Exploitation of Sugarcane Bagasse and Environmentally Sustainable Production, Purification, Characterization, and Application of Lovastatin by Aspergillus terreus AUMC 15760 under Solid-State Conditions
Abstract
1. Introduction
2. Results
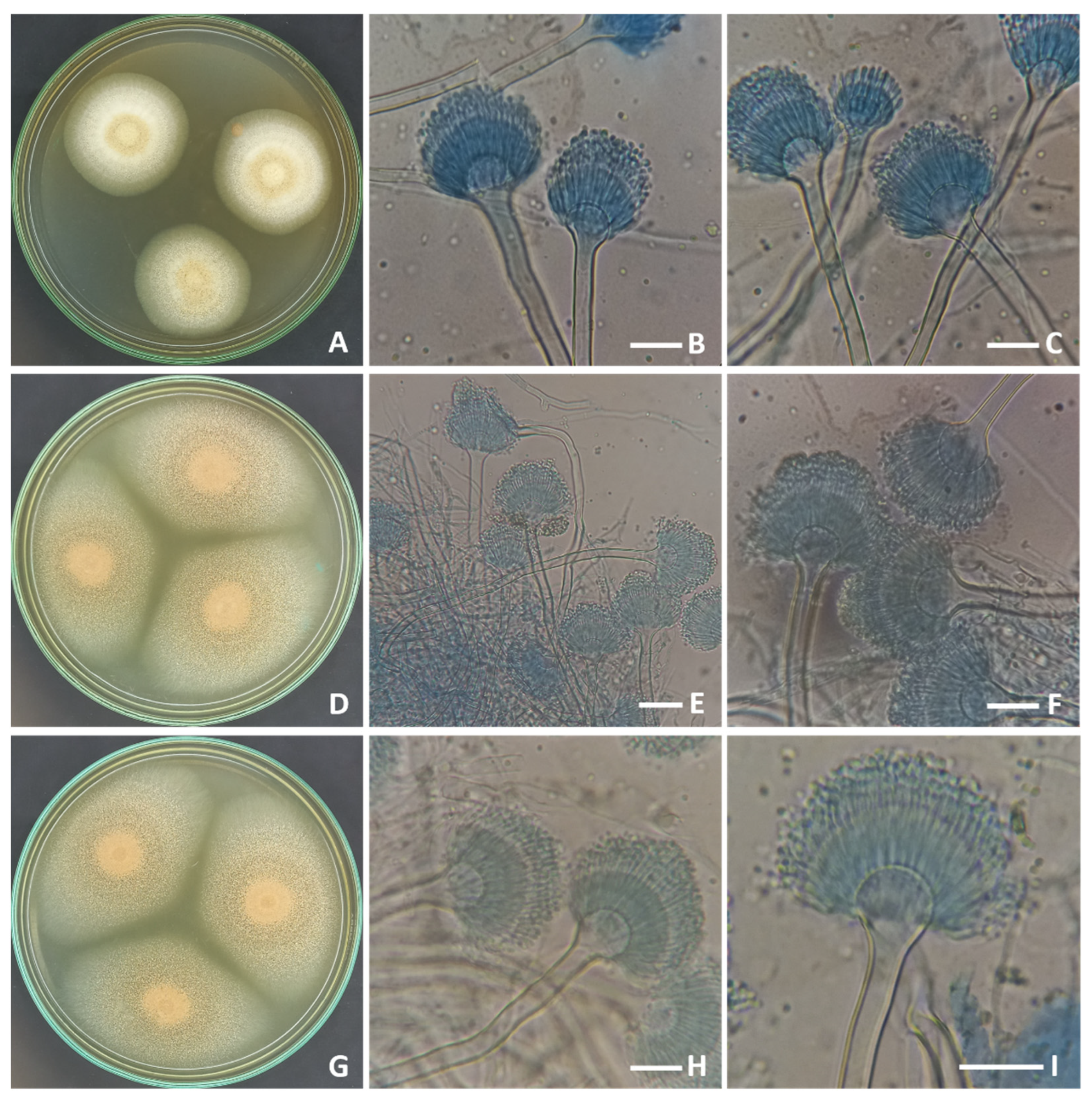
2.1. Morphological and Molecular Identification of the Strains of Aspergillus terreus
2.2. Screening Activity of Lovastatin Production by Strains of A. terreus Using Wheat Bran in SSF
2.3. GC-MS Detection of Lovastatin
2.4. Lovastatin Production by A. terreus AUMC 15760 from Different Lignocellulosic Wastes
2.5. Optimization of Lovastatin Production by A. terreus AUMC 15760
2.6. HPLC Analysis of Methanolic Extracts
2.7. Production and Purification of Lovastatin by Column Chromatography
2.8. LC-MS/MS and HR-ESI-MS Analysis
2.9. NMR
2.10. Antioxidant Activity of the Pure Lovastatin Produced by A. terreus AUMC 15760
2.11. Antimicrobial Ability of the Purified Lovastatin Produced by A. terreus AUMC 15760
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Strain Isolation and Preservation
4.3. Morphological and Molecular Identification of the Aspergillus terreus Strains
4.4. Screening of Lovastatin Production by Strains of A. terreus under SSF
Extraction of Lovastatin
4.5. GC-MS Analysis
4.6. Lovastatin Production by A. terreus AUMC 15760 from Different Lignocellulosic Wastes
4.7. Optimization of Lovastatin Production by A. terreus AUMC 15760 from Sugarcane Bagasse
4.8. HPLC Assay of the Produced Lovastatin by A. terreus AUMC 15760
4.9. Production of Lovastatin from Sugarcane Bagasse by A. terreus AUMC 15760 in SSF
4.10. Purification of Lovastatin
4.11. LC-MS/MS Analysis
4.12. Spectroscopic NMR
4.13. Thin Layer Chromatograph (TLC)
4.14. DPPH Radical Scavenging Activity
4.15. Antimicrobial Activity of Lovastatin Produced by A. terreus AUMC 15760
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AL-Kolaibe, A.M.; Moharram, A.M.; Al-Bedak, O.A. Worthwhile enzyme production and eco-friendly bioconversion of three agricultural residues by Aspergillus curvatus and Aspergillus gaarensis, promising enzyme-producers isolated from extreme environment. J. Basic Appl. Mycol. 2021, 12, 1–14. [Google Scholar]
- Wang, B.; Dong, F.; Chen, M.; Zhu, J.; Tan, J.; Fu, X.; Wang, Y.; Chen, S. Advances in recycling and utilization of agricultural wastes in China: Based on environmental risk, crucial pathways, influencing factors, policy mechanism. Procedia Environ. Sci. 2016, 31, 12–17. [Google Scholar] [CrossRef]
- Singh, R.; Kapoor, V.; Kumar, V. Utilization of agro-industrial wastes for the simultaneous production of amylase and xylanase by thermophilic actinomycetes. Braz. J. Microbiol. 2012, 43, 1545–1552. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Ismail, M.; Moubasher, A.; Mohamed, R.; Al-Beddak, O. Agro–industrial residues as alternative sources for cellulases and xylanases production and purification of xylanase produced by Aspergillus flavus AUMC 10331 isolated from extreme habitat. Curr. Res. Environ. Appl. Mycol. 2018, 8, 313–322. [Google Scholar] [CrossRef]
- Jeong, S.-M.; Choi, S.; Kim, K.; Kim, S.-M.; Lee, G.; Son, J.S.; Yun, J.-M.; Park, S.M. Association of change in total cholesterol level with mortality: A population-based study. PLoS ONE 2018, 13, e0196030. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; DeMets, D. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016, 388, 2532–2561. [Google Scholar] [CrossRef]
- Lai, L.-S.T.; Tsai, T.-H.; Cheng, T.-Y. The influence of culturing environments on lovastatin production by Aspergillus terreus in submerged cultures. Enzym. Microb. Technol. 2005, 36, 737–748. [Google Scholar] [CrossRef]
- Srinivasan, N.; Thangavelu, K.; Uthandi, S. Lovastatin production by an oleaginous fungus, Aspergillus terreus KPR12 using sago processing wastewater (SWW). Microb. Cell Factories 2022, 21, 1–14. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Q.; Peng, N.; Li, Y.; Qiu, D.; Yang, T.; Kang, R.; Usmani, A.; Amadasu, E.; Borlongan, C.V. Lovastatin Inhibits RhoA to Suppress Canonical Wnt/β-Catenin Signaling and Alternative Wnt-YAP/TAZ Signaling in Colon Cancer. Cell Transplantat. 2022, 31, 09636897221075749. [Google Scholar] [CrossRef]
- Endo, A. The Origin of the Statins, International Congress Series; Elsevier: Amsterdam, The Netherlands, 2004; pp. 3–8. [Google Scholar] [CrossRef]
- Barrios-González, J.; Miranda, R.U. Biotechnological production and applications of statins. Appl. Microbiol. Biotechnol. 2010, 85, 869–883. [Google Scholar] [CrossRef]
- Seenivasan, A.; Venkatesan, S.; Tapobrata, P. Cellular localization and production of lovastatin from Monascus purpureus. Indian J. Pharm. Sci. 2018, 80, 85–98. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, M.; Chen, M.; Zhou, L.; Zhao, L.; Hu, R.; Yan, R.; Dai, K. Lovastatin induces platelet apoptosis. Environ. Toxicol. Pharmacol. 2016, 42, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Seenivasan, A.; Subhagar, S.; Aravindan, R.; Viruthagiri, T. Microbial production and biomedical applications of lovastatin. Indian J. Pharm. Sci. 2008, 70, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Praveen, V.; Savitha, J. Solid state fermentation: An effective method for lovastatin production by fungi over submerged fermentation. E3 J. Biotechnol. Pharm. Res. 2012, 3, 15–21. [Google Scholar] [CrossRef]
- Javed, S.; Azeem, M.; Mahmood, S.; Al-Anazi, K.M.; Farah, M.A.; Ali, S.; Ali, B. Biotransformation of Agricultural Wastes into Lovastatin and Optimization of a Fermentation Process Using Response Surface Methodology (RSM). Agronomy 2022, 12, 2848. [Google Scholar] [CrossRef]
- Bizukojc, M.; Ledakowicz, S. Physiological, morphological and kinetic aspects of lovastatin biosynthesis by Aspergillus terreus. Biotechnol. J. 2009, 4, 647–664. [Google Scholar] [CrossRef]
- Dikshit, R.; Tallapragada, P. Bio-synthesis and screening of nutrients for lovastatin by Monascus sp. under solid-state fermentation. J. Food Sci. Technol. 2015, 52, 6679–6686. [Google Scholar] [CrossRef] [PubMed]
- Seydametova, E.; Salihon, J.; Zainol, N.; Convey, P. Production of Lovastatin by Penicillium spp. soil microfungi. Int. J. Chem. Eng. Appl. 2012, 3, 337–339. [Google Scholar] [CrossRef]
- Upendra, R.; Pratima, K.; Amiri, Z.; Shwetha, L. Screening and molecular characterization of natural fungal isolates producing lovastatin. J. Microb. Biochem. Technol. 2013, 5, 25–30. [Google Scholar] [CrossRef]
- Mouafi, F.E.; Ibrahim, G.S.; Elsoud, M.M.A. Optimization of lovastatin production from Aspergillus fumigatus. J. Genet. Eng. Biotechnol. 2016, 14, 253–259. [Google Scholar] [CrossRef]
- Manzoni, M.; Rollini, M. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl. Microbiol. Biotechnol. 2002, 58, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Kamath, P.V.; Dwarakanath, B.S.; Chaudhary, A.; Janakiraman, S. Optimization of culture conditions for maximal lovastatin production by Aspergillus terreus (KM017963) under solid state fermentation. HAYATI J. Biosci. 2015, 22, 174–180. [Google Scholar] [CrossRef]
- Mulder, K.C.; Mulinari, F.; Franco, O.L.; Soares, M.S.; Magalhães, B.S.; Parachin, N.S. Lovastatin production: From molecular basis to industrial process optimization. Biotechnol. Adv. 2015, 33, 648–665. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.F.; El-Hashemy, M.A.; Abdel-Latif, N.M.; Shetaya, W.H. Production of sugarcane bagasse-based activated carbon for formaldehyde gas removal from potted plants exposure chamber. J. Air Waste Manag. Assoc. 2015, 65, 1413–1420. [Google Scholar] [CrossRef]
- Moubarik, A.; Grimi, N. Valorization of olive stone and sugar cane bagasse by-products as biosorbents for the removal of cadmium from aqueous solution. Food Res. Int. 2015, 73, 169–175. [Google Scholar] [CrossRef]
- Peñafiel, M.E.; Matesanz, J.M.; Vanegas, E.; Bermejo, D.; Mosteo, R.; Ormad, M.P. Comparative adsorption of ciprofloxacin on sugarcane bagasse from Ecuador and on commercial powdered activated carbon. Sci. Total Environ. 2021, 750, 141498. [Google Scholar] [CrossRef]
- Tony, M.A. An industrial ecology approach: Green cellulose-based bio-adsorbent from sugar industry residue for treating textile industry wastewater effluent. Int. J. Environ. Anal. Chem. 2021, 101, 167–183. [Google Scholar] [CrossRef]
- Cardona, C.; Quintero, J.; Paz, I. Production of bioethanol from sugarcane bagasse: Status and perspectives. Bioresour. Technol. 2010, 101, 4754–4766. [Google Scholar] [CrossRef]
- Bhatia, L.; Paliwal, S. Ethanol producing potential of Pachysolen tannophilus from sugarcane bagasse. Int. J. Biotechnol. Bioeng. Res. 2011, 2, 271–276. [Google Scholar]
- Faisal, M.; Saeed, A. Sustainable Approaches Toward the Production of Bioethanol from Biomass. In Sustainable Ethanol and Climate Change; Springer: Berlin/Heidelberg, Germany, 2021; pp. 15–38. [Google Scholar] [CrossRef]
- Ntimbani, R.N.; Farzad, S.; Görgens, J.F. Furfural production from sugarcane bagasse along with co-production of ethanol from furfural residues. Biomass Convers. Biorefinery 2021, 12, 5257–5267. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T. Biotechnological potential of agro-industrial residues. I: Sugarcane bagasse. Bioresour. Technol. 2000, 74, 69–80. [Google Scholar] [CrossRef]
- Breccia, J.; Sineriz, F.; Bettucci, L.; Piaggio, M. Degradation of sugar cane bagasse by several white-rot fungi. Acta Biotechnol. 1997, 17, 177–184. [Google Scholar] [CrossRef]
- Al-Sa’ady, A.J.; Aziz, G.M. Optimization of Lovastatin Production from A Local Isolate of Aspergillus terreus A50 in Solid State Fermentation by Classical and Statistical Methods. Iraqi J. Sci. 2020, 61, 2525–2539. [Google Scholar] [CrossRef]
- Barrios-González, J.; Pérez-Sánchez, A.; Bibián, M.E. New knowledge about the biosynthesis of lovastatin and its production by fermentation of Aspergillus terreus. Appl. Microbiol. Biotechnol. 2020, 104, 8979–8998. [Google Scholar] [CrossRef]
- Zhgun, A.A.; Nuraeva, G.K.; Volkov, I.A. High-yielding lovastatin producer Aspergillus terreus shows increased resistance to inhibitors of polyamine biosynthesis. Appl. Sci. 2020, 10, 8290. [Google Scholar] [CrossRef]
- Sripalakit, P.; Saraphanchotiwitthaya, A. Lovastatin Production from Aspergillus Terreus ATCC 20542 Under Various Vegetable Oils Used as Sole and Supplementary Carbon Sources. Pharm. Chem. J. 2020, 54, 302–309. [Google Scholar] [CrossRef]
- El-Ramady, H.; El-Henawy, A.; Amer, M.; Omara, A.E.-D.; Elsakhawy, T.; Elbasiouny, H.; Elbehiry, F.; Abou Elyazid, D.; El-Mahrouk, M. Agricultural waste and its nano-management: Mini review. Egypt. J. Soil Sci. 2020, 60, 349–364. [Google Scholar] [CrossRef]
- Rodriguez Porcel, E.; Casas Lopez, J.; Sanchez Perez, J.; Chisti, Y. Enhanced production of lovastatin in a bubble column by Aspergillus terreus using a two-stage feeding strategy. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2007, 82, 58–64. [Google Scholar] [CrossRef]
- Wei, P.-L.; Xu, Z.-N.; Cen, P.-L. Lovastatin production by Aspergillus terreus in solid-state fermentation. J. Zhejiang Univ. Sci. A 2007, 8, 1521–1526. [Google Scholar] [CrossRef]
- Porcel, E.M.R.; Lopez, J.L.C.; Perez, J.A.S.; Chisti, Y. Lovastatin production by Aspergillus terreus in a two-staged feeding operation. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2008, 83, 1236–1243. [Google Scholar] [CrossRef]
- Lai, L.-S.T.; Pan, C.-C.; Tzeng, B.-K. The influence of medium design on lovastatin production and pellet formation with a high-producing mutant of Aspergillus terreus in submerged cultures. Process Biochem. 2003, 38, 1317–1326. [Google Scholar] [CrossRef]
- López, J.C.; Pérez, J.S.; Sevilla, J.F.; Fernández, F.A.; Grima, E.M.; Chisti, Y. Production of lovastatin by Aspergillus terreus: Effects of the C: N ratio and the principal nutrients on growth and metabolite production. Enzym. Microb. Technol. 2003, 33, 270–277. [Google Scholar] [CrossRef]
- Casas López, J.; Sanchez Perez, J.; Fernández Sevilla, J.; Acién Fernández, F.; Molina Grima, E.; Chisti, Y. Fermentation optimization for the production of lovastatin by Aspergillus terreus: Use of response surface methodology. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2004, 79, 1119–1126. [Google Scholar] [CrossRef]
- Porcel, E.R.; López, J.C.; Ferrón, M.V.; Pérez, J.S.; Sánchez, J.G.; Chisti, Y. Effects of the sporulation conditions on the lovastatin production by Aspergillus terreus. Bioprocess Biosyst. Eng. 2006, 29, 1–5. [Google Scholar] [CrossRef]
- Bizukojc, M.; Pawlowska, B.; Ledakowicz, S. Supplementation of the cultivation media with B-group vitamins enhances lovastatin biosynthesis by Aspergillus terreus. J. Biotechnol. 2007, 127, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.-S.T.; Hung, C.-S.; Lo, C.-C. Effects of lactose and glucose on production of itaconic acid and lovastatin by Aspergillus terreus ATCC 20542. J. Biosci. Bioeng. 2007, 104, 9–13. [Google Scholar] [CrossRef]
- Sripalakit, P.; Riunkesorn, J.; Saraphanchotiwitthaya, A. Utilisation of vegetable oils in the production of lovastatin by Aspergillus terreus ATCC 20542 in submerged cultivation. Maejo Int. J. Sci. Technol. 2011, 5, 231–240. [Google Scholar]
- Boruta, T.; Bizukojc, M. Induction of secondary metabolism of Aspergillus terreus ATCC 20542 in the batch bioreactor cultures. Appl. Microbiol. Biotechnol. 2016, 100, 3009–3022. [Google Scholar] [CrossRef]
- Rahim, M.H.A.; Hasan, H.; Harith, H.H.; Abbas, A. The effect of viscosity, friction, and sonication on the morphology and metabolite production from Aspergillus terreus ATCC 20542. Bioprocess Biosyst. Eng. 2017, 40, 1753–1761. [Google Scholar] [CrossRef]
- Novak, N.; Gerdin, S.; Berovic, M. Increased lovastatin formation by Aspergillus terreus using repeated fed-batch process. Biotechnol. Lett. 1997, 19, 947–948. [Google Scholar] [CrossRef]
- Faseleh Jahromi, M.; Liang, J.B.; Ho, Y.W.; Mohamad, R.; Goh, Y.M.; Shokryazdan, P. Lovastatin production by Aspergillus terreus using agro-biomass as substrate in solid state fermentation. J. Biomed. Biotechnol. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Saleem, Y.; Hussain, Z.; Zahoor, S.; Javed, M. Optimization of culture conditions for the production of lovastatin by Aspergillus terreus in submerged fermentation. Pharm. Chem. J. 2018, 52, 284–289. [Google Scholar] [CrossRef]
- Vilches Ferrón, M.; Casas López, J.; Sánchez Pérez, J.; Fernández Sevilla, J.; Chisti, Y. Rapid screening of Aspergillus terreus mutants for overproduction of lovastatin. World J. Microbiol. Biotechnol. 2005, 21, 123–125. [Google Scholar] [CrossRef]
- Oliveira, M.C.L.D.; Paulo, A.J.; Lima, C.D.A.; de Lima Filho, J.L.; Souza-Motta, C.M.; Vidal, E.E.; Nascimento, T.P.; Marques, D.D.A.V.; Porto, A.L.F. Lovastatin producing by wild strain of Aspergillus terreus isolated from Brazil. Prep. Biochem. Biotechnol. 2021, 51, 164–172. [Google Scholar] [CrossRef]
- Alberts, A.; Chen, J.; Kuron, G.; Hunt, V.; Huff, J.; Hoffman, C.; Rothrock, J.; Lopez, M.; Joshua, H.; Harris, E. Mevinolin: A highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. USA 1980, 77, 3957–3961. [Google Scholar] [CrossRef]
- Reddy, D.S.R.; Latha, D.P.; Latha, K. Production of lovastatin by solid state fermentation by Penicillium funiculosum NCIM 1174. Drug Invent Today 2011, 3, 75–77. [Google Scholar]
- Valera, H.; Gomes, J.; Lakshmi, S.; Gururaja, R.; Suryanarayan, S.; Kumar, D. Lovastatin production by solid state fermentation using Aspergillus flavipes. Enzym. Microb. Technol. 2005, 37, 521–526. [Google Scholar] [CrossRef]
- Alarcón, J.; Águila, S. Lovastatin production by Pleurotus ostreatus: Effects of the C: N ratio. Z. Für Nat. C 2006, 61, 95–98. [Google Scholar] [CrossRef]
- Pratheebaa, P.; Periasamy, R.; Palvannan, T. Factorial design for optimization of laccase production from Pleurotus ostreatus IMI 395545 and laccase mediated synthetic dye decolorization. Indian J. Biotechnol. 2013, 12, 236–245. [Google Scholar]
- Bizukojc, M.; Pawlak, M.; Boruta, T.; Gonciarz, J. Effect of pH on biosynthesis of lovastatin and other secondary metabolites by Aspergillus terreus ATCC 20542. J. Biotechnol. 2012, 162, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Samiee, S.M.; Moazami, N.; Haghighi, S.; Aziz Mohseni, F.; Mirdamadi, S.; Bakhtiari, M.R. Screening of Lovastatin Production by Filamentous Fungi. Iran. Biomed. J. 2003, 7, 29–33. [Google Scholar]
- Dewi, R.; Artanti, N.; Mulyani, H.; Lotulung, P.D.; Minarti, M. Production of Lovastatin and Sulochrin by Aspergillus terreus Using Solid State Fermentation. MAKARA Technol. Ser. 2011, 15, 1–4. [Google Scholar] [CrossRef]
- Raghunath, R.; Radhakrishna, A.; Manikandan, N.; Nathiya, K.; Palaniswamy, M. Optimized production of lovastatin through solid state fermentation by endophytic fungi. Int. J. Pharm. Bio Sci. 2012, 3, 562–570. [Google Scholar]
- Chaynika, P.; Srividya, S. Bioprospecting of Lovastatin Producing Fungi Isolated from Soil Samples. Int. Res. J. Biol. Sci. 2014, 3, 42–46. [Google Scholar]
- Gulyamova, T.; Ruzieva, D.; Masmetova, S.M.; Sattarova, R.S.; Kondrasheva, K.; Abdulmyanova, L.; Rasulova, G.A. Lovastatin production by Aspergillus terreus in solid state and submerged fermentations. Int. J. Eng. Sci. Technol. 2013, 5, 19–24. [Google Scholar] [CrossRef]
- Pansuriya, R.C.; Singhal, R.S. Response surface methodology for optimization of production of lovastatin by solid state fermentation. Braz. J. Microbiol. 2010, 41, 164–172. [Google Scholar] [CrossRef]
- Ahmed El-Gendy, M.M.A.; Al-Zahrani, H.A.; El-Bondkly, A.M.A. Genome shuffling of mangrove endophytic Aspergillus luchuensis MERV10 for improving the cholesterol-lowering agent lovastatin under solid state fermentation. Mycobiology 2016, 44, 171–179. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Sathiyabama, M. Lovastatin-producing endophytic fungus isolated from a medicinal plant Solanum xanthocarpum. Nat. Prod. Res. 2015, 29, 2282–2286. [Google Scholar] [CrossRef]
- Shurshalova, G.; Yulmetov, A.; Sharapova, D.; Aganov, A.; Klochkov, V. Interaction of lovastatin with model membranes by NMR data and from MD simulations. Bionanoscience 2020, 10, 493–501. [Google Scholar] [CrossRef]
- Alarcon, J.; Aguila, S.; Arancibia-Avila, P.; Fuentes, O.; Zamorano-Ponce, E.; Hernández, M. Production and purification of statins from Pleurotus ostreatus (Basidiomycetes) strains. Zeitschrift für Naturforschung C 2003, 58, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.H.; Zidan, S.A.; Samy, M.N.; Alian, A.; Abdelmohsen, U.R.; Fouad, M.A.; Kamel, M.S.; Matsunami, K. Cytotoxicity and chemical profiling of the Red Sea soft corals Litophyton arboreum. Nat. Prod. Res. 2022, 36, 4261–4265. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Xia, M.; Ito, Y. Preparation of Mevinolinic Acid from Monascus purpureus Using High-Speed Countercurrent Chromatography (HSCCC). J. Liq. Chromatogr. Relat. Technol. 2003, 26, 3085–3092. [Google Scholar] [CrossRef]
- Mahmoud, O.A.; Abdel_Hadi, S.Y. Extraction and Purification of Lovastatin from the Edible Mushroom Laetiporus sulphureus and its Antioxidant Activity. Egypt. J. Bot. 2022, 62, 169–175. [Google Scholar] [CrossRef]
- Kumar, S.; Srivastava, N.; Gomes, J. The effect of lovastatin on oxidative stress and antioxidant enzymes in hydrogen peroxide intoxicated rat. Food Chem. Toxicol. 2011, 49, 898–902. [Google Scholar] [CrossRef]
- Al-Saman, M.A.; Helmy, M.A.; Abdella, A.; Wilkins, M.R.; Gobba, N.A.E.K.; Mahrous, H. Optimization of lovastatin production by Aspergillus terreus ATCC 10020 using solid-state fermentation and its pharmacological applications. Biocatal. Agric. Biotechnol. 2021, 31, 101906. [Google Scholar] [CrossRef]
- Jeon, S.-M.; Bok, S.-H.; Jang, M.-K.; Lee, M.-K.; Nam, K.-T.; Park, Y.B.; Rhee, S.-J.; Choi, M.-S. Antioxidative activity of naringin and lovastatin in high cholesterol-fed rabbits. Life Sci. 2001, 69, 2855–2866. [Google Scholar] [CrossRef]
- Abou Ghalia, A.H.; Fouad, I.M. Glutathione and its metabolizing enzymes in patients with different benign and malignant diseases. Clin. Biochem. 2000, 33, 657–662. [Google Scholar] [CrossRef]
- Argani, H.; Ghorbani, A.; Rashtchizade, N.; Rahbaninobar, M. Effect of Lovastatin on Lipid peroxidation and total antioxidant concentrations in hemodialysis patients. Lipids Health Dis. 2004, 3, 6. [Google Scholar] [CrossRef]
- Stancu, C.; Sima, A. Statins: Mechanism of action and effects. J. Cell. Mol. Med. 2001, 5, 378–387. [Google Scholar] [CrossRef]
- Montazerifar, F.; Hashemi, M.; Karajibani, M.; Dikshit, M. Effect of antioxidant vitamins on lipid profile and total antioxidant capacity in hemodialysis patients. Rawal Med. J. 2010, 35, 120–123. [Google Scholar]
- Al-Fakih, A.A.; Almaqtri, W.Q.A. Overview on antibacterial metabolites from terrestrial Aspergillus spp. Mycology 2019, 10, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, G.; Huang, X.; Luo, Z. Fungal diversity study in the deep sea sediments of three oceans by culture-dependent approach. J. Appl. Oceanogr. 2015, 34, 103–110. [Google Scholar]
- Lorenz, R.; Parks, L. Effects of lovastatin (mevinolin) on sterol levels and on activity of azoles in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 1990, 34, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Macreadie, I.G.; Johnson, G.; Schlosser, T.; Macreadie, P.I. Growth inhibition of Candida species and Aspergillus fumigatus by statins. FEMS Microbiol. Lett. 2006, 262, 9–13. [Google Scholar] [CrossRef]
- Friesen, J.A.; Rodwell, V.W. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol. 2004, 5, 248. [Google Scholar] [CrossRef]
- Haeri, M.R.; White, K.; Qharebeglou, M.; Ansar, M.M. Cholesterol suppresses antimicrobial effect of statins. Iran. J. Basic Med. Sci. 2015, 18, 1253–1256. [Google Scholar]
- Qiao, J.; Kontoyiannis, D.P.; Wan, Z.; Li, R.; Liu, W. Antifungal activity of statins against Aspergillus species. Med. Mycol. 2007, 45, 589–593. [Google Scholar] [CrossRef]
- Graziano, T.S.; Cuzzullin, M.C.; Franco, G.C.; Schwartz-Filho, H.O.; de Andrade, E.D.; Groppo, F.C.; Cogo-Mueller, K. Statins and antimicrobial effects: Simvastatin as a potential drug against Staphylococcus aureus biofilm. PLoS ONE 2015, 10, e0128098. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Masadeh, M.; Mhaidat, N.; Alzoubi, K.; Al-Azzam, S.; Alnasser, Z. Antibacterial activity of statins: A comparative study of atorvastatin, simvastatin, and rosuvastatin. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.; Sommers, L. Plate-dilution frequency technique for assay of microbial ecology. Appl. Microbiol. 1968, 16, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-W.; Hyde, K.D.; Ho, W. Single spore isolation of fungi. Fungal Divers. 1999, 3, 29–38. [Google Scholar]
- Smith, D.; Onions, A.H. The Preservation and Maintenance of Living Fungi; CAB International: Egham, UK, 1994; p. 122. [Google Scholar]
- Raper, K.B.; Fennell, D.I. The Genus Aspergillus. Baltimore; Williams & Wilkins Co.: Philadelphia, PA, USA, 1965; p. 686. [Google Scholar]
- Moubasher, A.H.; Ismail, M.A.; Al-Bedak, O.A.; Mohamed, R.A. Ramophialophora chlamydospora, a new species from an alkaline lake of Wadi-El-Natron, Egypt. Asian J. Mycol. 2019, 2, 110–117. [Google Scholar] [CrossRef]
- Al-Bedak, O.A.; Moubasher, A.H. Aspergillus gaarensis, a new addition to section Circumdati from soil of Lake El-Gaar in Wadi-El-Natron, Egypt. Stud. Fungi 2020, 5, 59–65. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Posada, D.; Crandall, K.A. Modeltest: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef]
- Azeem, M.; Arshad, M.; Mahmood, S.; Abrar, S.; Zahoor, A.F.; Javed, S.; Tariq, B.; Hayyat, K. Development of One Pot Strategy for Hyper Production and In Vivo Evaluation of Lovastatin. Molecules 2020, 25, 4380. [Google Scholar] [CrossRef] [PubMed]
- Markopoulou, C.; Koundourelllis, J. Two derivative spectrophotometric methods for the simultaneous determination of lovastatin combined with three antioxidants. J. Pharm. Biomed. Anal. 2003, 33, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Valgas, C.; Souza, S.M.D.; Smânia, E.F.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- St, L.; Wold, S. Analysis of variance (ANOVA). Chemom. Intell. Lab. Syst. 1989, 6, 259–272. [Google Scholar] [CrossRef]






| No | δH, (J in Hz) | δC, Type | No | δH, (J in Hz) | δC, Type |
|---|---|---|---|---|---|
| 1 | 5.39, 1H, m | 69.5 d | 13 | 4.64, 1H, m | 78.0 d |
| 2a | 1.98, 1H, m | 33.6 t | 14a | 1.95, 1H, m | 36.6 t |
| 2b | 1.82, 1H, m | 14b | 1.79, 1H, m | ||
| 3 | 2.45, 1H, m | 28.8 d | 15 | 4.26, 1H, m | 63.3 d |
| 4 | 5.52, 1H, br.t (2.8) | 130.3 d | 16a | 2.73, 1H, dd (4.8, 16.8) | 39.1 t |
| 5 | - | 129.5 s | 16b | 2.56, 1H, ddd (1.6, 4.8, 16.8) | |
| 6 | 6.00, 1H, d (9.6) | 129.8 d | 17 | - | 173.4 s |
| 7 | 5.80, 1H, dd (6.0, 9.6) | 133.9 d | 18 | 0.94, 3H, d (7.2) | 14.1 q |
| 8 | 2.42, 1H, m | 31.9 d | 19 | 1.10, 3H, d (7.2) | 23.3 q |
| 9 | 1.75, 1H, m | 37.9 d | 1′ | - | 178.2 s |
| 10 | 2.38, 1H, dd (6.8, 7.2) | 38.5 d | 2′ | 2.36, 1H, m | 42.8 d |
| 11a | 1.52, 1H, m | 25.1 t | 3a′ | 1.68, 1H, m | 27.9 t |
| 11b | 1.45, 1H, m | 3b′ | 1.50, 1H, m | ||
| 12a | 1.91, 1H, m | 34.0 t | 4′ | 0.93, 3H, dd (2.0, 7.2) | 12.1 q |
| 12b | 1.37, 1H, m | 5′ | 1.12, 3H, d (7.2) | 16.6 q |
| Tested Organisms | Purified Lovastatin (mg/mL) | NS (50 mcg) | P/T (110 µg) | ||||
|---|---|---|---|---|---|---|---|
| 5 | 2.5 | 1.25 | 0.62 | 0.31 | |||
| C. albicans | 10.5 ± 0.2 c | 9.2 ± 0.2 e | 0 g | 0 g | 0 g | 21.0 ± 1.6 b | - |
| C. glabrata | 8.7 ± 0.4 d | 0 g | 0 g | 0 g | 0 g | 22.43 ± 1.2 a | - |
| C. krusei | 0 f | 0 f | 0 f | 0 g | 0 g | 16.2 ± 0.8 d | - |
| E. coli | 0 f | 0 f | 0 f | 0 f | 0 f | - | 15.3 ± 0.2 e |
| S. aureus | 20.3 ± 0.6 c | 15.6 ± 0.2 e | 11.5 ± 0.7 h | 0 f | 0 f | - | 18.0 ± 0.3 a |
| S. epidermidis | 19.4 ± 0.5 e | 15.3 ± 0.1 d | 11.4 ± 0.4 f | 0 f | 0 f | - | 15.36 ± 0.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramadan, A.M.A.A.; Shehata, R.M.; EL-Sheikh, H.H.; Ameen, F.; Stephenson, S.L.; Zidan, S.A.H.; Al-Bedak, O.A.M. Exploitation of Sugarcane Bagasse and Environmentally Sustainable Production, Purification, Characterization, and Application of Lovastatin by Aspergillus terreus AUMC 15760 under Solid-State Conditions. Molecules 2023, 28, 4048. https://doi.org/10.3390/molecules28104048
Ramadan AMAA, Shehata RM, EL-Sheikh HH, Ameen F, Stephenson SL, Zidan SAH, Al-Bedak OAM. Exploitation of Sugarcane Bagasse and Environmentally Sustainable Production, Purification, Characterization, and Application of Lovastatin by Aspergillus terreus AUMC 15760 under Solid-State Conditions. Molecules. 2023; 28(10):4048. https://doi.org/10.3390/molecules28104048
Chicago/Turabian StyleRamadan, Ahmed M. A. A., Reda M. Shehata, Hussein H. EL-Sheikh, Fuad Ameen, Steven L. Stephenson, Sabry A. H. Zidan, and Osama A. M. Al-Bedak. 2023. "Exploitation of Sugarcane Bagasse and Environmentally Sustainable Production, Purification, Characterization, and Application of Lovastatin by Aspergillus terreus AUMC 15760 under Solid-State Conditions" Molecules 28, no. 10: 4048. https://doi.org/10.3390/molecules28104048
APA StyleRamadan, A. M. A. A., Shehata, R. M., EL-Sheikh, H. H., Ameen, F., Stephenson, S. L., Zidan, S. A. H., & Al-Bedak, O. A. M. (2023). Exploitation of Sugarcane Bagasse and Environmentally Sustainable Production, Purification, Characterization, and Application of Lovastatin by Aspergillus terreus AUMC 15760 under Solid-State Conditions. Molecules, 28(10), 4048. https://doi.org/10.3390/molecules28104048









