Possibility of New Active Substrates (ASs) to Be Used to Prevent the Migration of Heavy Metals to the Soil and Water Environments
Abstract
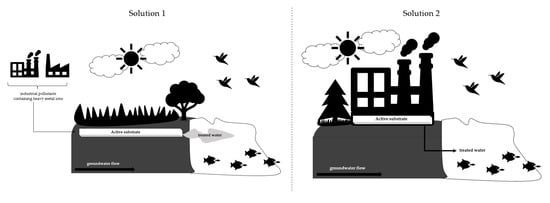
1. Introduction
2. Results
2.1. Processes of the Sorption of Metal Ions onto Active Substrates
Percentage of Metal Ion Removal from the Solutions (%Rads)
2.2. Quantification of Aliquat in Soil for Its Leaching from Active Substrates
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Procedure for Cleaning and Preparing the Soil for Research
4.3. Preparation of Active Substrates (ASs)
- Preparation of active substrates AS-1, AS-2, and AS-3
- Preparation of active substrate AS-4
- Preparation of AS-5
4.4. Preparation of Aqueous Solutions
- Solution 1 (S-1)
- Solution 2 (S-2)
- Solution 3 (S-3)
4.5. Methodology of the Sorption of Metal Ions onto Active Substrates
- Experiment 1
- Experiment 2
- Experiment 3
- Experiment 4
- Experiment 5
4.6. Quantification of Soil Using Gas Chromatography to Determine the Elution of Aliquat 336 from the Active Substrates
4.7. Determination of Metals in the Water Solution and Soil Filtrate by the AAS Method
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Okpashi, V.E. Health Risk of Ingested Heavy Metals in Fluidized Canned Milks: Are We Drinking Heavy Metals. J. Food Qual. 2022, 2683095. [Google Scholar] [CrossRef]
- Momodu, M.A.; Anyakora, C.A. Heavy metal contamination of ground water: The Surulere case study. Res. J. Environ. Earth Sci. 2010, 2, 39–43. [Google Scholar]
- Ali, I.; Hasan, M.A.; Alharbi, O.M.L. Toxic metal ions contamination in the groundwater. Kingd. Saudia Arabia J. Taibah Univ. Sci. 2020, 14, 1571–1579. [Google Scholar] [CrossRef]
- Sarfraz, S.; Abid, A.J.; Javed, M.; Iqbal, S.; Aljazzar, S.O.; Zahra, M.; Alrbyawi, H.; Elkaeed, E.B.; Somaily, H.H.; Pashameah, R.A.; et al. Chromium (III) ions were extracted from wastewater effluent using a synergistic green membrane with a binary combination of D2EHPA and kerosene. Catalysts 2022, 12, 1220. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef]
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation technologies for heavy metal contaminated groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barrier for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef]
- Moore, R.; Szecsody, J.; Rigali, M.; Vermuel, V.; Luellen, J.R. Assesment of hydroxyapatite permeable reactive barrier to remediate uranium at the old rifle site, Colorado—16193 Development of Sorbents and Permeable Reactive Barriers for Radionuclide Remediation. In Proceedings of the WM2016 Conference, Phoenix, AZ, USA, 6–10 March 2016. [Google Scholar]
- Han, W.; Fu, F.; Cheng, Z.; Tang, B.; Wu, S. Studies on the optimum conditions using acid-washed zero-valent iron/aluminum mixtures in permeable reactive barriers for the removal of different heavy metal ions from wastewater. J. Hazard. Mater. 2016, 302, 437–446. [Google Scholar] [CrossRef]
- Kornilovych, B.; Wireman, M.; Ubaldini, S.; Guglietta, D.; Koshik, Y.; Caruso, B.; Kovalchuk, I. Uranium removal from fround water by permeable reactive barrier with zero-valent iron and organic carbon mixtures: Laboratory and field studies. Metals 2018, 8, 408. [Google Scholar] [CrossRef]
- Wantanaphong, J.; Mooney, S.J.; Bailey, E.H. Natural and waste materials as metal sorbents in permeable reactive barriers (PRBs). Environ. Chem. Lett. 2005, 3, 19–23. [Google Scholar] [CrossRef]
- Komnitsas, K.; Bazdanis, G.; Bartzas, G.; Sahinkaya, E.; Zaharaki, D. Removal of heavy metals from leachates using organic/inorganic permeable reactive barriers. Desalin. Water Treat. 2013, 51, 3052–3059. [Google Scholar] [CrossRef]
- Faisal, A.H.; Abdul-Kareem, M.B.; Mohammed, A.K.; Naushed, M.; Ghfar, A.A.; Ahamad, T. Humic acid coated sand as a novel sorbent in permeable reactive barrier for environmental remediation of groundwater polluted with copper and cadmium ions. J. Water Process. Eng. 2020, 36, 101373. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, K.; Zhang, Y.; Zou, H. Remediation of persistent organic pollutant-contaminated soil using biosurfactant-enhanced electrokinetics coupled with a zero-valent iron/activated carbon permeable reactive barrier. Environ. Sci. Pollut. Res. 2017, 24, 28142–28151. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Vigneswaran, S.; Naidu, R. Permeable reactive barrier for groundwater remediation. J. Ind. Eng. Chem. 2008, 14, 145–156. [Google Scholar] [CrossRef]
- Xiao, J.; Pang, Z.; Zhou, S.; Chu, L.; Rong, L.; Liu, Y.; Li, J.; Tian, L. The mechanism of acid-washed zero-valent iron/activated carbon as permeable reactive barrier enhanced electrokinetic remediation of uranium-contaminated soil. Sep. Purif. Technol. 2020, 244, 116667. [Google Scholar] [CrossRef]
- Thakur, A.K.; Vithanage, M.; Das, D.B.; Kumar, M. A review on design, material selection, mechanism, and modeling of permeable reactive barrier for community-scale groundwater treatment. Environ. Technol. Innov. 2020, 19, 100917. [Google Scholar] [CrossRef]
- Harrup, M.K.; Jones, M.G.; Polson, L.; White, B. Polymer/silicate composites: New materials for subsurface permeable reactive barriers. J. Sol-Gel Sci. Technol. 2008, 47, 243–251. [Google Scholar] [CrossRef]
- Blowes, D.W.; Ptacek, C.J.; Benner, S.G.; McRae, C.W.T.; Bennett, T.A.; Plus, R.W. Treatment of inorganic contaminants using permeable reactive barriers. J. Contam. Hydrol. 2000, 45, 123–137. [Google Scholar] [CrossRef]
- Mikkola, J.-P.; Virtanen, P.; Sjoholm, R. Aliquat 336—A versatile and affordable cation for an entirely new family of hydrophobic ionic liquids. Green Chem. 2006, 8, 250–255. [Google Scholar] [CrossRef]
- Lee, L.Y.; Morad, N.; Ismail, N.; Talebi, A.; Rafatullah, M. Optimization for liquid-liquid extraction of Cd(II) over Cu(II) ions from aqueous solution using ionic liquid Aliquat 336 with Tributyl Phosphate. Int. J. Mol. Sci. 2020, 21, 6860. [Google Scholar] [CrossRef] [PubMed]
- Sellami, F.; Kebiche-Senhadjai, O.; Marais, S.; Couvrat, N.; Fatyeyeva, K. Polymer inclusion membranes based on CTA/PBAT blend containing Aliquat 336 as extractant for removal of Cr(VI): Efficiency, stability and selectivity. React. Funct. Pol. 2019, 139, 120–132. [Google Scholar] [CrossRef]
- Sellami, F.; Kebiche-Sendaji, O.; Marais, S.; Colasse, L.; Fatyeyeva, K. Enhance removal of Cr(VI) by polymer inclusion membrane based on poly(vinylidene fluoride) and Aliquat 336. Sep. Purif. Techn. 2020, 248, 117038. [Google Scholar] [CrossRef]
- Kumar, A.S.; Sharm, S.; Reddy, R.S.; Barathi, M.; Rajesh, N. Comprehending the interaction between chitosan and ionic liquid for the adsorption of palladium. Int. J. Biol. Macromol. 2015, 72, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ma, Z.; Li, D.; Tian, Q.; Xu, Z. Recovery of Re(VII) from aqueous solution with coated impregnated resins containing ionic liquid Aliquat 336. Hydrometallurgy 2019, 190, 105149. [Google Scholar] [CrossRef]
- Martins, R.; Pardo, R.; Boaventura, R. Cadmium(II) and zinc(II) adsorption by the aquatic moss Fontinalis antipyretic: Effect of temperature, pH and water hardness. Water Res. 2004, 38, 693–699. [Google Scholar] [CrossRef]
- Afolabi, F.O.; Musonge, P.; Bakare, B.F. Bio-sorption of copper and lead ions in single and binary systems onto banana peels. Cogent Eng. 2021, 8, 1886730. [Google Scholar] [CrossRef]
- Witt, K.; Bożejewicz, D.; Kaczorowska, M.A. N,N’-Bis(salicylidene)ethylenediamine (Salen) as an active compound for the recovery of Ni(II), Cu(II), and Zn(II) ions from aqueous solutions. Membranes 2020, 10, 60. [Google Scholar] [CrossRef]
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives OJ L 312, 22.11.2008, pp. 3–30. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008L0098 (accessed on 6 November 2022).
- Kumbasar, R.A. Transport of cadmium ions from zinc plant leach solutions through emulsion liquid membrane-containing Aliquat 336 as carrier. Sep. Purif. Technol. 2008, 63, 592–599. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Lee, M.S. Separation of Co(II), Cu(II), Ni(II) and Mn(II) from synthetic hydrochloric acid leaching solution of spent lithium ion batteries by solvent extraction. Physicochem. Probl. Miner. Process. 2020, 54, 599–610. [Google Scholar] [CrossRef]
- Zhu, F.; Tan, X.; Zhao, W.; Feng, L.; He, S.; Wei, L.; Yang, L.; Wang, K.; Zhao, Q. Efficiency assessment of ZVI-based media as fillers in permeable reactive barrier for multiple heavy metal-contaminated groundwater remediation. J. Hazard. Mater. 2022, 424, 127605. [Google Scholar] [CrossRef]
- Fedoročková, A.; Raschman, P.; Sučik, G.; Švandová, M.; Doráková, A. Reactive, Sparingly Soluble Calcined Magnesia, Tailor-Made as the Reactive Material for Heavy Metal Removal from Contaminated Groundwater Using Permeable Reactive Barrier. Minerals 2021, 11, 1153. [Google Scholar] [CrossRef]
- Bahrami, S.; Yaftian, M.R.; Najvak, P.; Dolatyari, L.; Shayani-Jam, H.; Kolev, S.D. PVDF-HFP based polymer inclusion membranes containing Cyphos IL 101 and Aliquat 336 for the removal Cr(VI) from sulfate solution. Sep. Purif. Technol. 2020, 250, 117251. [Google Scholar] [CrossRef]
- Annane, K.; Sahmoune, S.; Montels, P.; Tingry, S. Polymer inclusion membrane extraction of cadmium(II) with Aliquat 336 in micro-channel cel. Chem. Eng. Res. Des. 2015, 94, 605–610. [Google Scholar] [CrossRef]
- Khadivi, M.; Javaqnbakht, V. Emulsion ionic liquid membrane using edible paraffin oil for lead removal from aqueous solutions. J. Mol. Liq. 2020, 319, 114137. [Google Scholar] [CrossRef]
- Kagaya, S.; Maeno, T.; Ito, K.; Gemmei-Ide, M.; Cattrall, R.W.; Kolev, S.D. Improvement of chromium(VI) from acidic solutions using a poly(vinyl chloride)-based polymer inclusion membrane with Aliquat 336 as the carrier. Anal. Sci. 2017, 33, 643–646. [Google Scholar] [CrossRef]
- Semghouni, H.; Bey, S.; Figoli, A.; Criscuoli, A.; Benamor, M.; Drioli, E. Chromium(VI) removal by Aliquat-336 in a novel multiframe flat sheet membrane conactor. Chem. Eng. Process. 2020, 147, 1007765. [Google Scholar] [CrossRef]
- Yu, X.; Muhammad, F.; Yan, Y.; Yu, L.; Li, H.; Huang, X.; Jiao, B.; Lu, N.; Li, D. Effect of chemical additives on electrokinetic remediation of Cr-contaminated soil coupled with a permeable reactive barrier. R. Soc. Open Sci. 2019, 6, 182138182138. [Google Scholar] [CrossRef]
- Khalil, A.H.; Abdalwahedb, B. Remediation of nickel-contaminated clayey soil by electro-kinetic technology coupled with zeolite—A permeable reactive barrier. EREM 2017, 73, 58–69. [Google Scholar] [CrossRef][Green Version]
- Liu, T.; Yang, X.; Wang, Z.-L.; Yan, X. Enhanced chitosan beads-supported Fe0-nanoparticles for removal of heavy metals from electroplating wastewater in permeable reactive barriers. Water Res. 2013, 47, 6691–6700. [Google Scholar] [CrossRef]
- Lee, S.; Yun, J.-M.; Lee, J.-Y.; Hong, G.; Kim, J.-S.; Kim, D.; Han, J.-G. The remediation characteristic of heavy metals (copper, and lead) on applying recycled food waste ash and electrokinetic remediation techniques. Apply. Sci. 2021, 11, 7437. [Google Scholar] [CrossRef]
- Rajas, C.M.M.; Valasquez, M.F.R.; Tavolaro, A.; Molinari, A.; Fallico, C. Use of vegetable fibers for PRB to remove heavy metals from contaminated aquifers-comparisons among Cabuya fibers, broom fibers and ZVI. Int. J. Environ. Res. Public Health 2017, 14, 684. [Google Scholar] [CrossRef]







| Metal Ions | qt, mg/g | ||||
|---|---|---|---|---|---|
| AS-1 | AS-2 | AS-3 | AS-4 | AS-5 | |
| Cr(VI) | 1.34 | 0.70 | 1.81 | 1.32 | 1.01 |
| Ni(II) | 0.78 | 0.00 | 0.83 | 0.57 | 0.94 |
| Zn(II) | 0.40 | 0.57 | 0.00 | 0.00 | 0.00 |
| Cd(II) | 0.81 | 0.11 | 1.72 | 1.51 | 1.34 |
| Pb(II) | 0.82 | 0.19 | 1.31 | 0.94 | 1.43 |
| Cu(II) | 0.00 | 0.00 | 0.00 | 0.71 | 0.84 |
| Experiment No./Active Substrates | % Rads, % | |||||
|---|---|---|---|---|---|---|
| Cr(VI) | Ni(II) | Cu(II) | Zn(II) | Cd(II) | Pb(II) | |
| 3/AS-3 | 63.22 | 37.77 | 0 | 0 | 78.01 | 45.91 |
| 4/AS-4 | 60.59 | 26.06 | 65.11 | 0 | 69.83 | 42.92 |
| %Rads, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Metal Ions | AS-1 | AS-2 | AS-3 | AS-4 | AS-5 | PRB | PIM | ELM | SE |
| Cr(VI) | 93.50 | 81.66 | 63.22 | 60.59 | 54.77 | 95.65 | 77.34 | - | - |
| Ni(II) | 54.30 | 0 | 37.77 | 26.06 | 50.96 | 5–80 | - | 1.50 | 92.20 |
| Zn(II) | 28.10 | 66.67 | 0 | 0 | 0 | 5–98 | - | 28.00 | - |
| Cd(II) | 56.70 | 13.23 | 78.01 | 69.38 | 72.76 | - | 11–90 | 90.50 | - |
| Pb(II) | 57.10 | 22.33 | 45.91 | 42.92 | 77.12 | - | - | 95 | - |
| Cu(II) | 0 | 0 | 0 | 65.11 | 0 | 92–99 | - | - | 71.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witt, K.; Studziński, W.; Bożejewicz, D. Possibility of New Active Substrates (ASs) to Be Used to Prevent the Migration of Heavy Metals to the Soil and Water Environments. Molecules 2023, 28, 94. https://doi.org/10.3390/molecules28010094
Witt K, Studziński W, Bożejewicz D. Possibility of New Active Substrates (ASs) to Be Used to Prevent the Migration of Heavy Metals to the Soil and Water Environments. Molecules. 2023; 28(1):94. https://doi.org/10.3390/molecules28010094
Chicago/Turabian StyleWitt, Katarzyna, Waldemar Studziński, and Daria Bożejewicz. 2023. "Possibility of New Active Substrates (ASs) to Be Used to Prevent the Migration of Heavy Metals to the Soil and Water Environments" Molecules 28, no. 1: 94. https://doi.org/10.3390/molecules28010094
APA StyleWitt, K., Studziński, W., & Bożejewicz, D. (2023). Possibility of New Active Substrates (ASs) to Be Used to Prevent the Migration of Heavy Metals to the Soil and Water Environments. Molecules, 28(1), 94. https://doi.org/10.3390/molecules28010094