fraction of radiolabelled monoclonal antibodies: What is an appropriate method? Cancer Biother. Radiopharm. 2004, 19, 706–715. [Google Scholar]
- Denoël, T.; Pedrelli, L.; Pantaleo, G.; Prio, J.O. A robust method for assaying the immunoreactive fraction in nonequilibrium systems. Pharmaceuticals 2019, 12, 177. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 6th ed.; Elsevier: Oxford, UK, 2009. [Google Scholar]
- Pilichowski, J.F.; Borel, M.; Meyniel, G. Synthesis of macrocyclic polyamines: Application to the increase of urinary excretion of copper in rats. Eur. J. Med. Chem. 1984, 19, 425–431. [Google Scholar]
- Dudkin, V.; Goldberg, S.; Erhardt, J.; Salter, R.; Mcdevitt, T. Radiolabeling of Polypeptides. Patent WO2019125982, 27 June 2019. [Google Scholar]
- Frindel, M.; Le Saëc, P.; Beyler, M.; Navarro, A.S.; Saï-Maurel, C.; Alliot, C.; Chérel, M.; Gestin, J.F.; Faivre-Chauvet, A.; Tripier, R. Cyclam te1pa for 64Cu PET imaging. Bioconjugation to antibody, radiolabeling and preclinical application in xenografted colorectal cancer. RSC Adv. 2017, 7, 9272–9283. [Google Scholar] [CrossRef]
- Chadwick, R.C.; Van Gyzen, S.; Adronov, A. Scalable synthesis of strained cyclooctyne derivatives. Synthesis 2014, 46, 669–677. [Google Scholar] [CrossRef]
- McNelles, S.A.; Adronov, A. Rapid synthesis of functionalized high-generation polyester dendrimers via strain-promoted alkyne-azide cycloaddition. Macromolecules 2017, 50, 7993–8001. [Google Scholar] [CrossRef]
- Campbell-Verduyn, L.S.; Mirfeizi, L.; Schoonen, A.K.; Dierckx, R.A.; Elsinga, P.H.; Feringa, B.L. Strain promoted copper-free "click" chemisty for 18F-radiolabeling of bombesin. Angew. Chem. Int. Ed. 2011, 50, 11117–11120. [Google Scholar] [CrossRef] [PubMed]
- Yale, H.L.; Sowinski, F.A. 5-(Substituted amino-lower alkylene)-5,6-dihydrodibenz[b,f]azocines. Patent United States Patent 3514443, 26 May 1970. [Google Scholar]
- von Wantoch Rekowski, M.; Kumar, V.; Zhou, Z.; Moschner, J.; Marazioti, A.; Bantzi, M.; Spyroulias, G.A.; van den Akker, F.; Giannis, A.; Papapetropoulos, A. Insights into soluble guanylyl cyclase activation derived from improved heme-mimetics. J. Med. Chem. 2013, 56, 8948–8952. [Google Scholar] [CrossRef]
- Wei, W.H.; Tomohiro, T.; Kodaka, M.; Okuno, H. Selective synthesis and kinetic measurement of 1:1 and 2:2 cyclic compounds containing 1,4,7,10-tetraazacyclododecane and azobenzene units. J. Org. Chem. 2000, 65, 8979–8987. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.A. Selective trialkylation of cyclen with tert-butyl bromoacetate [1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid, tri-tert-butyl ester hydrobromide]. Org Synth. 2008, 85, 10–14. [Google Scholar] [CrossRef][Green Version]
- Jagadish, B.; Brickert-Albrecht, G.L.; Nichol, G.S.; Mash, E.A.; Raghunand, N. On the synthesis of 1,4,7-tris(tert-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane. Tetrahedron Lett. 2011, 52, 2058–2061. [Google Scholar] [CrossRef]
- Strauch, R.C.; Mastarone, D.J.; Sukerkar, P.A.; Song, Y.; Ipsaro, J.J.; Meade, T.J. Reporter protein-targeted probes for magnetic resonance imaging. J. Am. Chem. Soc. 2011, 133, 16346–16349. [Google Scholar] [CrossRef]
- Kohl, S.W.; Kuse, K.; Hummert, M.; Schumann, H.; Mügge, C.; Janek, K.; Weisshoff, H. New synthetic routes for 1-benzyl-1,4,7,10-tetraazacyclododecane and 1,4,7,10-tetraazacyclododecane-1-acetic acid ethyl ester, important starting materials for metal-coded DOTA-based affinity tags. Z. Naturforsch. B 2007, 62b, 397–406. [Google Scholar] [CrossRef][Green Version]
- Grate, J.W.; Mo, K.F.; Daily, M.D. Triazine-based sequence-defined polymers with side-chain diversity and backbone-backbone interaction motifs. Angew. Chem. Int. Ed. Engl. 2016, 55, 3925–3930. [Google Scholar] [CrossRef]
- Montagner, D.; Gandin, V.; Marzano, C.; Erxleben, A. Phosphate diester cleavage, DNA interaction and cytotoxic activity of a bimetallic bis(1,4,7-triazacyclononane) zinc complex. Eur. J. Inorg. Chem. 2014, 2014, 4084–4092. [Google Scholar] [CrossRef]
- Searle, G.H.; Geue, R.J. Improved Richman-Atkins syntheses of cyclic polyamines particularly 1,4,7-triazacyclononane (tacn) and 1,4,7-triazacyclodecane (tacd), with the aid of cation-exchange in purification and isolation. Aust. J. Chem. 1984, 37, 959–970. [Google Scholar] [CrossRef]
- Zhang, J.; Voegtle, F. Synthesis of N,N’,N’’-trisubstituted 1,4,7-triazacyclononane. Chin. J. Org. Chem. 1987, 273–277. [Google Scholar]
- Stavila, V.; Allali, M.; Canaple, L.; Stortz, Y.; Franc, C.; Maurin, P.; Beuf, O.; Dufay, O.; Samarut, J.; Janier, M.; et al. Significant relaxivity gap between a low-spin and a high-spin iron(II) complex of structural similarity: An attractive off-on system for the potential design of responsive MRI probes. New J. Chem. 2008, 32, 428–435. [Google Scholar] [CrossRef]
- Yoshida, M.; Narita, M.; Hara, S. Asymmetric Michael addition of malonates to enones catalyzed by a primary β-amino acid and its lithium salt. J. Org. Chem. 2011, 76, 8513–8517. [Google Scholar] [CrossRef] [PubMed]
- Albert, R.; Danklmaier, J.; Hönig, H.; Kandolf, H. A simple and convenient synthesis of β-aspartates and γ-glutamates. Synthesis 1987, 1987, 635–637. [Google Scholar] [CrossRef]
- Lamarque, L.; Montgomery, C.; Parker, D. Pyridyl-aza(thio)xanthone Sensitizer Comprizing Lanthanide(III) ion Complexing Compounds, Their Luminescent Lanthanide (III) ion Complexes and use Thereof as Fluorescent Labels. Patent WO2010084090, 29 July 2010. [Google Scholar]
- Shetty, D.; Choi, S.Y.; Jeong, J.M.; Lee, J.Y.; Hoigebazar, L.; Lee, Y.S.; Lee, D.S.; Chung, J.K.; Lee, M.C.; Chung, Y.K. Stable aluminium fluoride chelates with triazacyclononane derivatives proved by X-ray crystallography and 18F-labeling study. Chem. Commun. 2011, 47, 9732–9734. [Google Scholar] [CrossRef] [PubMed]



| Radioimmunoconjugate | Average Number of Chelators per mAb a | IRF (%) a |
|---|---|---|
| [125I]I-trastuzumab | n.a. b | 93.8 ± 0.6 c |
| [64Cu]Cu-DOTA-trastuzumab | 3.74 ± 0.18 | 91.2 ± 6.8 |
| [64Cu]Cu-15-5-trastuzumab | 3.64 ± 0.16 | 87.9 ± 1.2 |
| [64Cu]Cu-NODAGA-trastuzumab | 3.89 ± 0.22 | 93.8 ± 4.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maisonial-Besset, A.; Witkowski, T.; Quintana, M.; Besse, S.; Gaumet, V.; Cordonnier, A.; Alliot, C.; Vidal, A.; Denevault-Sabourin, C.; Tarrit, S.; et al. Synthesis and In Vitro Comparison of DOTA, NODAGA and 15-5 Macrocycles as Chelators for the 64Cu-Labelling of Immunoconjugates. Molecules 2023, 28, 75. https://doi.org/10.3390/molecules28010075
Maisonial-Besset A, Witkowski T, Quintana M, Besse S, Gaumet V, Cordonnier A, Alliot C, Vidal A, Denevault-Sabourin C, Tarrit S, et al. Synthesis and In Vitro Comparison of DOTA, NODAGA and 15-5 Macrocycles as Chelators for the 64Cu-Labelling of Immunoconjugates. Molecules. 2023; 28(1):75. https://doi.org/10.3390/molecules28010075
Chicago/Turabian StyleMaisonial-Besset, Aurélie, Tiffany Witkowski, Mercedes Quintana, Sophie Besse, Vincent Gaumet, Axel Cordonnier, Cyrille Alliot, Aurélien Vidal, Caroline Denevault-Sabourin, Sébastien Tarrit, and et al. 2023. "Synthesis and In Vitro Comparison of DOTA, NODAGA and 15-5 Macrocycles as Chelators for the 64Cu-Labelling of Immunoconjugates" Molecules 28, no. 1: 75. https://doi.org/10.3390/molecules28010075
APA StyleMaisonial-Besset, A., Witkowski, T., Quintana, M., Besse, S., Gaumet, V., Cordonnier, A., Alliot, C., Vidal, A., Denevault-Sabourin, C., Tarrit, S., Levesque, S., Miot-Noirault, E., & Chezal, J.-M. (2023). Synthesis and In Vitro Comparison of DOTA, NODAGA and 15-5 Macrocycles as Chelators for the 64Cu-Labelling of Immunoconjugates. Molecules, 28(1), 75. https://doi.org/10.3390/molecules28010075