Visualization of Phototherapy Evolution by Optical Imaging
Abstract
:1. Introduction
2. Phototherapy
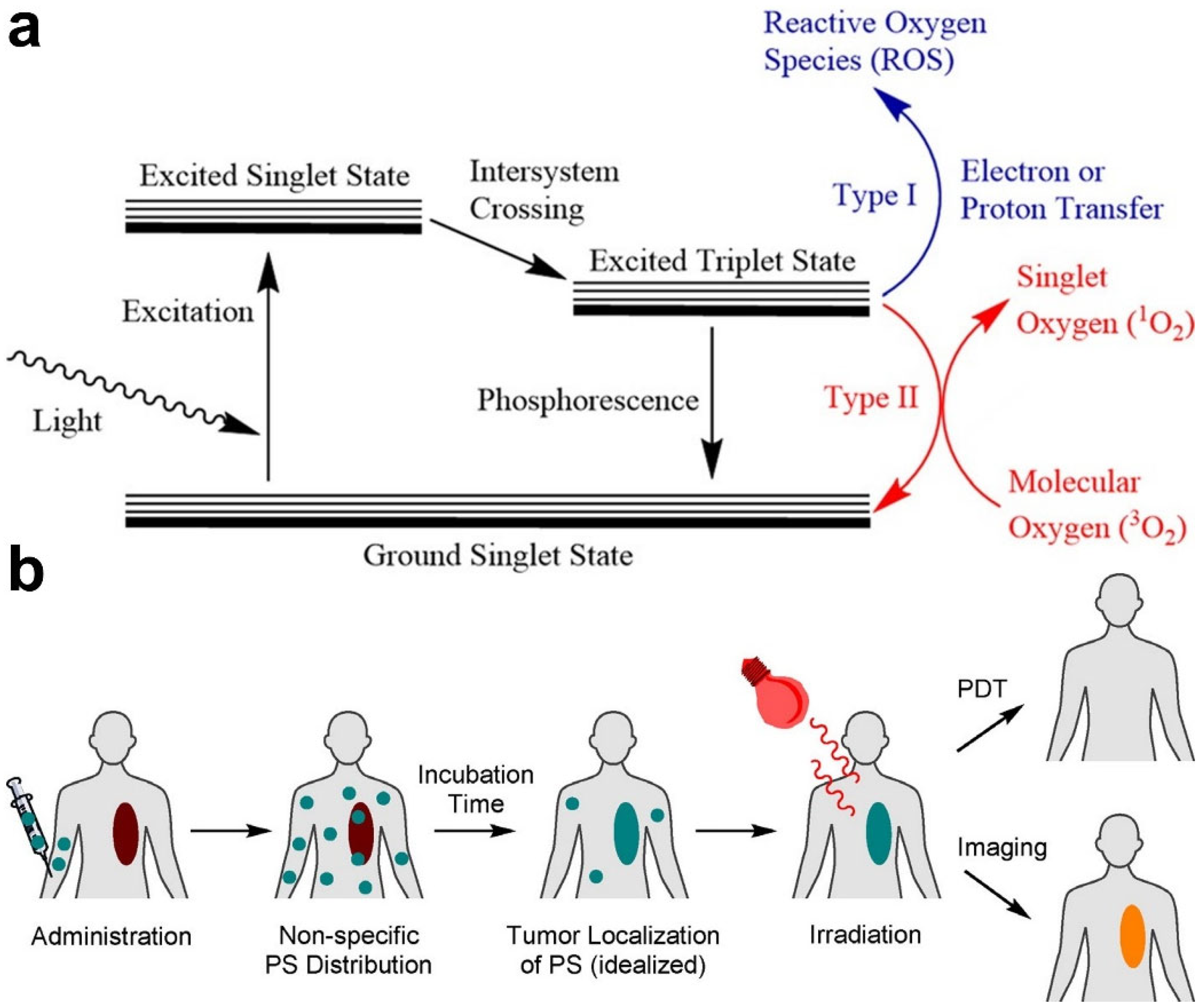
2.1. PDT
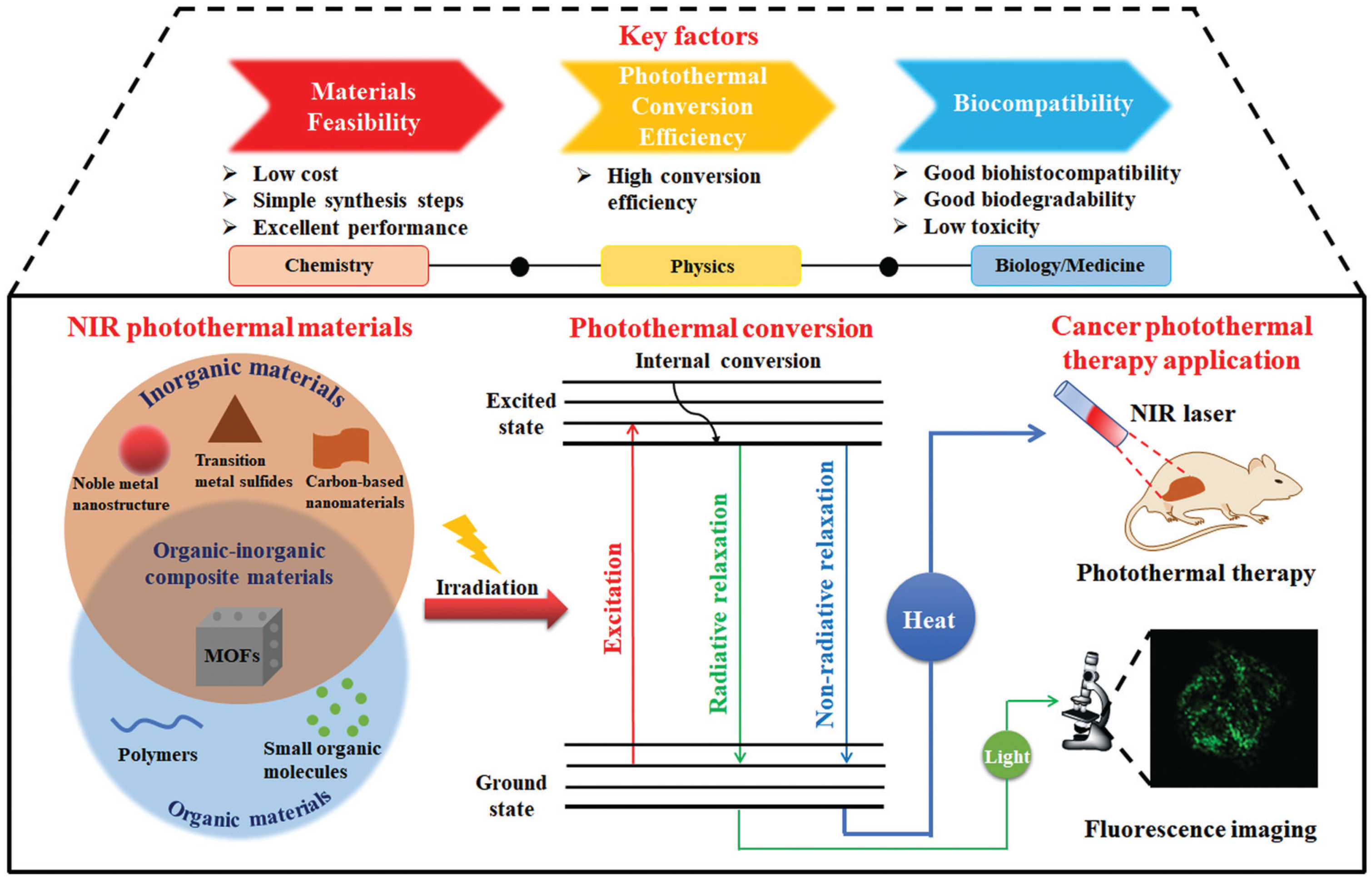
2.2. PTT
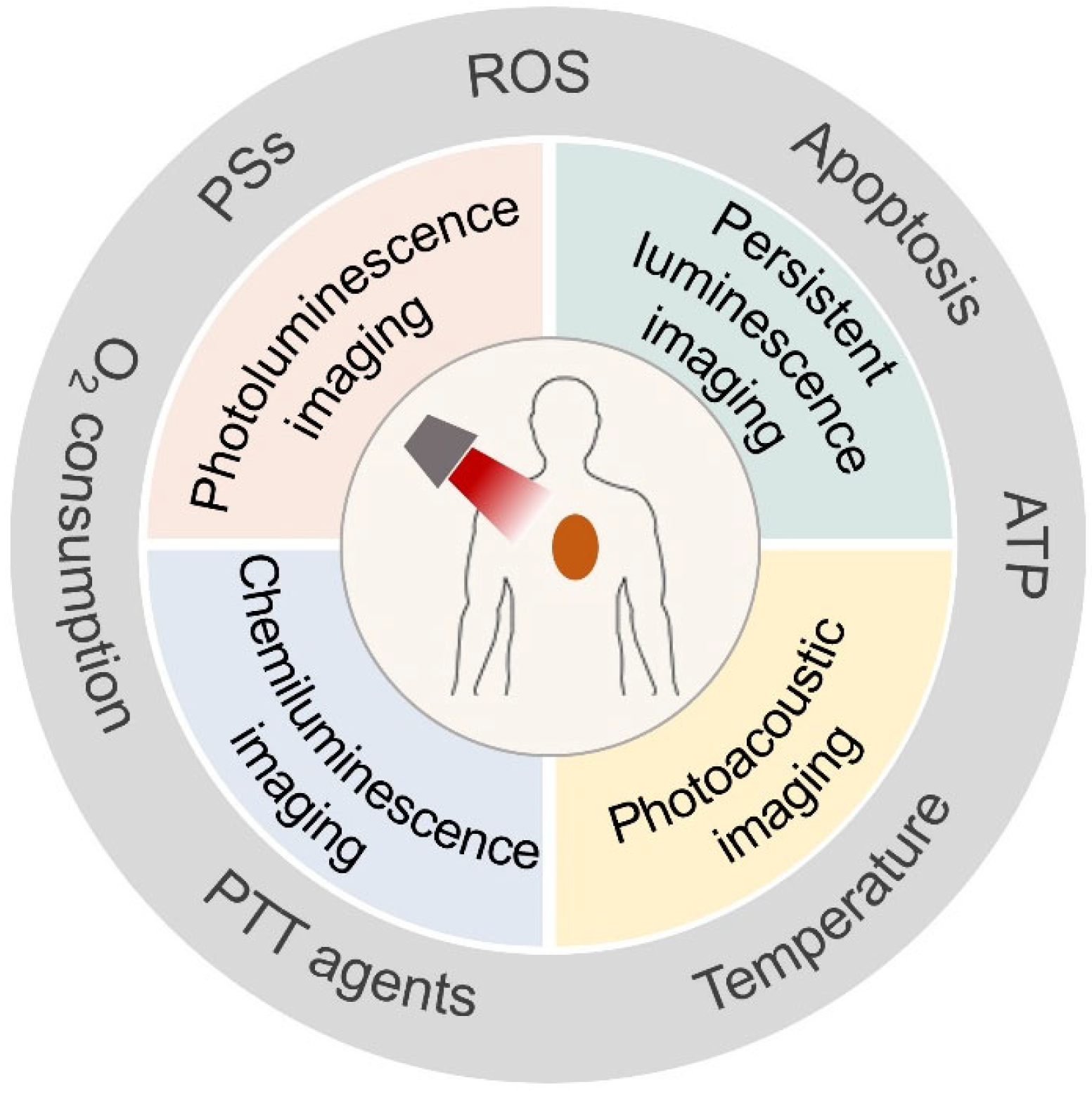
3. Optical Imaging Technology
3.1. Photoluminescence Imaging
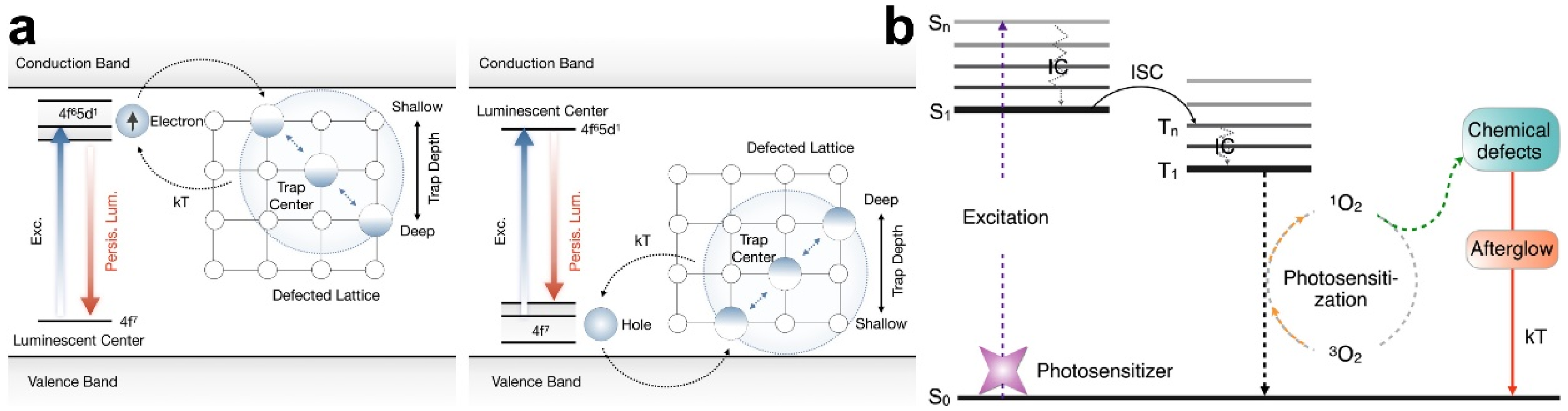
3.2. Persistent Luminescence Imaging
3.3. Chemiluminescence Imaging
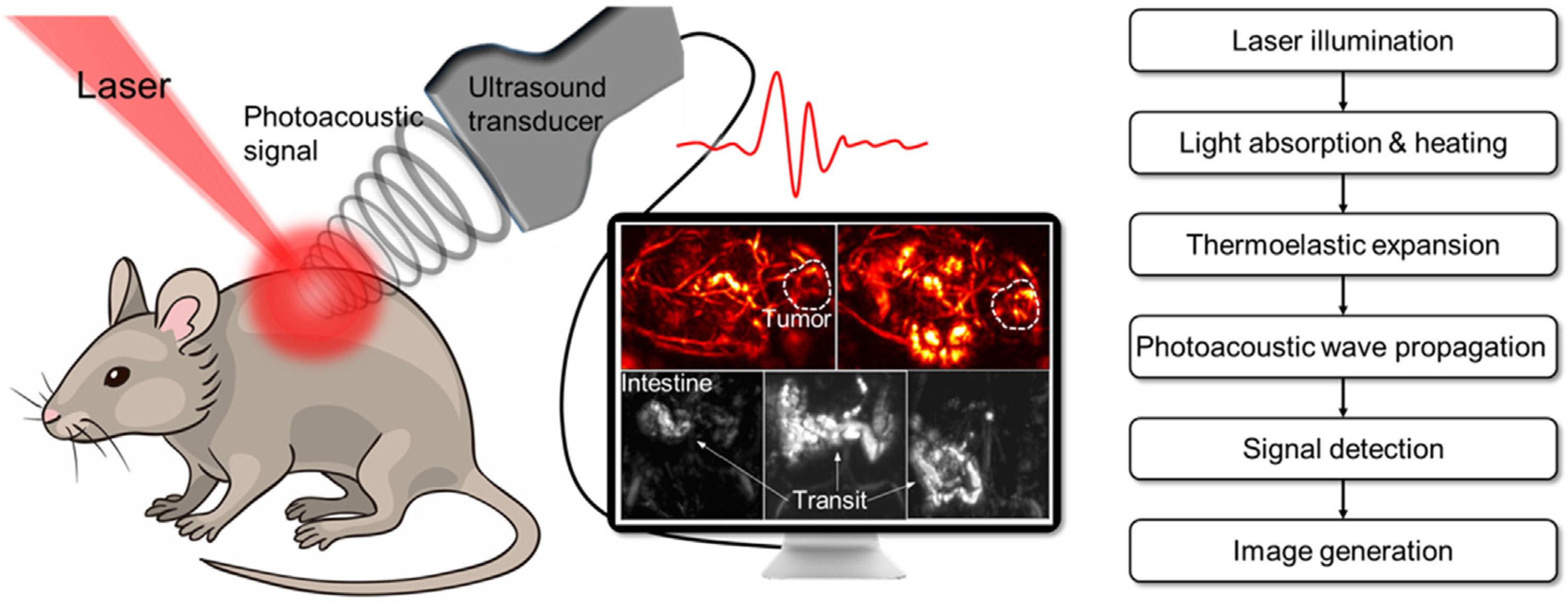
3.4. Photoacoustic Imaging
4. PDT Evaluation
4.1. Monitoring of PSs
4.2. Monitoring of ROS
4.3. Monitoring of Cell Apoptosis
4.4. Monitoring of Other Biomarkers
5. PTT Evaluation
5.1. Monitoring of PTT Agents
5.2. Monitoring of Temperature
5.3. Monitoring of Cell Apoptosis
6. Conclusions and Perspectives
- (1)
- Improving the biocompatibility of therapeutic agents. The therapeutic agents are usually composed of metal ions and a polycyclic structure, which inevitably causes unexpected damage to the skin, normal tissues, or organs during blood circulation. It is highly necessary to synthesize more safe and innoxious agents or design agents using natural drugs to obtain therapeutic agents with high biocompatibility and degradability levels for safer cancer therapy.
- (2)
- Enhancing the specificity of therapeutic agents toward tumors. Most of the therapeutic agents display side effects due to the always-on model under light and a lack of specificity toward tumors, resulting in undesired damage to normal tissues. In order to create precise medicine, developing activatable self-reporting agents that can only be activated in the tumor microenvironment is of great significance for the improvement of therapeutic accuracy and minimization of side effects.
- (3)
- Evaluating therapy performance based on the simultaneous imaging of multiple biomarkers. The therapy process and dynamic changes in tumors are complex. The signal produced by a single parameter may lead to a false-positive signal or limited signal–noise ratio, resulting in an incorrect evaluation of the therapy’s performance. Designing self-reporting agents for the simultaneous monitoring of multiple pathological parameters is crucial to enhance the precision of cancer therapy evaluations.
- (4)
- Increasing the penetration depth of therapeutic agents. The limited penetration depth of visible-emitting agents hinders in vivo applications. The luminescent units in agents need to be synthesized with extended absorption and emission wavelengths to the NIR, even NIR-II, window for deep-seated tumor therapy and high-resolution imaging.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The trends projection analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.; Warsame, C.; Seenivasagam, R.K.; Katiyar, N.K.; Aleem, E.; Goel, S. Nanoparticle-mediated cancer cell therapy: Basic science to clinical applications. Cancer Metastasis Rev. 2023, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Diener, M.K.; Mihaljevic, A.L.; Strobel, O.; Loos, M.; Schmidt, T.; Schneider, M.; Berchtold, C.; Mehrabi, A.; Müller-Stich, B.P.; Jiang, K. Periarterial divestment in pancreatic cancer surgery. Surgery 2021, 169, 1019–1025. [Google Scholar] [CrossRef]
- Lakkad, M.; Martin, B.; Li, C.; Harrington, S.; Dayer, L.; Painter, J.T. The use of gabapentinoids and opioids and risk of developing opioid-induced respiratory depression among older breast cancer survivors with neuropathic pain. J. Cancer Surviv. 2023, 1–11. [Google Scholar] [CrossRef]
- Cornen, S.; Vivier, E. Chemotherapy and tumor immunity. Science 2018, 362, 1355–1356. [Google Scholar] [CrossRef]
- Liu, S.; Khan, A.R.; Yang, X.; Dong, B.; Ji, J.; Zhai, G. The reversal of chemotherapy-induced multidrug resistance by nanomedicine for cancer therapy. J. Control. Release 2021, 335, 1–20. [Google Scholar] [CrossRef]
- Yang, K.; Yang, Z.; Yu, G.; Nie, Z.; Wang, R.; Chen, X. Polyprodrug Nanomedicines: An Emerging Paradigm for Cancer Therapy. Adv. Mater. 2022, 34, e2107434. [Google Scholar] [CrossRef]
- Jing, Z.; Du, Q.; Zhang, X.; Zhang, Y. Nanomedicines and nanomaterials for cancer therapy: Progress, challenge and perspectives. Chem. Eng. J. 2022, 446, 137147. [Google Scholar] [CrossRef]
- Lan, G.; Ni, K.; Lin, W. Nanoscale Metal-Organic Frameworks for Phototherapy of Cancer. Coord. Chem. Rev. 2019, 379, 65–81. [Google Scholar] [CrossRef]
- Shu, Y.; Linghu, X.; Zhao, Y.; Chen, Z.; Zhang, J.; Shan, D.; Liu, W.; Di, M.; Wang, B. Photodynamic and photothermal therapy-driven synergistic cancer treatment assisted by zeolitic imidazolate framework-8: A review. J. Drug Deliv. Sci. Technol. 2023, 81, 104272. [Google Scholar] [CrossRef]
- Wu, H.; Liu, L.; Song, L.; Ma, M.; Gu, N.; Zhang, Y. Enhanced Tumor Synergistic Therapy by Injectable Magnetic Hydrogel Mediated Generation of Hyperthermia and Highly Toxic Reactive Oxygen Species. ACS Nano 2019, 13, 14013–14023. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, H.; Bian, J.; Zhang, X.; Fu, Y.; Li, Z.; Wei, S.; Xu, Z.; Liu, X.; Liu, Z.; et al. Ag/Pd bimetal nanozyme with enhanced catalytic and photothermal effects for ROS/hyperthermia/chemotherapy triple-modality antitumor therapy. Chem. Eng. J. 2020, 397, 125438. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.; Wei, Z.; Feng, W.; Li, L.; Ma, L.; Li, F.; Zhou, J. Customized Photothermal Therapy of Subcutaneous Orthotopic Cancer by Multichannel Luminescent Nanocomposites. Adv. Mater. 2021, 33, e2008615. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, C.; Han, L.; Zhang, X.; Song, G. Optical-Magnetic probe for evaluating cancer therapy. Coord. Chem. Rev. 2021, 441, 213978. [Google Scholar] [CrossRef]
- Bensch, F.; van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.J.N.; van der Wekken, A.J. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M. New response evaluation criteria in solid tumours: Revised RECIST guideline. Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Neves, A.A.; Brindle, K.M. Imaging cell death. J. Nucl. Med. 2014, 55, 1–4. [Google Scholar] [CrossRef]
- Lindsay, K.E.; Bhosle, S.M.; Zurla, C.; Beyersdorf, J.; Rogers, K.A.; Vanover, D.; Xiao, P.; Araínga, M.; Shirreff, L.M.; Pitard, B. Visualization of early events in mRNA vaccine delivery in non-human primates via PET-CT and near-infrared imaging. Nat. Biomed. Eng. 2019, 3, 371–380. [Google Scholar] [CrossRef]
- Brennan, J.A.; Mao, L.; Hruban, R.H.; Boyle, J.O.; Eby, Y.J.; Koch, W.M.; Goodman, S.N.; Sidransky, D. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 1995, 332, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Moller, K.I.; Kongshoj, B.; Philipsen, P.A.; Thomsen, V.O.; Wulf, H.C. How Finsen’s light cured lupus vulgaris. Photodermatol. Photoimmunol. Photomed. 2005, 21, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Maisels, M.J.; McDonagh, A.F. Phototherapy for neonatal jaundice. N. Engl. J. Med. 2008, 358, 920–928. [Google Scholar] [CrossRef]
- Kemeny, L.; Varga, E.; Novak, Z. Advances in phototherapy for psoriasis and atopic dermatitis. Expert. Rev. Clin. Immunol. 2019, 15, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Diogo, M.L.G.; Campos, T.M.; Fonseca, E.S.R.; Pavani, C.; Horliana, A.; Fernandes, K.P.S.; Bussadori, S.K.; Fantin, F.; Leite, D.P.V.; Yamamoto, A.T.A.; et al. Effect of Blue Light on Acne Vulgaris: A Systematic Review. Sensors 2021, 21, 6943. [Google Scholar] [CrossRef]
- Zubair, R.; Hamzavi, I.H. Phototherapy for Vitiligo. Dermatol. Clin. 2020, 38, 55–62. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Zhao, Z.; Tang, B.Z.; Yoon, J. Organic photosensitizers for antimicrobial phototherapy. Chem. Soc. Rev. 2022, 51, 3324–3340. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, Z.; Almatrafi, E.; Huang, D.; Zhang, C.; Xiong, W.; Cheng, M.; Zhou, C.; Wang, W.; Song, B.; et al. Core-shell structured nanoparticles for photodynamic therapy-based cancer treatment and related imaging. Coord. Chem. Rev. 2022, 458, 214427. [Google Scholar] [CrossRef]
- Sun, J.; Xing, F.; Braun, J.; Traub, F.; Rommens, P.M.; Xiang, Z.; Ritz, U. Progress of Phototherapy Applications in the Treatment of Bone Cancer. Int. J. Mol. Sci. 2021, 22, 11354. [Google Scholar] [CrossRef]
- Hak, A.; Ravasaheb Shinde, V.; Rengan, A.K. A review of advanced nanoformulations in phototherapy for cancer therapeutics. Photodiagn. Photodyn. Ther. 2021, 33, 102205. [Google Scholar] [CrossRef]
- Karges, J. Clinical Development of Metal Complexes as Photosensitizers for Photodynamic Therapy of Cancer. Angew. Chem. Int. Ed. 2022, 61, e202112236. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J. Photodynamic therapy (PDT) of malignant tumors. Crit. Rev. Oncol. Hematol. 1984, 2, 83–116. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, S.; Chen, Y.; He, W.; Guo, Z. Recent Advances of Noble Metal Complex based Photodynamic Therapy. Chem. Sci. 2022, 13, 5085–5106. [Google Scholar] [CrossRef] [PubMed]
- Aires-Fernandes, M.; Botelho Costa, R.; Rochetti do Amaral, S.; Mussagy, C.U.; Santos-Ebinuma, V.C.; Primo, F.L. Development of Biotechnological Photosensitizers for Photodynamic Therapy: Cancer Research and Treatment-From Benchtop to Clinical Practice. Molecules 2022, 27, 6848. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Sun, I.-C.; Sook Hwang, H.; Yeol Yoon, H.; Kim, K. Light-triggered photodynamic nanomedicines for overcoming localized therapeutic efficacy in cancer treatment. Adv. Drug Deliv. Rev. 2022, 186, 114344. [Google Scholar] [CrossRef]
- Zhen, X.; Cheng, P.; Pu, K. Recent Advances in Cell Membrane-Camouflaged Nanoparticles for Cancer Phototherapy. Small 2019, 15, 1804105. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Ji, W.; Wang, J.; Wang, N.; Wu, W.; Wu, Q.; Hou, X.; Hu, W.; Li, L. Near infrared photothermal conversion materials: Mechanism, preparation, and photothermal cancer therapy applications. J. Mater. Chem. B 2021, 9, 7909–7926. [Google Scholar] [CrossRef]
- Suo, X.; Zhang, J.; Zhang, Y.; Liang, X.J.; Zhang, J.; Liu, D. A nano-based thermotherapy for cancer stem cell-targeted therapy. J. Mater. Chem. B 2020, 8, 3985–4001. [Google Scholar] [CrossRef]
- Ding, Z.; Gu, Y.; Zheng, C.; Gu, Y.; Yang, J.; Li, D.; Xu, Y.; Wang, P. Organic small molecule-based photothermal agents for cancer therapy: Design strategies from single-molecule optimization to synergistic enhancement. Coord. Chem. Rev. 2022, 464, 214564. [Google Scholar] [CrossRef]
- Chang, M.; Hou, Z.; Wang, M.; Li, C.; Lin, J. Recent Advances in Hyperthermia Therapy-Based Synergistic Immunotherapy. Adv. Mater. 2020, 33, 2004788. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Li, L.; Yang, Z.; Chen, X. Phototherapy meets immunotherapy: A win-win strategy to fight against cancer. Nanophotonics 2021, 10, 3229–3245. [Google Scholar] [CrossRef]
- Huang, J.; Pu, K. Activatable Molecular Probes for Second Near-Infrared Fluorescence, Chemiluminescence, and Photoacoustic Imaging. Angew. Chem. Int. Ed. 2020, 59, 11717–11731. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, W.; Zhang, B. ROS-responsive probes for low-background optical imaging: A review. Biomed. Mater. 2021, 16, 022002. [Google Scholar] [CrossRef]
- Li, J.; Pu, K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem. Soc. Rev. 2019, 48, 38–71. [Google Scholar] [CrossRef]
- Meng, X.; Yang, F.; Dong, H.; Dou, L.; Zhang, X. Recent advances in optical imaging of biomarkers in vivo. Nano Today 2021, 38, 101156. [Google Scholar] [CrossRef]
- Yang, S.; Dai, W.; Zheng, W.; Wang, J. Non-UV-activated persistent luminescence phosphors for sustained bioimaging and phototherapy. Coord. Chem. Rev. 2023, 475, 214913. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Shen, R.; Chen, N.; Qin, X.; Wang, W.; Yuan, Q. Topological Radiated Dendrites Featuring Persistent Bactericidal Activity for Daily Personal Protection. Small 2021, 17, e2100562. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Q.; Wang, Y.; Ma, Q.; Wang, J.; Li, Z.; Li, Y.; Lv, X.; Wei, W.; Chen, L.; et al. Background-free latent fingerprint imaging based on nanocrystals with long-lived luminescence and pH-guided recognition. Nano Res. 2018, 11, 6167–6176. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Luo, Q.; Ma, Q.; Chen, N.; Fu, L.; Wang, J.; Yang, R.; Yuan, Q. Facile Synthesis of Luminous Nanoparticles with Tunable Size and Long-Lived Luminescence for Lifetime-Based Biosensing. Cryst. Growth Des. 2019, 19, 2322–2328. [Google Scholar] [CrossRef]
- Jiang, Y.; Pu, K. Molecular Probes for Autofluorescence-Free Optical Imaging. Chem. Rev. 2021, 121, 13086–13131. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Cheon, S.Y.; Park, S.; Lee, D.; Lee, Y.; Han, S.; Kim, M.; Koo, H. Recent advances in optical imaging through deep tissue: Imaging probes and techniques. Biomater. Res. 2022, 26, 57. [Google Scholar] [CrossRef] [PubMed]
- Hananya, N.; Shabat, D. Recent Advances and Challenges in Luminescent Imaging: Bright Outlook for Chemiluminescence of Dioxetanes in Water. ACS Cent. Sci. 2019, 5, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Park, S.; Kim, J.; Kim, C. Listening to drug delivery and responses via photoacoustic imaging. Adv. Drug Deliv. Rev. 2022, 184, 114235. [Google Scholar] [CrossRef]
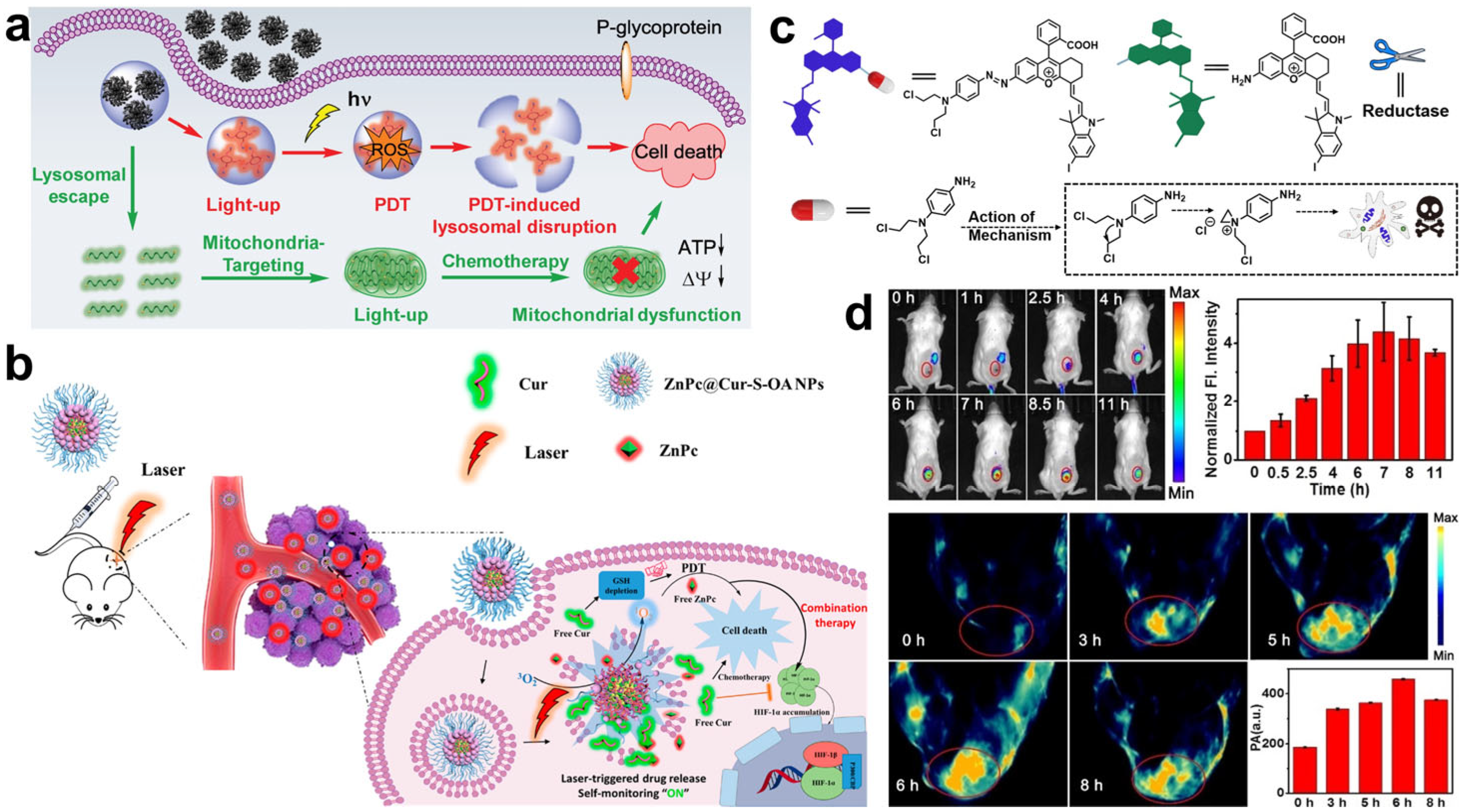
- Chen, X.; Li, Y.; Li, S.; Gao, M.; Ren, L.; Tang, B.Z. Mitochondria- and Lysosomes-Targeted Synergistic Chemo-Photodynamic Therapy Associated with Self-Monitoring by Dual Light-Up Fluorescence. Adv. Funct. Mater. 2018, 28, 1804362. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, R.; Huang, X.; Luo, R.; Xue, J.; Gao, J.; Liu, W.; Liu, F.; Feng, F.; Qu, W. Self-Delivered and Self-Monitored Chemo-Photodynamic Nanoparticles with Light-Triggered Synergistic Antitumor Therapies by Downregulation of HIF-1α and Depletion of GSH. ACS Appl. Mater. Interfaces 2020, 12, 5680–5694. [Google Scholar] [CrossRef]
- Yuan, J.; Zhou, Q.H.; Xu, S.; Zuo, Q.P.; Li, W.; Zhang, X.X.; Ren, T.B.; Yuan, L.; Zhang, X.B. Enhancing the Release Efficiency of a Molecular Chemotherapeutic Prodrug by Photodynamic Therapy. Angew. Chem. Int. Ed. 2022, 61, e202206169. [Google Scholar] [CrossRef]
- Hananya, N.; Green, O.; Blau, R.; Satchi-Fainaro, R.; Shabat, D. A Highly Efficient Chemiluminescence Probe for the Detection of Singlet Oxygen in Living Cells. Angew. Chem. Int. Ed. 2017, 56, 11793–11796. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Li, Y.; Xu, T.; Zhang, Q.; Yang, M.; Wang, P.; Gu, Y. A Novel Theranostic Nanoprobe for In Vivo Singlet Oxygen Detection and Real-Time Dose-Effect Relationship Monitoring in Photodynamic Therapy. Small 2019, 15, e1902185. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, F.; Guan, K.; Wang, Y.; Fu, X.; Yang, Y.; Yin, X.; Song, G.; Zhang, X.B.; Tan, W. In vivo therapeutic response monitoring by a self-reporting upconverting covalent organic framework nanoplatform. Chem. Sci. 2019, 11, 1299–1306. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, X.; Cui, J.; Peng, X.; Song, F. Three-in-One Functional Silica Nanocarrier with Singlet Oxygen Generation, Storage/Release, and Self-Monitoring for Enhanced Fractional Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 25750–25757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, R.; Luo, R.; Zhu, J.; Huang, X.; Liu, W.; Liu, F.; Feng, F.; Qu, W. An Activatable Theranostic Nanoprobe for Dual-Modal Imaging-Guided Photodynamic Therapy with Self-Reporting of Sensitizer Activation and Therapeutic Effect. ACS Nano 2021, 15, 5366–5383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, J.; Lu, C.; Yang, T.; Zhao, Y.; Teng, L.; Yang, Y.; Song, G.; Zhang, X.-B. H2S-Activated “One-Key Triple-Lock” Bis-Metal Coordination Network for Visualizing Precise Therapy of Colon Cancer. CCS Chem. 2021, 3, 2126–2142. [Google Scholar] [CrossRef]
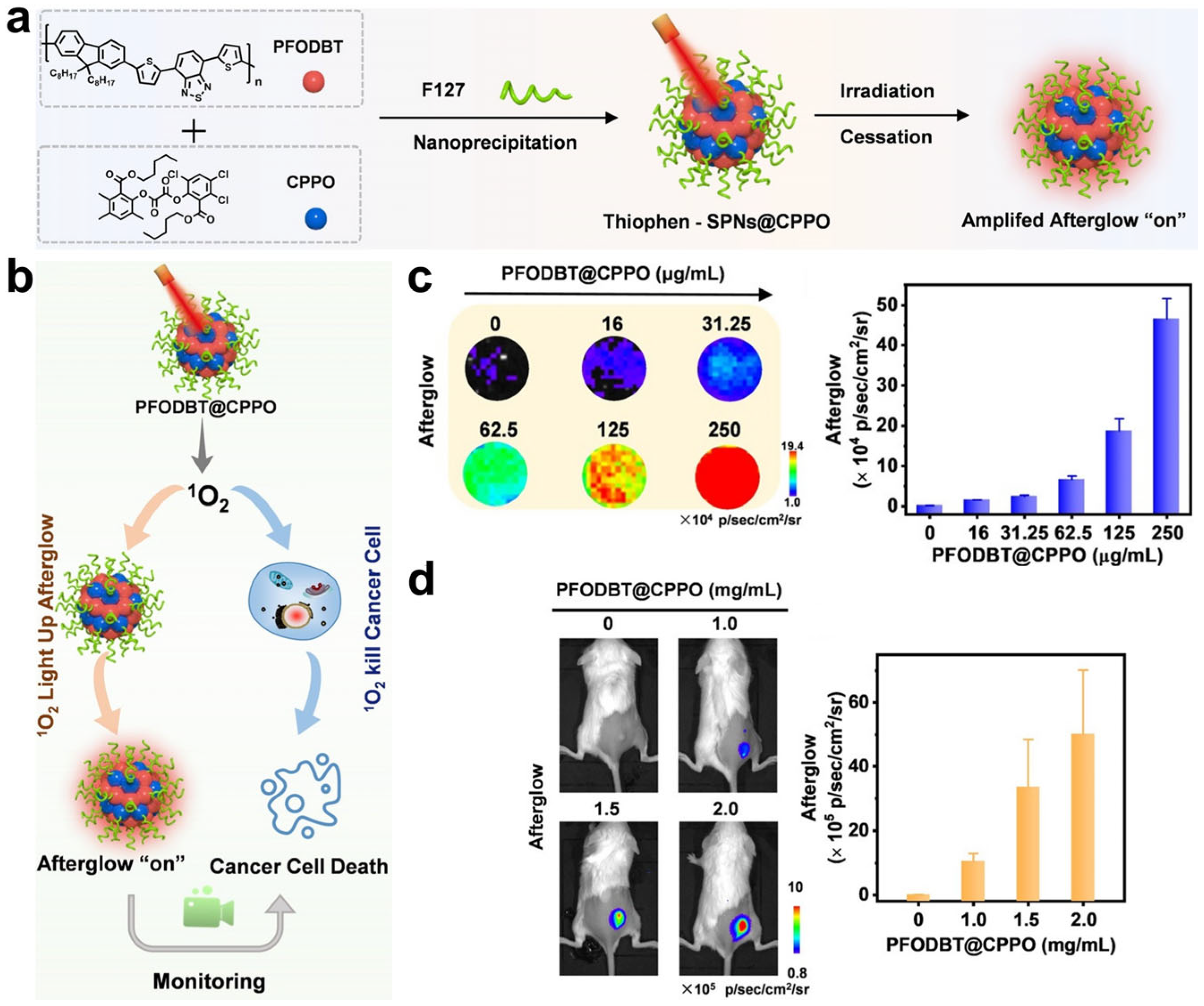
- Wang, Y.; Song, G.; Liao, S.; Qin, Q.; Zhao, Y.; Shi, L.; Guan, K.; Gong, X.; Wang, P.; Yin, X.; et al. Cyclic Amplification of the Afterglow Luminescent Nanoreporter Enables the Prediction of Anti-cancer Efficiency. Angew. Chem. Int. Ed. 2021, 60, 19779–19789. [Google Scholar] [CrossRef]
- Liao, S.; Wang, Y.; Li, Z.; Zhang, Y.; Yin, X.; Huan, S.; Zhang, X.B.; Liu, S.; Song, G. A novel afterglow nanoreporter for monitoring cancer therapy. Theranostics 2022, 12, 6883–6897. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, J.; Shabat, D.; Fan, J.; Peng, X. Near-Infrared Chemiluminescent Probe for Real-Time Monitoring Singlet Oxygen in Cells and Mice Model. ACS Sens. 2020, 5, 3158–3164. [Google Scholar] [CrossRef]
- Wei, J.; Liu, M.; Liu, H.; Wang, H.; Wang, F.; Zhang, Y.; Han, L.; Lin, X. Oleanolic acid arrests cell cycle and induces apoptosis via ROS-mediated mitochondrial depolarization and lysosomal membrane permeabilization in human pancreatic cancer cells. J. Appl. Toxicol. 2013, 33, 756–765. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; He, X.; He, Z.; Yang, X.; Tian, S.; Meng, F.; Ding, D.; Luo, L.; Tang, B.Z. A Dual-Functional Photosensitizer for Ultraefficient Photodynamic Therapy and Synchronous Anticancer Efficacy Monitoring. Adv. Funct. Mater. 2019, 29, 1902673. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Zheng, Z.; Ye, R.; Zhang, Y.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. In Situ Monitoring Apoptosis Process by a Self-Reporting Photosensitizer. J. Am. Chem. Soc. 2019, 141, 5612–5616. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, J.; Shang, D.; Chai, C.; Li, Y.; Sun, H.; Li, Y.; Gao, M.; Li, M. Mitochondria-anchoring and AIE-active photosensitizer for self-monitored cholangiocarcinoma therapy. Mater. Chem. Front. 2020, 4, 3201–3208. [Google Scholar] [CrossRef]
- Niu, J.; Meng, F.; Hao, Q.; Fu, J.; Zong, C.; Tian, M.; Yu, X. A self-reporting photosensitizer for inducing and in-situ monitoring lysosomal damage and cell apoptosis. Sens. Actuators B 2023, 382, 133482. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Xie, X.; Zhang, Y.; Chao, Z.; Ma, H.; Liu, Y.; Ju, H. Energy Pumping by Surface Collectors on Upconversion Nanoparticles for Extended Transfer and Efficient Self-Evaluable Photodynamic Therapy. CCS Chem. 2022, 4, 1251–1262. [Google Scholar] [CrossRef]
- Yang, Z.; Wen, J.; Wang, Q.; Li, Y.; Zhao, Y.; Tian, Y.; Wang, X.; Cao, X.; Zhang, Y.; Lu, G.; et al. Sensitive, Real-Time, and In-Vivo Oxygen Monitoring for Photodynamic Therapy by Multifunctional Mesoporous Nanosensors. ACS Appl. Mater. Interfaces 2019, 11, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Bartman, C.R.; Weilandt, D.R.; Shen, Y.; Lee, W.D.; Han, Y.; TeSlaa, T.; Jankowski, C.S.R.; Samarah, L.; Park, N.R.; da Silva-Diz, V.; et al. Slow TCA flux and ATP production in primary solid tumours but not metastases. Nature 2023, 614, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Qian, M.; Qi, H.; Gao, Q.; Zhang, C. Multifunctional zeolitic imidazolate framework-8 for real-time monitoring ATP fluctuation in mitochondria during photodynamic therapy. Nanoscale 2020, 12, 15663–15669. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Lei, H.; Kang, J.; Leng, X.; Ma, R.; Wang, D.; Zhou, Q.; Yu, J.; Lu, T.; Xing, J. Application of a Dual-Probe Coloading Nanodetection System in the Process Monitoring and Efficacy Assessment of Photodynamic Therapy: An In Vitro Study. ACS Biomater. Sci. Eng. 2023, 9, 1089–1103. [Google Scholar] [CrossRef]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef]
- Patel, S.A.; Nilsson, M.B.; Le, X.; Cascone, T.; Jain, R.K.; Heymach, J.V. Molecular mechanisms and future implications of VEGF/VEGFR in cancer therapy. Clin. Cancer Res. 2023, 29, 30–39. [Google Scholar] [CrossRef]
- Shao, L.; Li, Q.; Zhao, C.; Lu, J.; Li, X.; Chen, L.; Deng, X.; Ge, G.; Wu, Y. Auto-fluorescent polymer nanotheranostics for self-monitoring of cancer therapy via triple-collaborative strategy. Biomaterials 2019, 194, 105–116. [Google Scholar] [CrossRef]
- Wan, Y.; Lu, G.; Zhang, J.; Wang, Z.; Li, X.; Chen, R.; Cui, X.; Huang, Z.; Xiao, Y.; Chelora, J.; et al. A Biocompatible Free Radical Nanogenerator with Real-Time Monitoring Capability for High Performance Sequential Hypoxic Tumor Therapy. Adv. Funct. Mater. 2019, 29, 1903436. [Google Scholar] [CrossRef]
- Sun, J.; Xu, W.; Li, L.; Fan, B.; Peng, X.; Qu, B.; Wang, L.; Li, T.; Li, S.; Zhang, R. Ultrasmall endogenous biopolymer nanoparticles for magnetic resonance/photoacoustic dual-modal imaging-guided photothermal therapy. Nanoscale 2018, 10, 10584–10595. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, L.; Wang, Q.; Zou, B.; Ren, S.; Xin, L.; Gao, J.; Zhang, R. Construction of Artificial Controllable Aggregation Trojan Horse-Like Nanoplatform for Enhanced NIR-II Photothermal Therapy. ACS Appl. Mater. Interfaces 2023, 15, 4903–4910. [Google Scholar] [CrossRef]
- He, M.; Wang, Y.; Li, D.; Zhang, M.; Wang, T.; Zhi, F.; Ji, X.; Ding, D. Recent applications of phase change materials in tumor therapy and Theranostics. Biomater. Adv. 2023, 147, 213309. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, J.; Qiu, X.; Liu, Y.; Feng, W.; Li, F. Upconversion nanocomposite for programming combination cancer therapy by precise control of microscopic temperature. Nat. Commun. 2018, 9, 2176. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sun, J.; Dong, B.; Huang, G.; Zhang, L.; Zhou, W.; Lv, J.; Zhang, X.; Liu, M.; Xu, L.; et al. Noninvasive temperature monitoring for dual-modal tumor therapy based on lanthanide-doped up-conversion nanocomposites. Biomaterials 2019, 201, 42–52. [Google Scholar] [CrossRef]
- Xu, M.; Xue, B.; Wang, Y.; Wang, D.; Gao, D.; Yang, S.; Zhao, Q.; Zhou, C.; Ruan, S.; Yuan, Z. Temperature-Feedback Nanoplatform for NIR-II Penta-Modal Imaging-Guided Synergistic Photothermal Therapy and CAR-NK Immunotherapy of Lung Cancer. Small 2021, 17, e2101397. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Z.; Sun, W.; Lin, X.; Wang, R.; Li, C.; Zong, L.; Fu, Z.; Liu, H.; Xu, S. Robust emission in near-infrared II of lanthanide nanoprobes conjugated with Au (LNPs-Au) for temperature sensing and controlled photothermal therapy. Chem. Eng. J. 2023, 452, 139504. [Google Scholar] [CrossRef]
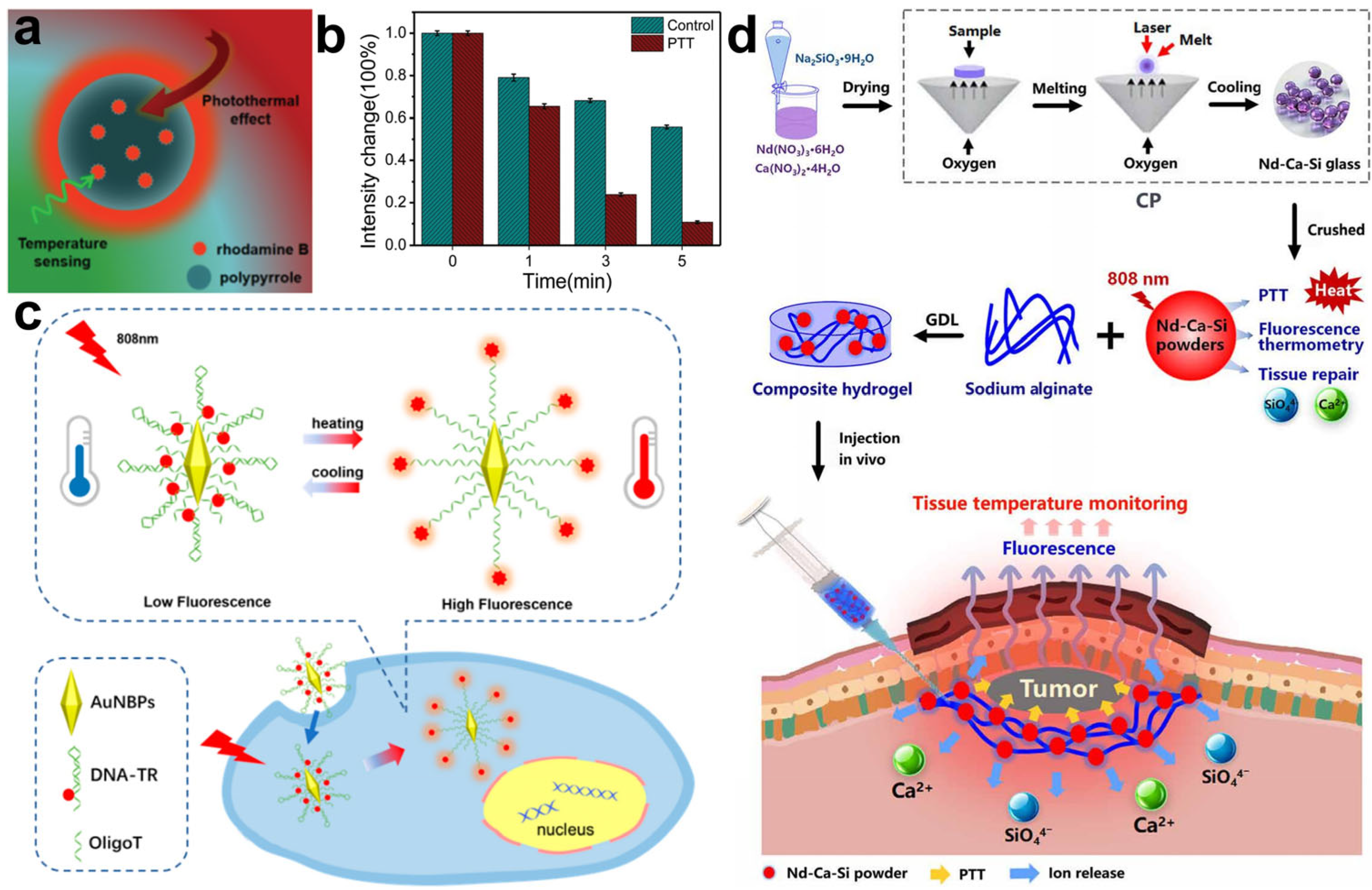
- Wang, X.H.; Chen, X.Q.; Peng, H.S.; Wei, X.F.; Wang, X.J.; Cheng, K.; Liu, Y.A.; Yang, W. Facile synthesis of polypyrrole-rhodamine B nanoparticles for self-monitored photothermal therapy of cancer cells. J. Mater. Chem. B 2020, 8, 1033–1039. [Google Scholar] [CrossRef]
- Wu, X.; Mu, L.; Chen, M.; Liang, S.; Wang, Y.; She, G.; Shi, W. Bifunctional Gold Nanobipyramids for Photothermal Therapy and Temperature Monitoring. ACS Appl. Bio Mater. 2019, 2, 2668–2675. [Google Scholar] [CrossRef]
- Ma, L.L.; Zhou, Y.L.; Zhang, Z.W.B.; Liu, Y.Q.; Zhai, D.; Zhuang, H.; Li, Q.; Yuye, J.D.; Wu, C.T.; Chang, J. Multifunctional bioactive Nd-Ca-Si glasses for fluorescence thermometry, photothermal therapy, and burn tissue repair. Sci. Adv. 2020, 6, eabb1311. [Google Scholar] [CrossRef]
- Zhen, X.; Xie, C.; Pu, K. Temperature-Correlated Afterglow of a Semiconducting Polymer Nanococktail for Imaging-Guided Photothermal Therapy. Angew. Chem. Int. Ed. 2018, 57, 3938–3942. [Google Scholar] [CrossRef]
- Meng, Y.; Yang, J.; Jiang, R.; Wang, S.; Zheng, L.; Wang, G.; Tian, X.; Zhu, H.; Yan, D.; Liu, C.; et al. Biocompatible PLNP-GNR composite nanoplatforms for monitoring deep-tissue photothermal therapy process. Appl. Surf. Sci. 2021, 562, 150189. [Google Scholar] [CrossRef]
- Yang, S.; Sun, B.; Liu, F.; Li, N.; Wang, M.; Wu, P.; Wu, G.L.; Fang, H.; He, Y.; Zhou, W.; et al. NIR-II Imaging-Guided Mitochondrial-Targeting Organic Nanoparticles for Multimodal Synergistic Tumor Therapy. Small 2023, 2207995. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhan, W.; Gao, G.; Jiang, Q.; Zhang, X.; Zhang, H.; Sun, X.; Han, W.; Wu, F.G.; Liang, G. Apoptosis-Amplified Assembly of Porphyrin Nanofiber Enhances Photodynamic Therapy of Oral Tumor. J. Am. Chem. Soc. 2023, 145, 7918–7930. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, W.; Zhang, T.; Zhu, Y.; Ni, Y.; Wang, C.; Sierra Raya, F.M.; Zou, L.; Wang, L.; Liang, G. A Self-Evaluating Photothermal Therapeutic Nanoparticle. ACS Nano 2020, 14, 9585–9593. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, X.; Zhang, H.; Chen, H.; Abualrejal, M.M.A.; Song, D.; Wang, Z. Six-in-one peptide functionalized upconversion@polydopamine nanoparticle-based ratiometric fluorescence sensing platform for real-time evaluating anticancer efficacy through monitoring caspase-3 activity. Sens. Actuators B 2021, 333, 129554. [Google Scholar] [CrossRef]
- Li, Q.Y.; Yu, X.; Li, X.; Bao, L.N.; Zhang, Y.; Wang, S.L.; Jiang, M.; Huang, K.; Xu, L. Congo Red-Derived Carbon Dots: Simultaneously as Fluorescence Probe for Protein Aggregates, Inhibitor for Protein Aggregation, and Scavenger of Free Radicals. Small 2023, 19, e2205634. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Jin, T.; Sun, K.; Yang, J. pH/Viscosity dual-response fluorescent probes as highly selective tumor visualization tools. Sens. Actuators B Chem. 2023, 375, 132935. [Google Scholar] [CrossRef]
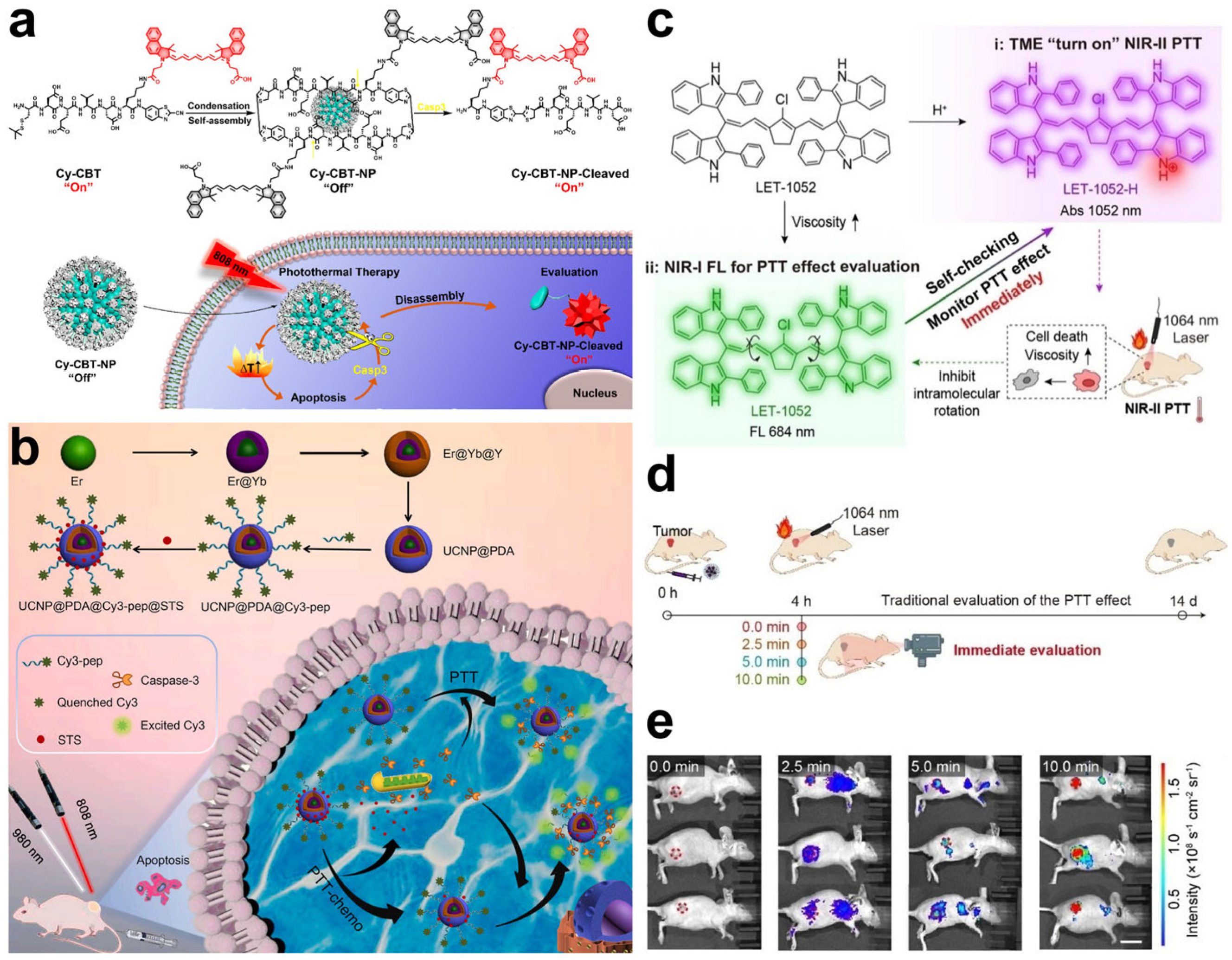
- Li, B.; Liu, H.; He, Y.; Zhao, M.; Ge, C.; Younis, M.R.; Huang, P.; Chen, X.; Lin, J. A “Self-Checking” pH/Viscosity-Activatable NIR-II Molecule for Real-Time Evaluation of Photothermal Therapy Efficacy. Angew. Chem. Int. Ed. 2022, 61, e202200025. [Google Scholar]
- Chaudhuri, A.; Venkatesh, Y.; Das, J.; Behara, K.K.; Mandal, S.; Maiti, T.K.; Singh, N.D.P. Squaric Acid-Coumarin-Chlorambucil: Photoresponsive Single-Component Fluorescent Organic Nanoconjugates for Self-Monitored Therapeutics. ACS Appl. Nano Mater. 2018, 1, 6312–6319. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Y.; Li, N.; Luo, C.; Hou, X. Anthracene-based fluorescent MOFs decorated by platinum nanozymes as a multifunctional nanoplatform for enhanced photodynamic therapy and self-monitoring of real-time singlet oxygen. Chem. Eng. J. 2022, 446, 137333. [Google Scholar] [CrossRef]
- Chen, K.; He, P.; Wang, Z.; Tang, B.Z. A Feasible Strategy of Fabricating Type I Photosensitizer for Photodynamic Therapy in Cancer Cells and Pathogens. ACS Nano 2021, 15, 7735–7743. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, X.; Zhang, J.; Wang, H.; Liu, G.; Bu, Y.; Yu, J.; Tian, Y.; Zhou, H. AIE-Based Theranostic Agent: In Situ Tracking Mitophagy Prior to Late Apoptosis To Guide the Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ren, J.; Xiong, Y.; Yang, Z.; Zhu, W.; He, Q.; Xu, Z.; He, W.; Wang, J. Enhancing magnetic resonance/photoluminescence imaging-guided photodynamic therapy by multiple pathways. Biomaterials 2019, 199, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ren, T.B.; Xu, S.; Wang, C.J.; Zhang, X.B.; Yuan, L. A Unique Multifunctional Luminescent Probe for Self-Monitoring Photodynamic Therapy by Detecting H2S in Cancer Cells. ACS Appl. Bio Mater. 2021, 4, 6016–6022. [Google Scholar] [CrossRef]
- Kang, Y.F.; Zheng, B.; Li, C.Y.; Zhang, Z.L.; Tang, H.W.; Wu, Q.S.; Pang, D.W. Real-Time Monitoring of Temperature Variations around a Gold Nanobipyramid Targeted Cancer Cell under Photothermal Heating by Actively Manipulating an Optically Trapped Luminescent Upconversion Microparticle. Anal. Chem. 2020, 92, 1292–1300. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Suo, H.; Chen, Y.; Xiang, J.; Guo, C. Temperature self-monitoring photothermal nano-particles of Er3+/Yb3+ Co-doped zircon-tetragonal BiVO4. Ceram. Int. 2021, 47, 409–415. [Google Scholar] [CrossRef]
- Suo, H.; Zhao, X.; Zhang, Z.; Wu, Y.; Guo, C. Upconverting LuVO4:Nd3+/Yb3+/Er3+@SiO2@Cu2S Hollow Nanoplatforms for Self-monitored Photothermal Ablation. ACS Appl. Mater. Interfaces 2018, 10, 39912–39920. [Google Scholar] [CrossRef]
- Dai, W.B.; Li, H.; Chen, Y.; Fan, Y.M.; Shen, F. Multifunctional up/down-conversion luminescence of core/shell nanocomposite for self-monitored heating and fluorescence imaging. J. Lumin. 2021, 234, 117960. [Google Scholar] [CrossRef]
- Shi, H.; Yan, R.; Wu, L.; Sun, Y.; Liu, S.; Zhou, Z.; He, J.; Ye, D. Tumor-targeting CuS nanoparticles for multimodal imaging and guided photothermal therapy of lymph node metastasis. Acta Biomater. 2018, 72, 256–265. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, Y.; Lian, M.; Xie, X.; Lu, Q.; Zhang, Q. Drug self-framework delivery system-coated gold nanorods for multi-modal imaging and combination therapy for breast cancer. Chem. Commun. 2023, 59, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Fu, G.; Yan, X.; Liu, J.; Wang, X.; Cheng, L.; Zhang, F.; Sun, P.Z.; Liu, G. Noninvasive magnetic resonance/photoacoustic imaging for photothermal therapy response monitoring. Nanoscale 2018, 10, 5864–5868. [Google Scholar] [CrossRef] [PubMed]




| Optical Imaging Technology | Chemicals | Biomarkers | Phototherapy Evolution | Ref. |
|---|---|---|---|---|
| Photoluminescence imaging | Organic PS AlPcSNa4 | PS release | PDT | [55] |
| ZnPc@Cur-S-OA self-assembly nanoparticles | PS release | PDT | [56] | |
| Squaric acid–coumarin–chlorambucil (Sq-Cou-Cbl) nanoconjugates | PS release | PDT | [100] | |
| FRET-based UCNPs nanoplatform | ROS, caspase-3 | PDT | [59,60,72] | |
| Protoporphyrin IX, 2-pyridone, and Cy7 embedded in silica nanocarrier | ROS | PDT | [61] | |
| ZnPc@TPCB nanoparticles | ROS | PDT | [62] | |
| Gd/Cu nanosheets | ROS | PDT | [63] | |
| TCPP@DPA-MOF-Pt nanoplatform | ROS | PDT | [101] | |
| AIE molecule TPCI, TPE-4EP+, TTVPHE, TIdBO, and TPA3 | Cell Apoptosis | PDT | [68,69,70,102,103] | |
| Organic PS NSLN | Cell Apoptosis | PDT | [71] | |
| Photoluminescence imaging | HMON-Ce6-[(Ru(dpp)3)]Cl2 nanoparticles | Oxygen | PDT | [73] |
| Rhodamine B@ZIF-8 nanoprobe | ATP | PDT | [75] | |
| MSNTH@PDAApt fluorescent probe | VEGF, ROS | PDT | [76] | |
| Organic PS protoporphyrin IX | PS biosynthesis | PDT | [104] | |
| Ru-NBD probe | H2S | PDT | [105] | |
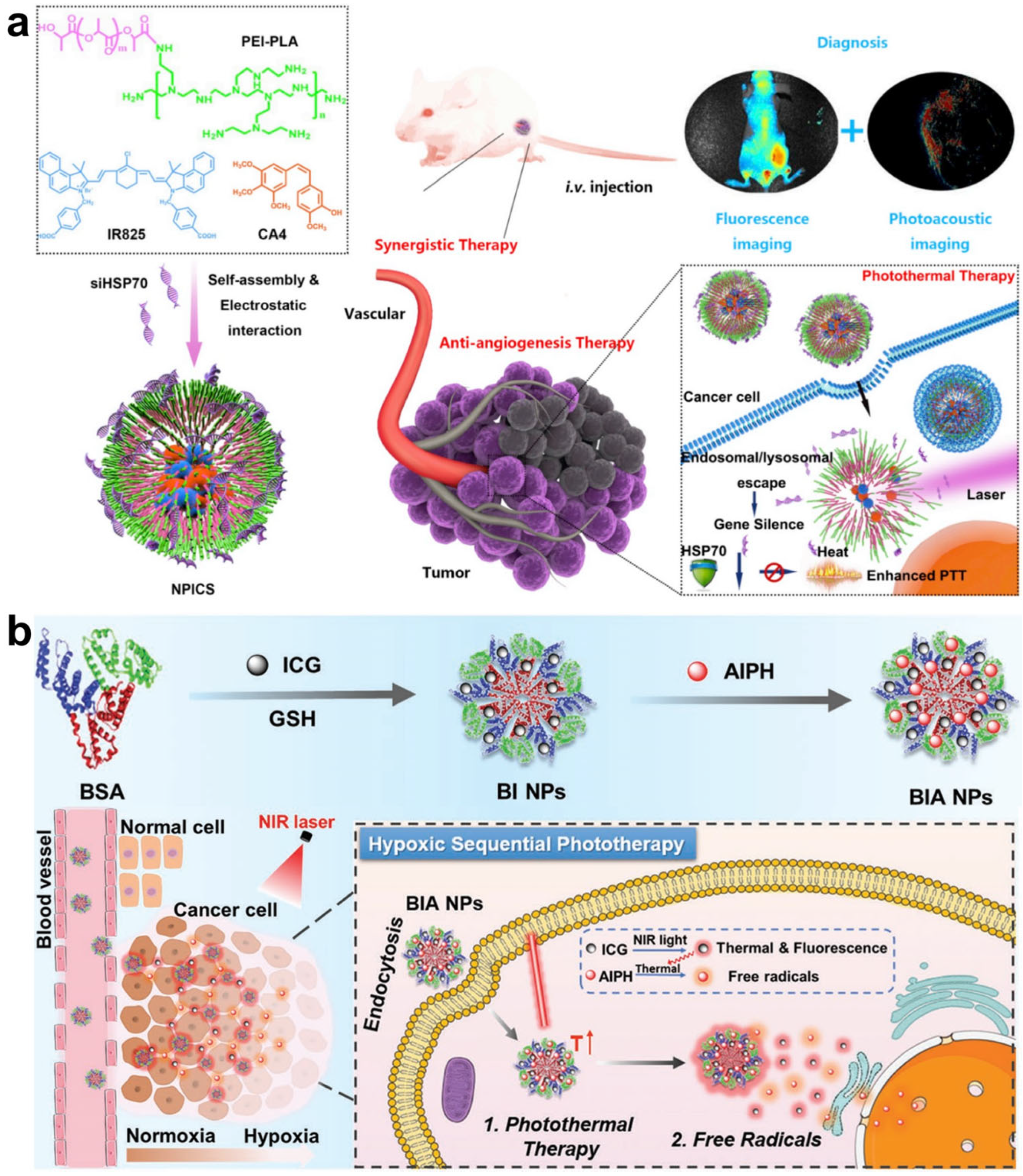
| NPICS, BIA self-assembly nanoparticles | PTT agent release | PTT | [79,80] | |
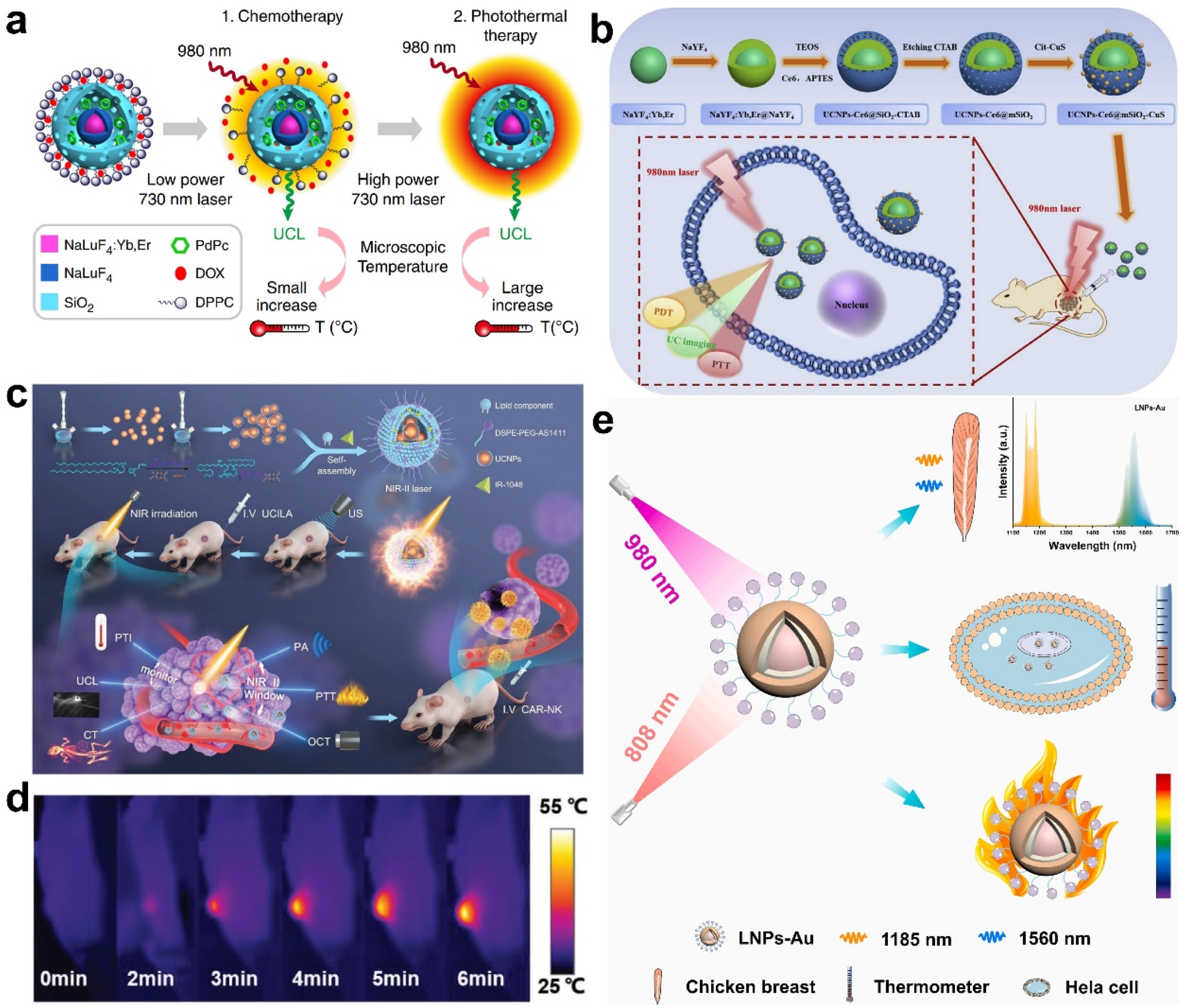
| TR-UCNS nanoplatform | Temperature | PTT | [84] | |
| UCNPs-based nanoplatform | Temperature | PTT | [85,86,106,107,108,109] | |
| LNPs-Au nanoplatform | Temperature | PTT | [87] | |
| Polypyrrole–rhodamine B polymer nanoparticles | Temperature | PTT | [88] | |
| Gold nanobipyramids–DNA–Texas Red nanoplatform | Temperature | PTT | [89] | |
| Bioactive Nd-Ca-Si glasses | Temperature | PTT | [90] | |
| Cy-CBT self-assembly nanoparticles | Caspase-3 | PTT | [95] | |
| UCNP@PDA@Cy3-pep nanoplatform | Caspase-3 | PTT | [96] | |
| Polymethine dye LET-1052 | Viscosity | PTT | [99] | |
| RGD-CuS-Cy5.5 nanoparticle | Sentinel lymph node metastasis | PTT | [110] | |
| Persistent luminescence imaging | PFODBT@CPPO semiconducting polymer | ROS | PDT | [64] |
| Pyrido pyrazine-thiophene semiconducting polymer | ROS | PDT | [65] | |
| SPNCT semiconducting polymer | Temperature | PTT | [91] | |
| ZGGO:Cr3+ PLNP-GNR composite nanoplatforms | Temperature | PTT | [92] | |
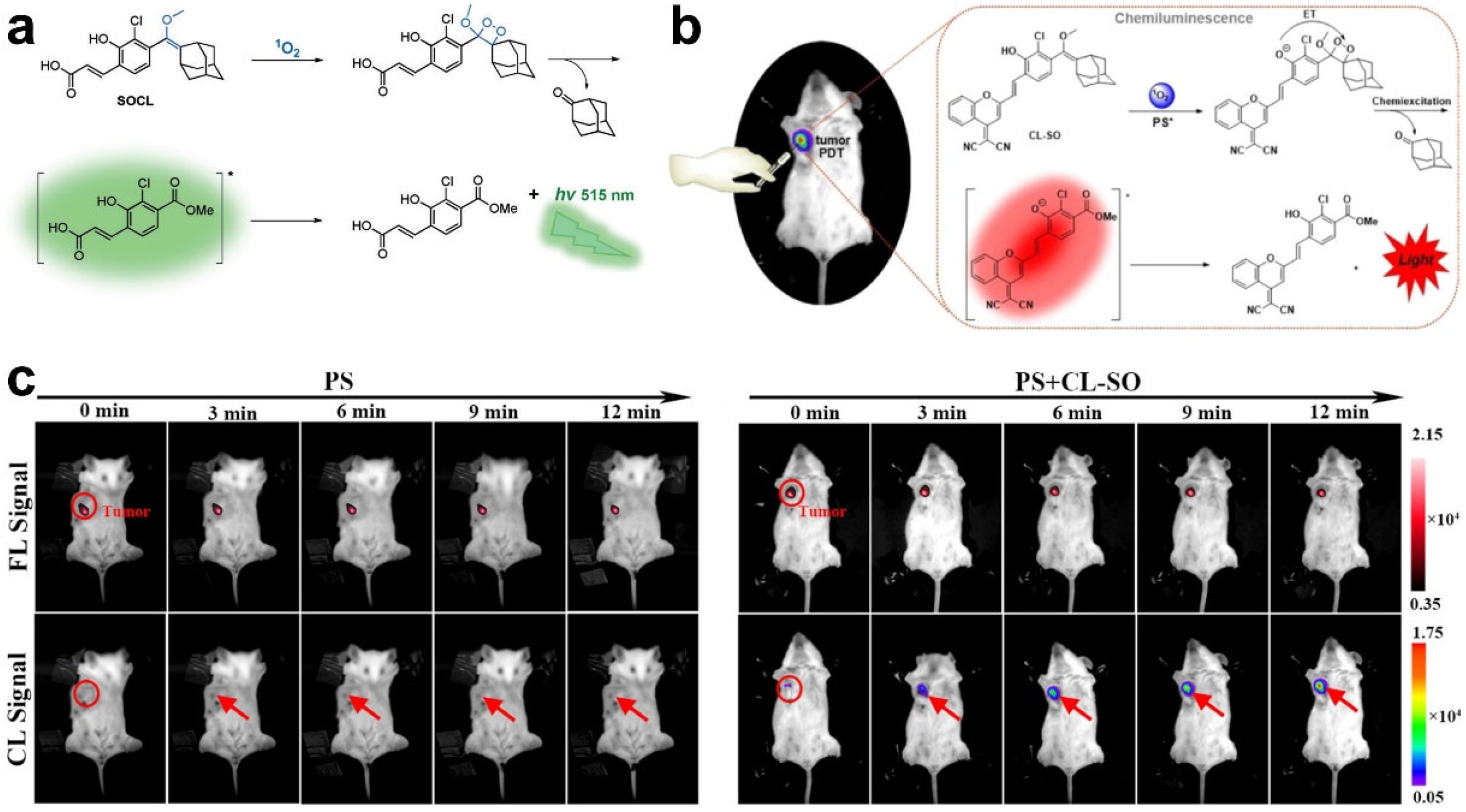
| Chemiluminescence imaging | SOCL-CPP | ROS | PDT | [58] |
| CL-SO | ROS | PDT | [66] | |
| Photoacoustic imaging | Organic PS CS-P | PS release | PDT | [57] |
| Mn2+-biopolymer melanin | PTT agent release | PTT | [81] | |
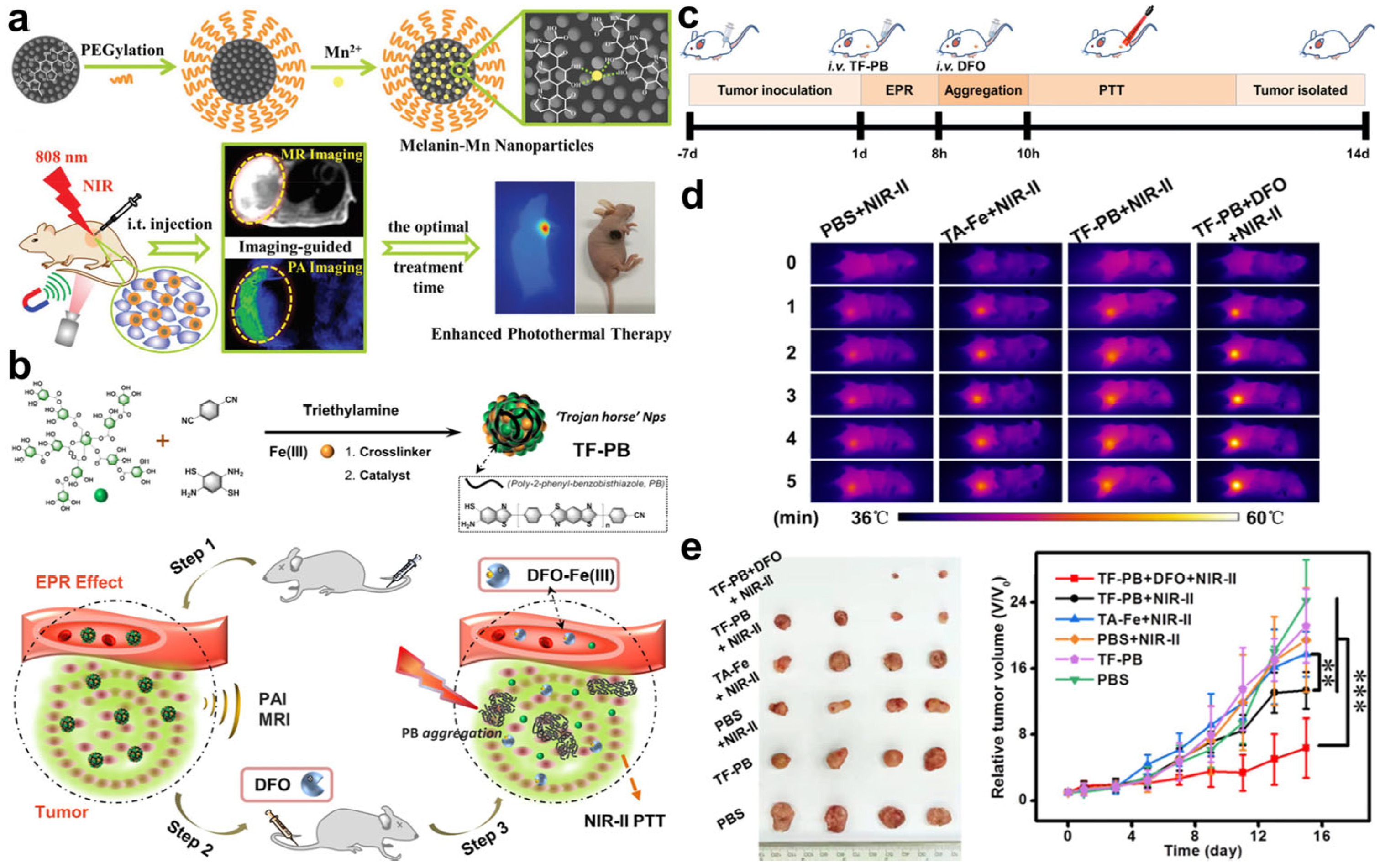
| TF-PB self-assembly nanoparticles | PTT agent release | PTT | [82] | |
| Au NRs@DSFDSs self-assembly nanoparticles | PTT agent release | PTT | [111] | |
| WS2-PEG nanosheets | Temperature | PTT | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, Z.; Wang, J. Visualization of Phototherapy Evolution by Optical Imaging. Molecules 2023, 28, 3992. https://doi.org/10.3390/molecules28103992
Li Z, Li Z, Wang J. Visualization of Phototherapy Evolution by Optical Imaging. Molecules. 2023; 28(10):3992. https://doi.org/10.3390/molecules28103992
Chicago/Turabian StyleLi, Zhiheng, Zheng Li, and Jie Wang. 2023. "Visualization of Phototherapy Evolution by Optical Imaging" Molecules 28, no. 10: 3992. https://doi.org/10.3390/molecules28103992
APA StyleLi, Z., Li, Z., & Wang, J. (2023). Visualization of Phototherapy Evolution by Optical Imaging. Molecules, 28(10), 3992. https://doi.org/10.3390/molecules28103992







