Layered-Oxide Cathode Materials for Fast-Charging Lithium-Ion Batteries: A Review
Abstract
1. Introduction
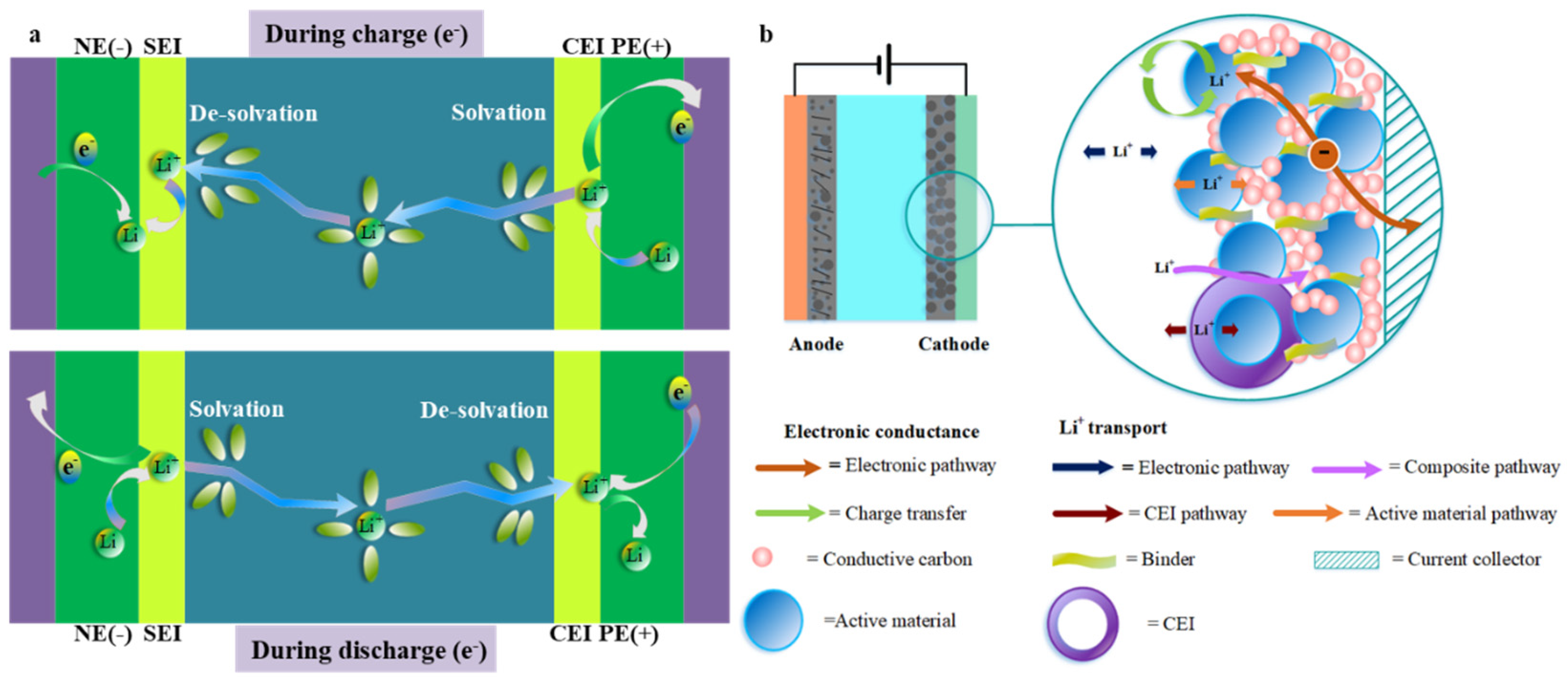
2. LIB Fast Charging Principles
3. Application of Layered Oxides in Fast-Charging LIBs
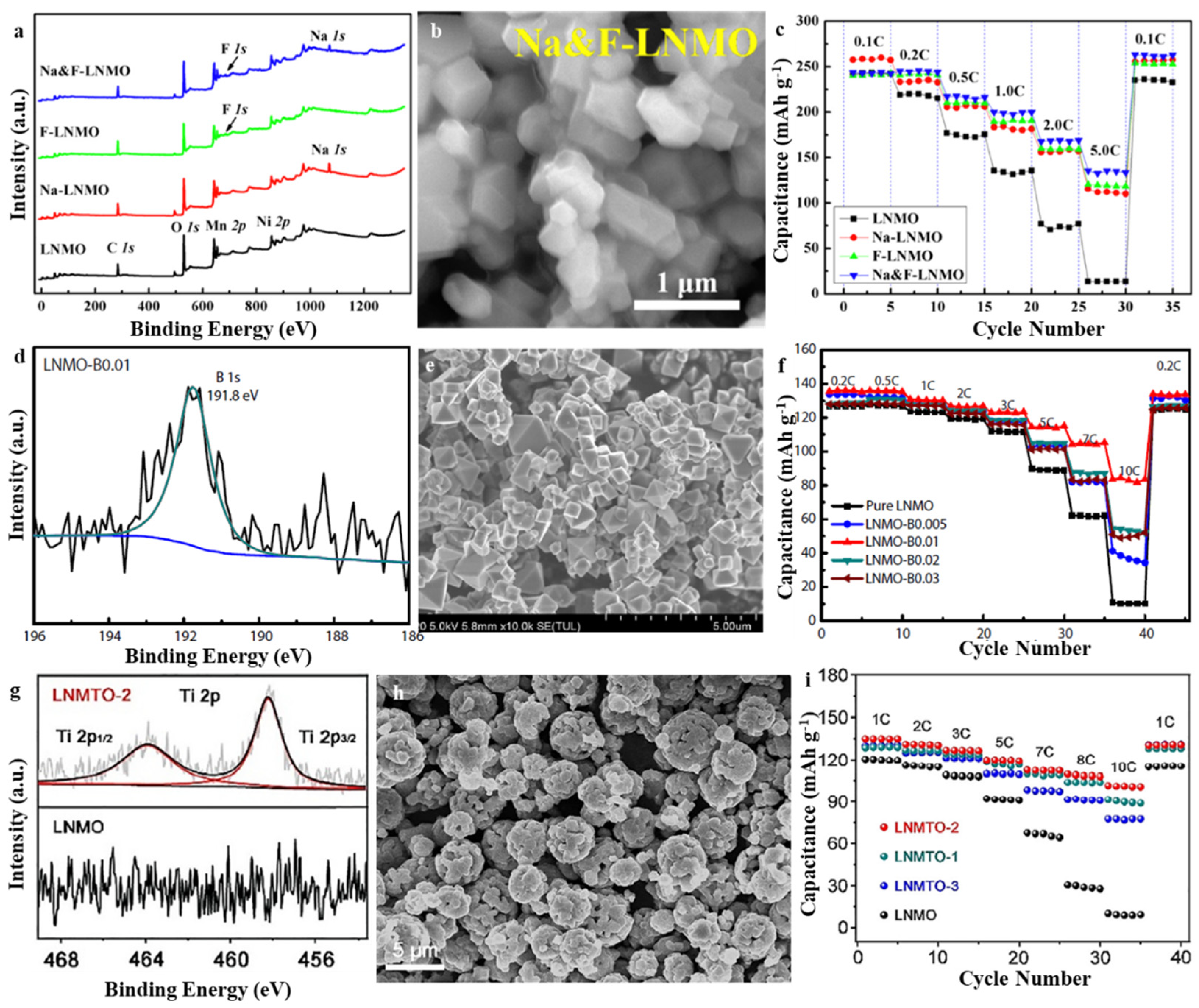
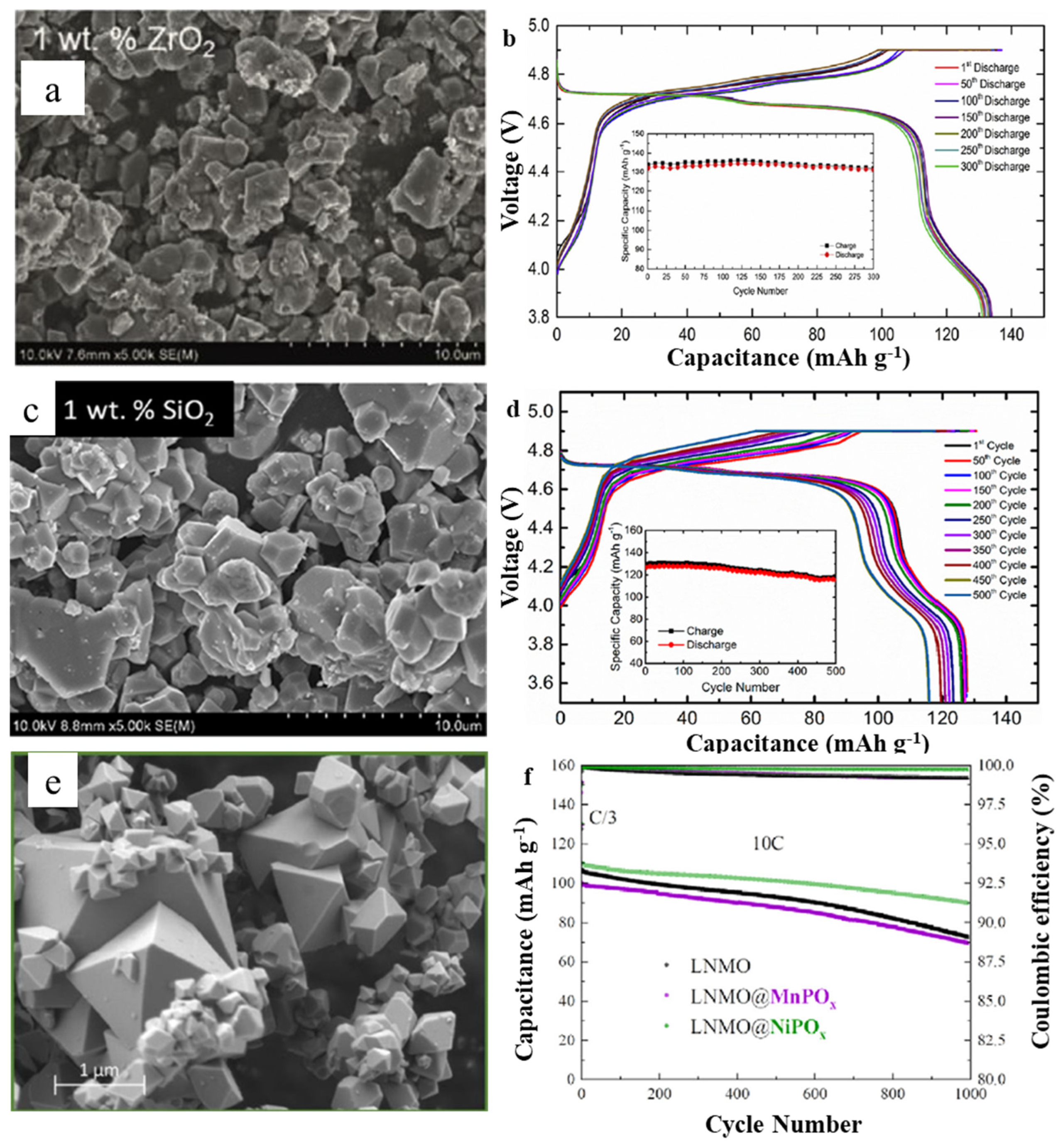
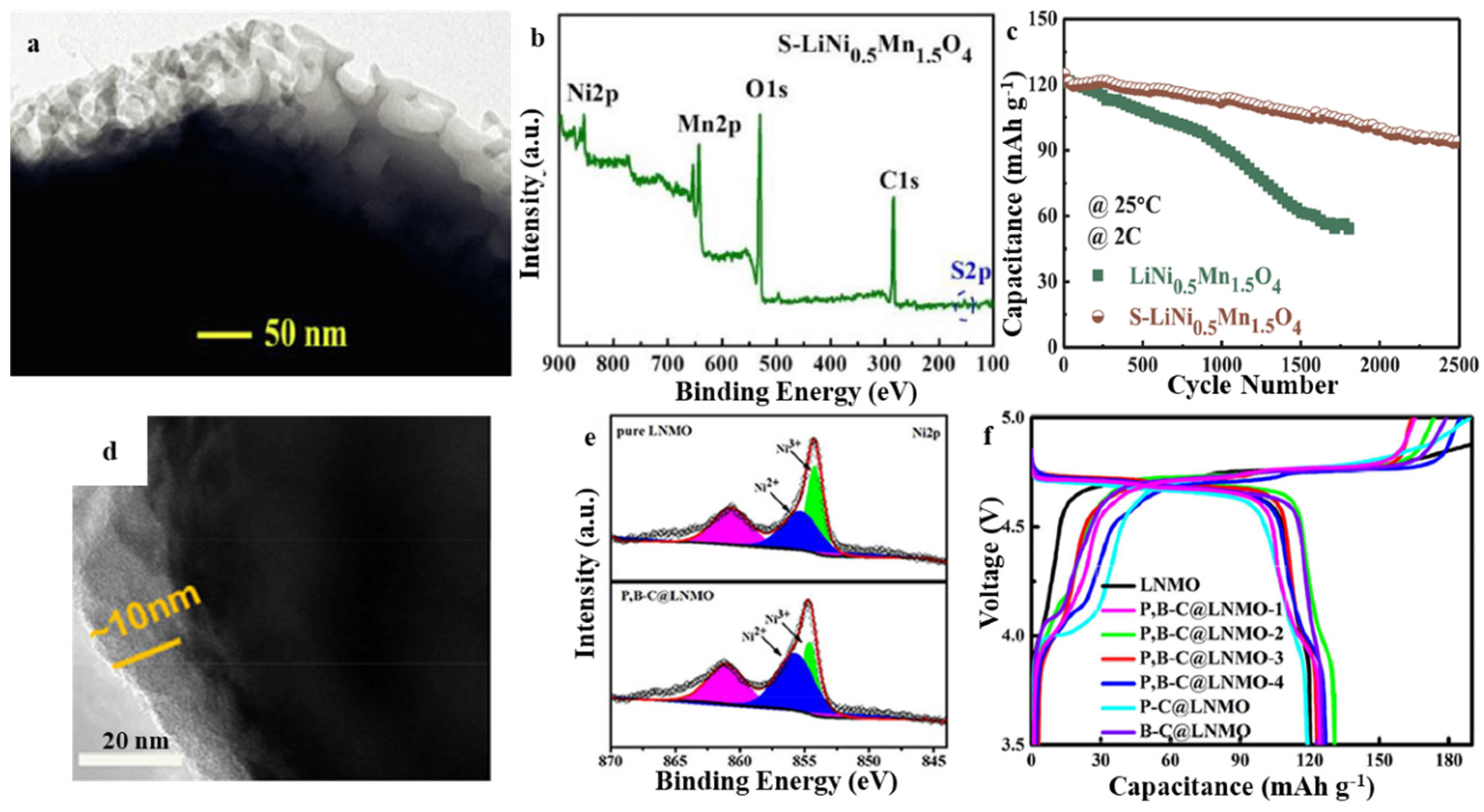
3.1. Spinel LiNi0.5Mn1.5O4 (LNMO)
3.2. LiNixCoyMnzO2 (NCM)
3.3. LiNixCoyAlzO2 (NCA)
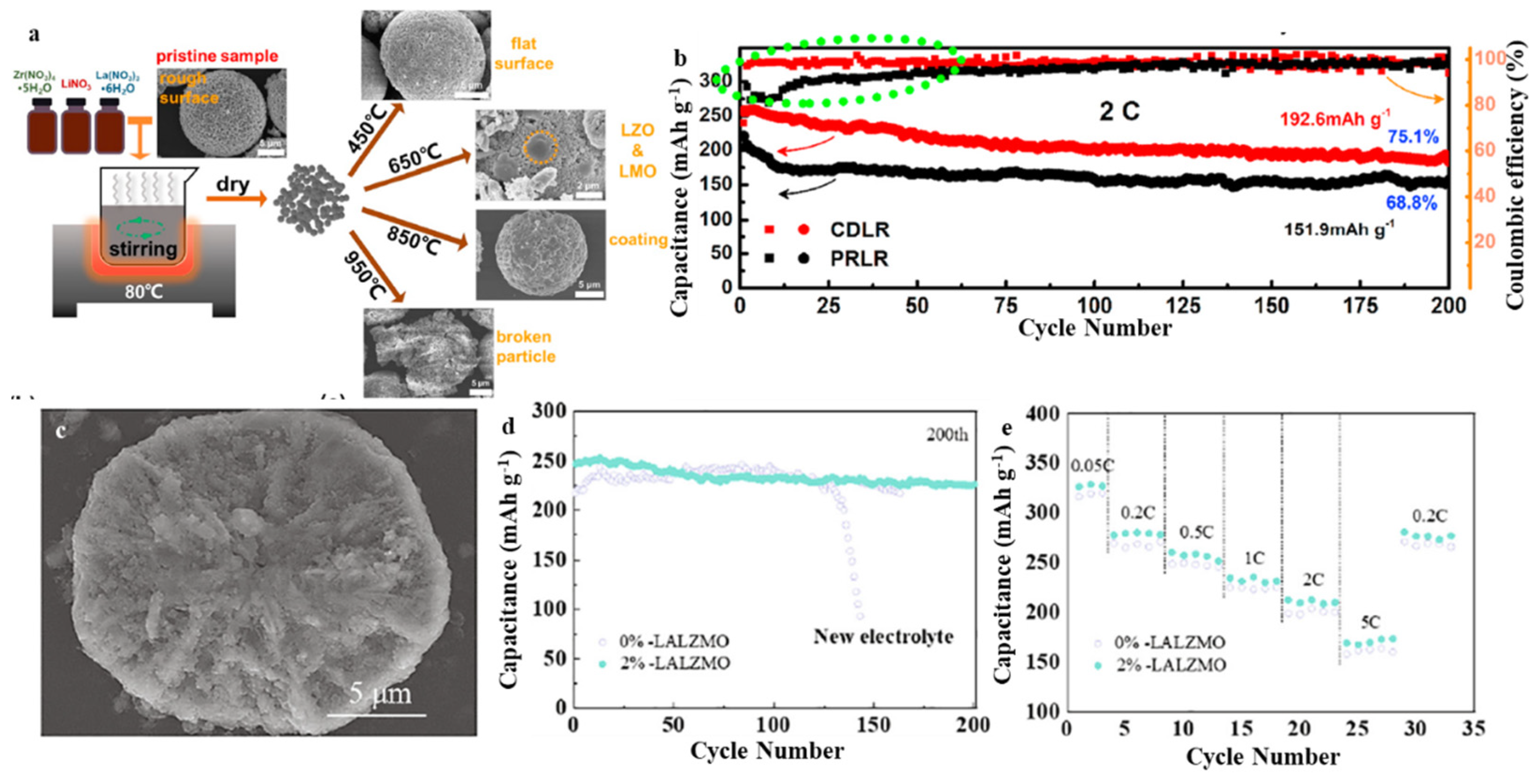
3.4. Li-Rich Mn-Based (LRM)
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Obama, B. The irreversible momentum of clean energy. Science 2017, 355, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, L.; Young, S.B.; Fowler, M.; Fraser, R.A.; Achachlouei, M.A. A cascaded life cycle: Reuse of electric vehicle lithium-ion battery packs in energy storage systems. Int. J. Life Cycle Assess. 2017, 22, 111–124. [Google Scholar] [CrossRef]
- Li, L.; Jia, S.; Cao, M.; Ji, Y.; Qiu, H.; Zhang, D. Research progress of “rocking chair” type zinc-ion batteries with zinc metal-free anodes. Chin. Chem. Lett. 2023, 2023, 108307. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Zhang, W.; Cao, M.; Qiu, H.; Ji, X. Research progress on electrolytes for fast-charging lithium-ion batteries. Chin. Chem. Lett. 2022, 34, 107122. [Google Scholar] [CrossRef]
- Li, L.; Jia, S.; Cheng, Z.; Zhang, C. Improved strategies for separators in zinc ion batteries. ChemSusChem 2023, 16, e202202330. [Google Scholar] [CrossRef]
- Li, L.; Jia, S.; Cheng, Z.; Zhang, C. Improved strategies for ammonium vanadate-based zinc ion batteries. Nanoscale 2023. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, C.; Ou, T.; Li, L.; Ji, X. Constructing Advanced Electrode Materials for Low-temperature Lithium-ion batteries. Energy Rep. 2022, 8, 4525–4534. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Pan, W.; Wang, M.; Zhang, H.; Zhang, D.; Zhang, D. Application of expanded graphite-based materials for rechargeable batteries beyond lithium-ion. Nanoscale 2021, 13, 19291–19305. [Google Scholar] [CrossRef]
- Li, L.; Zhang, D.; Deng, J.; Gou, Y.; Fang, J.; Cui, H.; Zhao, Y.; Cao, M. Carbon-based materials for fast charging lithium-ion batteries. Carbon 2021, 183, 721–734. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Zhang, Y. Metal chalcogenides-based materials for high-performance metal ion capacitors. J. Alloys Compd. 2021, 869, 159352. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Zhang, W.; Cao, M.; Qiu, H.; Ji, X. Synthesis of expanded graphite-based materials for application in lithium-based batteries. J. Energy Stroge 2023, 60, 106678. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Whittingham, M.S. Electrical Energy Storage and Intercalation Chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, A. The Birth of the Lithium-Ion Battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef] [PubMed]
- Turcheniuk, K.; Bondarev, D.; Amatucci, G.G.; Yushin, G. Battery materials for low-cost electric transportation. Mater. Today 2020, 42, 57–72. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, K.; Zhang, J.; Sun, B.; Wang, G. Reaction Mechanisms of Layered Lithium-Rich Cathode Materials for High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 2208–2220. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, Z.; Yan, K.; Wan, S.; He, F.; Sun, B.; Wang, G. Towards high-energy-density lithium-ion batteries: Strategies for developing high-capacity lithium-rich cathode materials. Energy Storage Mater. 2021, 34, 716–734. [Google Scholar] [CrossRef]
- Ji, X.; Xia, Q.; Xu, Y.; Feng, H.; Wang, P.; Tan, Q. A review on progress of lithium-rich manganese-based cathodes for lithium ion batteries. J. Power Sources 2021, 487, 229362. [Google Scholar] [CrossRef]
- Hao, G.; Lai, Q.; Zhang, H. Nanostructured Mn-based oxides as high-performance cathodes for next generation Li-ion batteries. J. Energy Chem. 2021, 59, 547–571. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J. An overview of modification strategies to improve LiNi0.8Co0.1Mn0.1O2 (NCM811) cathode performance for automotive lithium-ion batteries. eTransportation 2021, 7, 100105. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Ni, Y.; Zhang, K.; Cheng, F.; Chen, J. Recent breakthroughs and perspectives of high-energy layered oxide cathode materials for lithium ion batteries. Mater. Today 2021, 43, 132–165. [Google Scholar] [CrossRef]
- Zheng, H.; Han, X.; Guo, W.; Lin, L.; Xie, Q.; Liu, P.; He, W.; Wang, L.; Peng, D.L. Recent developments and challenges of Li-rich Mn-based cathode materials for high-energy lithium-ion batteries. Mater. Today Energy 2020, 18, 100518. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, M.; Wu, T.; Zhang, J.; Wang, P.; Zhang, L.; Yang, C.; Peng, C.; Lu, H. A Bifunctional-Modulated Conformal Li/Mn-Rich Layered Cathode for Fast-Charging, High Volumetric Density and Durable Li-Ion Full Cells. Nano-Micro Lett. 2021, 13, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.H.; Park, J.H.; Aishova, A.; Ribas, R.M.; Monteiro, R.S.; Griffith, K.J.; Yoon, C.S.; Sun, Y.K. Microstructure Engineered Ni-Rich Layered Cathode for Electric Vehicle Batteries. Adv. Energy Mater. 2021, 11, 2100884. [Google Scholar] [CrossRef]
- Shen, Y.; Xue, H.; Wang, S.; Zhang, D.; Yin, D.; Wang, L.; Cheng, Y. Ammonia-low coprecipitation synthesis of lithium layered oxide cathode material for high-performance battery. Chem. Eng. J. 2021, 411, 128487. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, Y.; Li, X.; Luo, J.; Deng, S.; Zhao, Y.; Sun, Y.; Wu, D.; Hu, Y.; Li, W.; et al. A gradient oxy-thiophosphate-coated Ni-rich layered oxide cathode for stable all-solid-state Li-ion batteries. Nat. Commun. 2023, 14, 1461. [Google Scholar] [CrossRef]
- Xiao, B.; Liu, H.; Chen, N.; Banis, M.N.; Yu, H.; Liang, J.; Sun, Q.; Sham, T.K.; Li, R.; Cai, M.; et al. Size-Mediated Recurring Spinel Sub-nanodomains in Li- and Mn-Rich Layered Cathode Materials. Angew. Chem. Int. Ed. 2020, 59, 14313–14320. [Google Scholar] [CrossRef]
- Zhang, S.S. Understanding of performance degradation of LiNi0.80Co0.10Mn0.10O2 cathode material operating at high potentials. J. Energy Chem. 2020, 41, 135–141. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen Release and Its Effect on the Cycling Stability of LiNixMnyCozO2 (NMC) Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A1361–A1377. [Google Scholar] [CrossRef]
- Weiss, M.; Ruess, R.; Kasnatscheew, J.; Levartovsky, Y.; Levy, N.R.; Minnmann, P.; Stolz, L.; Waldmann, T.; Wohlfahrt-Mehrens, M.; Aurbach, D.; et al. Fast Charging of Lithium-Ion Batteries: A Review of Materials Aspects. Adv. Energy Mater. 2021, 11, 2101126. [Google Scholar] [CrossRef]
- Yang, X.G.; Zhang, G.; Ge, S.; Wang, C.Y. Fast charging of lithium-ion batteries at all temperatures. Proc. Natl. Acad. Sci. USA 2018, 115, 7266–7271. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Trask, S.E.; Bauer, C.; Woehrle, T.; Lux, S.F.; Tschech, M.; Lamp, P.; Polzin, B.J.; Ha, S.; Long, B.; et al. Optimizing areal capacities through understanding the limitations of lithium-ion electrodes. J. Electrochem. Soc. 2016, 163, 138–149. [Google Scholar] [CrossRef]
- Arora, P.; Doyle, M.; White, R.E. Mathematical modeling of the lithium deposition overcharge reaction in lithium-ion batteries using carbon-based negative electrodes. J. Electrochem. Soc. 1999, 146, 3543. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’Kane, S.; Liu, X.; Chen, J.; Ji, C.; Endler, E.; Li, R.; Liu, L.; et al. Lithium-ion battery fast charging: A review. eTransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Jow, T.R.; Delp, S.A.; Allen, J.L.; Jones, J.P.; Smart, M.C. Factors limiting Li+ charge transfer kinetics in Li-ion batteries. J. Electrochem. Soc. 2018, 165, 361–367. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Rodehorst, U.; Streipert, B.; Wiemers-Meyer, S.; Jakelski, R.; Wagner, R.; Laskovic, I.C.; Wintera, M. Learning from Overpotentials in Lithium Ion Batteries: A Case Study on the LiNi1/3Co1/3Mn1/3O2 (NCM) Cathode. J. Electrochem. Soc. 2016, 163, A2943–A2950. [Google Scholar] [CrossRef]
- Aurbach, D.; Levi, M.D.; Levi, E.; Teller, H.; Markovsky, B.; Salitra, G.; Heider, U.; Heider, L. Common Electroanalytical Behavior of Li Intercalation Processes into Graphite and Transition Metal Oxides. J. Electrochem. Soc. 1998, 145, 3024. [Google Scholar] [CrossRef]
- Jow, T.R.; Marx, M.B.; Allen, J.L. Distinguishing Li+ Charge Transfer Kinetics at NCA/Electrolyte and Graphite/Electrolyte Interfaces, and NCA/Electrolyte and LFP/Electrolyte Interfaces in Li-Ion Cells. J. Electrochem. Soc. 2012, 159, A604. [Google Scholar] [CrossRef]
- Nisar, U.; Amin, R.; Essehli, R.; Shakoor, R.A.; Kahraman, R.; Kim, D.K.; Khaleel, M.A.; Belharouak, I. Extreme fast charging characteristics of zirconia modified LiNi0.5Mn1.5O4 cathode for lithium ion batteries. J. Power Sources 2018, 396, 774–781. [Google Scholar] [CrossRef]
- Nisar, U.; Al-Hail, S.A.J.A.; Petla, R.K.; Shakoor, R.A.; Essehli, R.; Kahraman, R.; AlQaradawi, S.Y.; Kim, D.K.; Belharouak, I.; Amin, M.R. Understanding the Origin of the Ultrahigh Rate Performance of a SiO2-Modified LiNi0.5Mn1.5O4 Cathode for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 7263–7271. [Google Scholar] [CrossRef]
- Park, M.; Zhang, X.; Chung, M.; Less, G.B.; Sastry, A.M. A review of conduction phenomena in Li-ion batteries. J. Power Sources 2010, 195, 7904–7929. [Google Scholar] [CrossRef]
- Liu, D.; Fan, X.; Li, Z.; Liu, T.; Sun, M.; Qian, C.; Ling, M.; Liu, Y.; Liang, C. A cation/anion co-doped Li1.12Na0.08Ni0.2Mn0.6O1.95F0.05 cathode for lithium ion batteries. Nano Energy 2019, 58, 786–796. [Google Scholar] [CrossRef]
- Wei, A.; Mu, J.; He, R.; Bai, X.; Liu, Z.; Zhang, L.; Wang, Y.; Liu, Z. Enhancing electrochemical performance and structural stability of LiNi0.5Mn1.5O4 cathode material for rechargeable lithium-ion batteries by boron doping. Ceram. Int. 2021, 47, 226–237. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, H.; Hu, Y.; Zhu, H.; Zhu, Y.; Jiang, H.; Li, C. Pomegranate-like Ti-doped LiNi0.4Mn1.6O4 5 V-class cathode with superior high-voltage cycle and rate performance for Li-ion batteries. Chem. Eng. Sci. 2021, 231, 116297. [Google Scholar] [CrossRef]
- Kuenzel, M.; Kim, G.T.; Zarrabeitia, M.; Lin, S.D.; Schuer, A.R.; Geiger, D.; Kaiser, U.; Bresser, D.; Passerini, S. Crystal engineering of TMPOx-coated LiNi0.5Mn1.5O4 cathodes for high-performance lithium-ion batteries. Mater. Today 2020, 39, 127–136. [Google Scholar] [CrossRef]
- Gu, T.; Wang, J.; Tian, J.H.; Zheng, X.; Lu, K.; Xin, Y.; Wang, H.; Yang, R. Phosphorus and Boron Co-Doped Carbon Coating of LiNi0.5Mn1.5O4 Cathodes for Advanced Lithium-ion Batteries. ChemElectroChem 2019, 6, 2224–2230. [Google Scholar] [CrossRef]
- Wei, L.; Tao, J.; Yang, Y.; Fan, X.; Ran, X.; Li, J.; Lin, Y.; Huang, Z. Surface sulfidization of spinel LiNi0.5Mn1.5O4 cathode material for enhanced electrochemical performance in lithium-ion batteries. Chem. Eng. J. 2020, 384, 123268. [Google Scholar] [CrossRef]
- Liang, R.; Wu, Z.Y.; Yang, W.M.; Tang, Z.Q.; Xiong, G.G.; Cao, Y.C.; Hu, S.R.; Wang, Z.B. A simple one step molten salt method for synthesis of micro sized single primary LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries. Ionics 2020, 26, 1635. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, W.; Ming, J.; Li, M.; Xie, L.; He, X.; Wang, J.; Liang, S.; Wu, Y. An Exploration of New Energy Storage System: High Energy Density, High Safety, and Fast Charging Lithium Ion Battery. Adv. Funct. Mater. 2019, 29, 1805978. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, B.; Yang, J.; Hou, J.; Wang, Y.; Zhu, Y. Synthesizing LiNi0.5Co0.2Mn0.3O2 with microsized peanut-like structure for enhanced electrochemical properties of lithium ion batteries. J. Alloy. Compd. 2020, 832, 154464. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Wu, C.Y.; Duh, J.G. Synthesis of Ni-rich NMC cathode material by re dox-assiste d deposition method for lithium ion batteries. Electrochim. Acta 2021, 381, 138244. [Google Scholar] [CrossRef]
- Zhou, P.; Meng, H.; Zhang, Z.; Chen, C.; Lu, Y.; Cao, J.; Cheng, F.; Chen, J. Stable layered Ni-rich LiNi0.9Co0.07Al0.03O2 microspheres assembled with nanoparticles as high-performance cathode materials for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 2724–2731. [Google Scholar] [CrossRef]
- Choi, J.U.; Voronina, N.; Sun, Y.K.; Myung, S.T. Recent Progress and Perspective of Advanced High-Energy Co-Less Ni-Rich Cathodes for Li-Ion Batteries: Yesterday, Today, and Tomorrow. Adv. Energy Mater. 2020, 10, 2002027. [Google Scholar] [CrossRef]
- Nam, G.W.; Park, N.Y.; Park, K.J.; Yang, J.; Liu, J.; Yoon, C.S.; Sun, Y.K. Capacity Fading of Ni-Rich NCA Cathodes: Effect of Microcracking Extent. ACS Energy Lett. 2019, 4, 2995. [Google Scholar] [CrossRef]
- Watanabe, S.; Kinoshita, M.; Hosokawa, T.; Morigaki, K.; Nakura, K. Capacity fade of LiAlyNi1−x−yCoxO2 cathode for lithium-ion batteries during accelerated calendar and cycle life tests (surface analysis of LiAlyNi1−x−yCoxO2 cathode after cycle tests in restricted depth of discharge ranges). J. Power Sources 2014, 258, 210–217. [Google Scholar] [CrossRef]
- Miller, D.J.; Proff, C.; Wen, J.G.; Abraham, D.P.; Bareño, J. Observation of Microstructural Evolution in Li Battery Cathode Oxide Particles by In Situ Electron Microscopy. Adv. Energy Mater. 2013, 3, 1098. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Gu, M.; Xiao, J.; Zhang, J.G.; Wang, C.M. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat. Commun. 2017, 8, 14101. [Google Scholar] [CrossRef]
- Ryu, H.H.; Park, K.J.; Yoon, C.S.; Sun, Y.K. Capacity Fading of Ni-Rich Li[NixCoyMn1–x–y]O2 (0.6 ≤ x ≤ 0.95) Cathodes for High-Energy-Density Lithium-Ion Batteries: Bulk or Surface Degradation? Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Park, K.J.; Hwang, J.Y.; Ryu, H.H.; Maglia, F.; Kim, S.J.; Lamp, P.; Yoon, C.S.; Sun, Y.K. Degradation Mechanism of Ni-Enriched NCA Cathode for Lithium Batteries: Are Microcracks Really Critical? ACS Energy Lett. 2019, 4, 1394–1400. [Google Scholar] [CrossRef]
- Kim, J.H.; Ryu, H.H.; Kim, S.J.; Yoon, C.S.; Sun, Y.K. Degradation Mechanism of Highly Ni-Rich Li[NixCoyMn1–x–y]O2 Cathodes with x > 0.9. ACS Appl. Mater. Interfaces 2019, 11, 30936–30942. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.H.; Ryu, H.H.; Kim, J.H.; Mücke, R.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.K. Microstructure-Controlled Ni-Rich Cathode Material by Microscale Compositional Partition for Next-Generation Electric Vehicles. Adv. Energy Mater. 2019, 9, 1803902. [Google Scholar] [CrossRef]
- Nie, Y.; Xiao, W.; Miao, C.; Fang, R.; Kou, Z.; Wang, D.; Xu, M.; Wang, C. Boosting the electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode materials in-situ modified with Li1.3Al0.3Ti1.7(PO4)3 fast ion conductor for lithium-ion batteries. Electrochim. Acta 2020, 353, 136477. [Google Scholar] [CrossRef]
- Nie, Y.; Xiao, W.; Miao, C.; Wang, J.; Tan, Y.; Xu, M.; Wang, C. Improving the structural stability of Ni-rich LiNi0.81Co0.15Al0.04O2 cathode materials with optimal content of trivalent Al ions doping for lithium ions batteries. Ceram. Int. 2021, 47, 9717–9726. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, H. High-Energy Cathode Materials (Li2MnO3–LiMO2) for Lithium-Ion Batteries. J. Phys. Chem. Lett. 2013, 4, 1268–1280. [Google Scholar] [CrossRef]
- Ding, X.; Li, Y.; Deng, M.; Wang, S.; Aqsa, Y.; Hu, Q.; Chen, C. Cesium doping to improve the electrochemical performance of layered Li1.2Ni0.13Co0.13Mn0.54O2 cathode material. J. Alloys Compd. 2019, 791, 100–108. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Kang, S.H.; Johnson, C.S.; Vaughey, J.T.; Benedek, R.; Hackney, S.A. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 2007, 17, 3112–3125. [Google Scholar] [CrossRef]
- Rossouw, M.H.; Thackeray, M.M. Lithium manganese oxides from Li2MnO3 for rechargeable lithium battery applications. Mater. Res. Bull. 1991, 26, 463–497. [Google Scholar] [CrossRef]
- Rossouw, M.H.; Liles, D.C.; Thackeray, M.M. Synthesis and Structural Characterization of a Novel Layered Lithium Manganese Oxide, Li0.36Mn0.91O2, and Its Lithiated Derivative, Li1.09Mn0.91O2. J. Solid. State Chem. 1993, 104, 464–466. [Google Scholar] [CrossRef]
- Numata, K.; Sakaki, C.; Yamanaka, S. Synthesis of Solid Solutions in a System of LiCoO2-Li2MnO3 for Cathode Materials of Secondary Lithium Batteries. Chem. Lett. 1997, 26, 725–726. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Allen, J. LiNiPO4–LiCoPO4 solid solutions as cathodes. J. Power Sources 2004, 136, 150–153. [Google Scholar] [CrossRef]
- Yang, D.; Liao, X.Z.; Huang, B.; Shen, J.; He, Y.S.; Ma, Z.F. A Na4Fe(CN)6/NaCl solid solution cathode material with an enhanced electrochemical performance for sodium ion batteries. J. Mater. Chem. 2013, 1, 13417–13421. [Google Scholar] [CrossRef]
- Neelakantaiah, R.R.; Dasari, B.B.; Ette, P.M.; Ramesha, K. Improving the Electrochemical Performance of Li2RuO3 through Chemical Substitution: A Case Study of (x)LiCoO2-(1−x)Li2RuO3 Solid Solution (x ≤ 0.4). Chemelectrochem 2020, 7, 328–335. [Google Scholar] [CrossRef]
- He, W.; Liu, P.; Zhang, Y.; Lin, J.; Qu, B.; Zheng, Z.; Wang, J.; Zhang, Y.; Sa, B.; Wang, L.; et al. Utilizing the different distribution habit of La and Zr in Li-rich Mn-based cathode to achieve fast lithium-ion diffusion kinetics. J. Power Sources 2021, 499, 229915. [Google Scholar] [CrossRef]
- Kang, Y.; Guo, X.; Guo, Z.; Li, J.; Zhou, Y.; Liang, Z.; Han, C.; He, X.; Zhao, Y.; Tavajohi, N.; et al. Phosphorus-doped lithium- and manganese-rich layered oxide cathode material for fast charging lithium-ion batteries. J. Energy Chem. 2021, 62, 538–545. [Google Scholar] [CrossRef]
- Guan, D.; Shi, C.; Xu, H.; Gu, Y.; Zhong, J.; Sha, Y.; Hu, Z.; Ni, M.; Shao, Z. Simultaneously mastering operando strain and reconstruction effects via phase-segregation strategy for enhanced oxygen-evolving electrocatalysis. J. Energy Chem. 2023. [Google Scholar] [CrossRef]
- Guan, D.; Zhong, J.; Xu, H.; Huang, Y.C.; Hu, Z.; Chen, B.; Zhang, Y.; Ni, M.; Xu, X.; Zhou, W.; et al. A universal chemical-induced tensile strain tuning strategy to boost oxygen-evolving electrocatalysis on perovskite oxides. Appl. Phys. Rev. 2022, 9, 011422. [Google Scholar] [CrossRef]

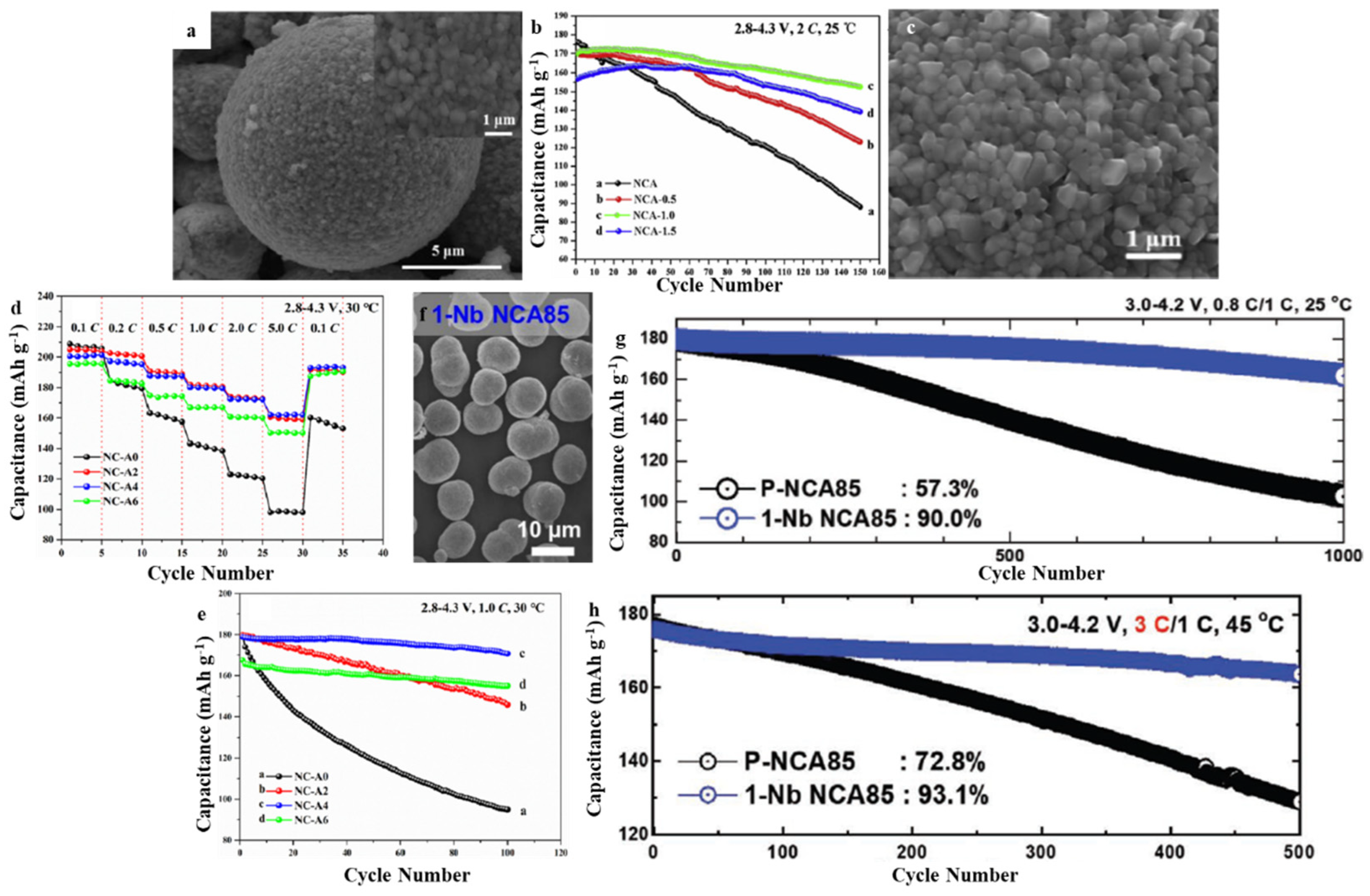
| Layered-Oxide Materials | Voltage Window (V) | Maximum Capacity (mAh g−1) | Capacity@High c-Rate (mAh g−1) | Capacity Retention | Ref. |
|---|---|---|---|---|---|
| Co-doped Na and F-LNMO | 2.0–4.8 | 245 (0.2 C) | 167 (5 C) | 100% after 100 cycles (0.2 C) | [44] |
| LNMO-B0.01 | 3.5–5.0 | 136.1 (0.2 C) | 82.9 (10 C) | 83% after 500 cycles (3 C) | [45] |
| LNMTO | 3.5–5.0 | 134 (1 C) | 101 (10 C) | 84.4% after 500 cycles (1 C) | [46] |
| ZrO2-Modified LNMO | 4.0–4.8 | 133 (0.5 C) | 101 (80 C) | 85.6% after 1200 cycles (40 C) | [41] |
| SiO2-Modified LNMO | 3.4–5.0 | 132 (0.5 C) | 105 (80 C) | 96.7% after 400 cycles (10 C) | [42] |
| LNMO@TMPOx | 1.5–4.8 | 128 (0.1 C) | 110 (10 C) | 82% after 1000 cycles (10 C) | [47] |
| S-LNMO | 3.0–4.8 | 125 (0.5 C) | 116 (6 C) | 74.9% after 2500 cycles (2 C) | [44] |
| P, B-C@LNMO | 3.5–5.0 | 130.7 (0.1 C) | 111 (5 C) | 96.7% after 200 cycles (5 C) | [48] |
| NCM-H | 0.5–3.5 | 175 (0.1 C) | - | 89.7% after 100 cycles (0.5 C) | [51] |
| Peanut-like H-NCM | 3.0–4.3 | 176.5 (0.2 C) | 159.9 (10 C) | 90% after 100 cycles (10 C) | [52] |
| MNC | 2.5–4.8 | 276 (0.1 C) | 189 (10 C) | 70.3% after 1000 cycles (20 C) | [25] |
| NMC-R | 2.4–4.3 | 197 (0.1 C) | 68 (10 C) | 93% after 100 cycles (0.5 C) | [53] |
| NCA | 2.8–4.3 | 198.5 (0.1 C) | 153.2 (0.5 C) | 89.5% after 1500 cycles (2 C) | [64] |
| NCA | 2.8–4.3 | 196.3 (1 C) | 162.1 (5 C) | 95.5% after 100 cycles (1 C) | [65] |
| NCA85 | 2.7–4.3 | - | - | 90% after 1000 cycles (1 C) | [26] |
| PRLR | 2.0–4.8 | 268 (0.1 C) | 150 (5 C) | 75.1% after 200 cycles (2 C) | [75] |
| P5+-LMR | 2.0–4.7 | 317 (0.05 C) | 175 (5 C) | 90.5% after 200 cycles (5 C) | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Wang, J.; Li, L. Layered-Oxide Cathode Materials for Fast-Charging Lithium-Ion Batteries: A Review. Molecules 2023, 28, 4007. https://doi.org/10.3390/molecules28104007
Meng X, Wang J, Li L. Layered-Oxide Cathode Materials for Fast-Charging Lithium-Ion Batteries: A Review. Molecules. 2023; 28(10):4007. https://doi.org/10.3390/molecules28104007
Chicago/Turabian StyleMeng, Xin, Jiale Wang, and Le Li. 2023. "Layered-Oxide Cathode Materials for Fast-Charging Lithium-Ion Batteries: A Review" Molecules 28, no. 10: 4007. https://doi.org/10.3390/molecules28104007
APA StyleMeng, X., Wang, J., & Li, L. (2023). Layered-Oxide Cathode Materials for Fast-Charging Lithium-Ion Batteries: A Review. Molecules, 28(10), 4007. https://doi.org/10.3390/molecules28104007






