Light Control-Induced Oxygen Vacancy Generation and In Situ Surface Heterojunction Reconstruction for Boosting CO2 Reduction
Abstract
:1. Introduction
2. Results and Discussion
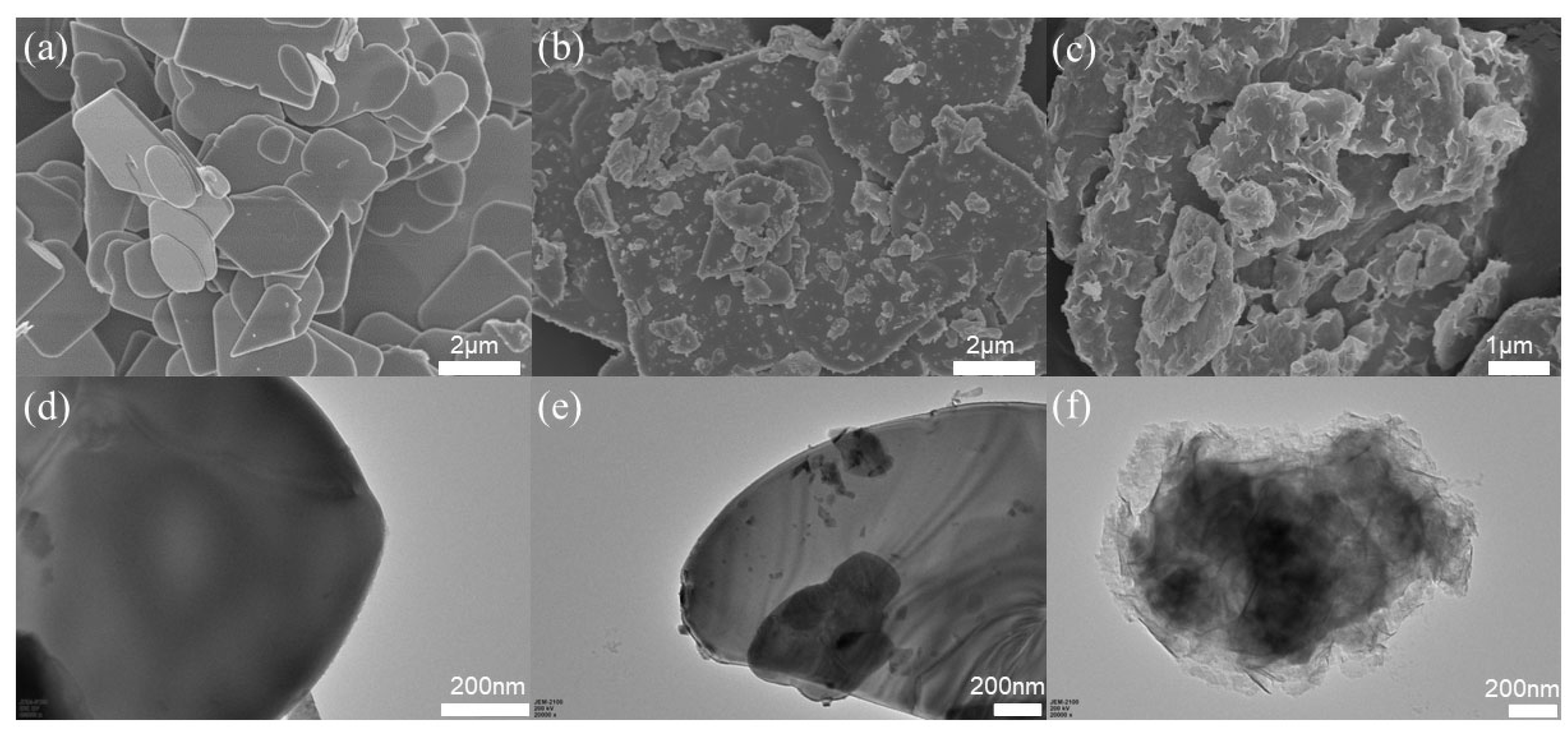
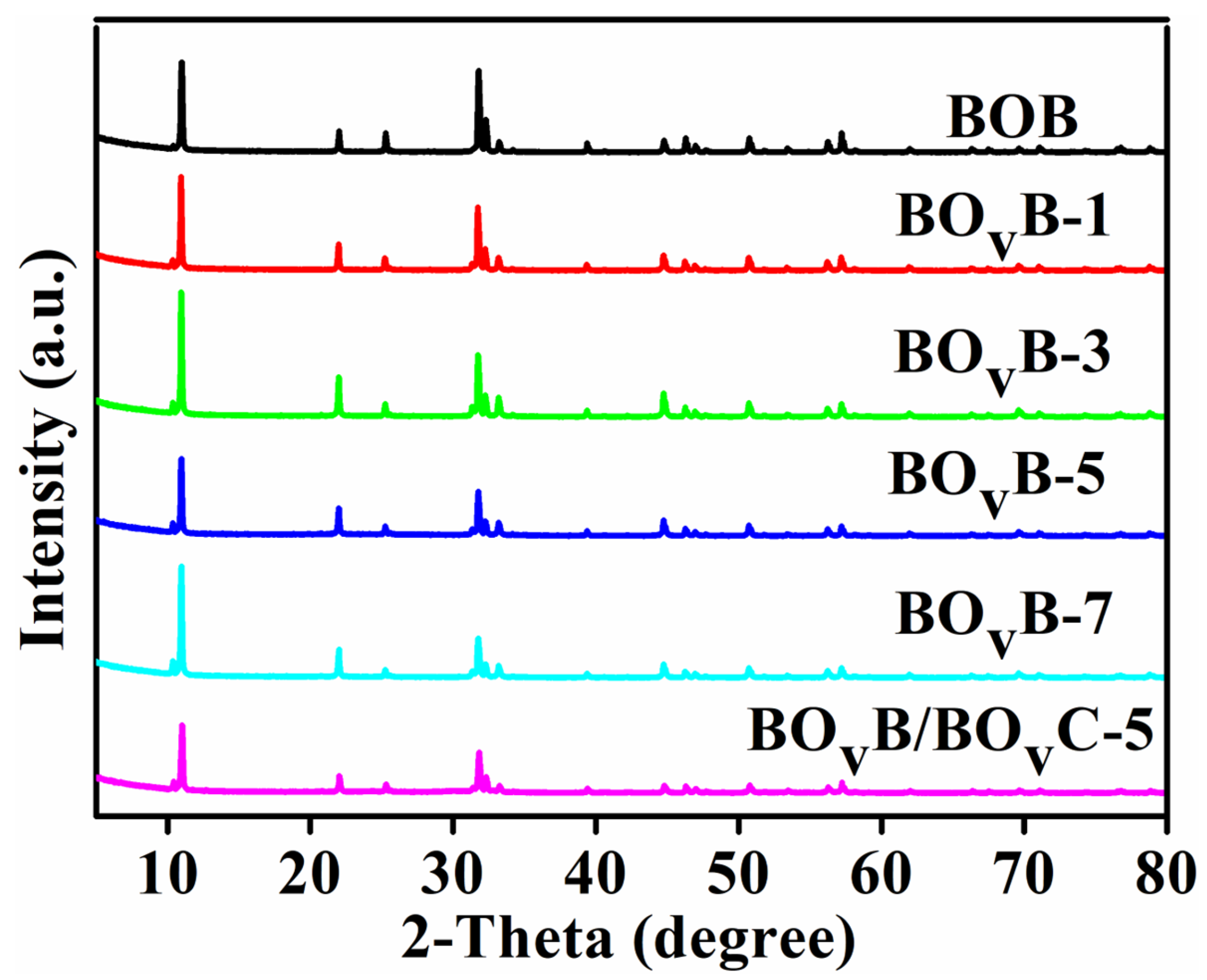
2.1. Structural Characterization and Morphological Analysis
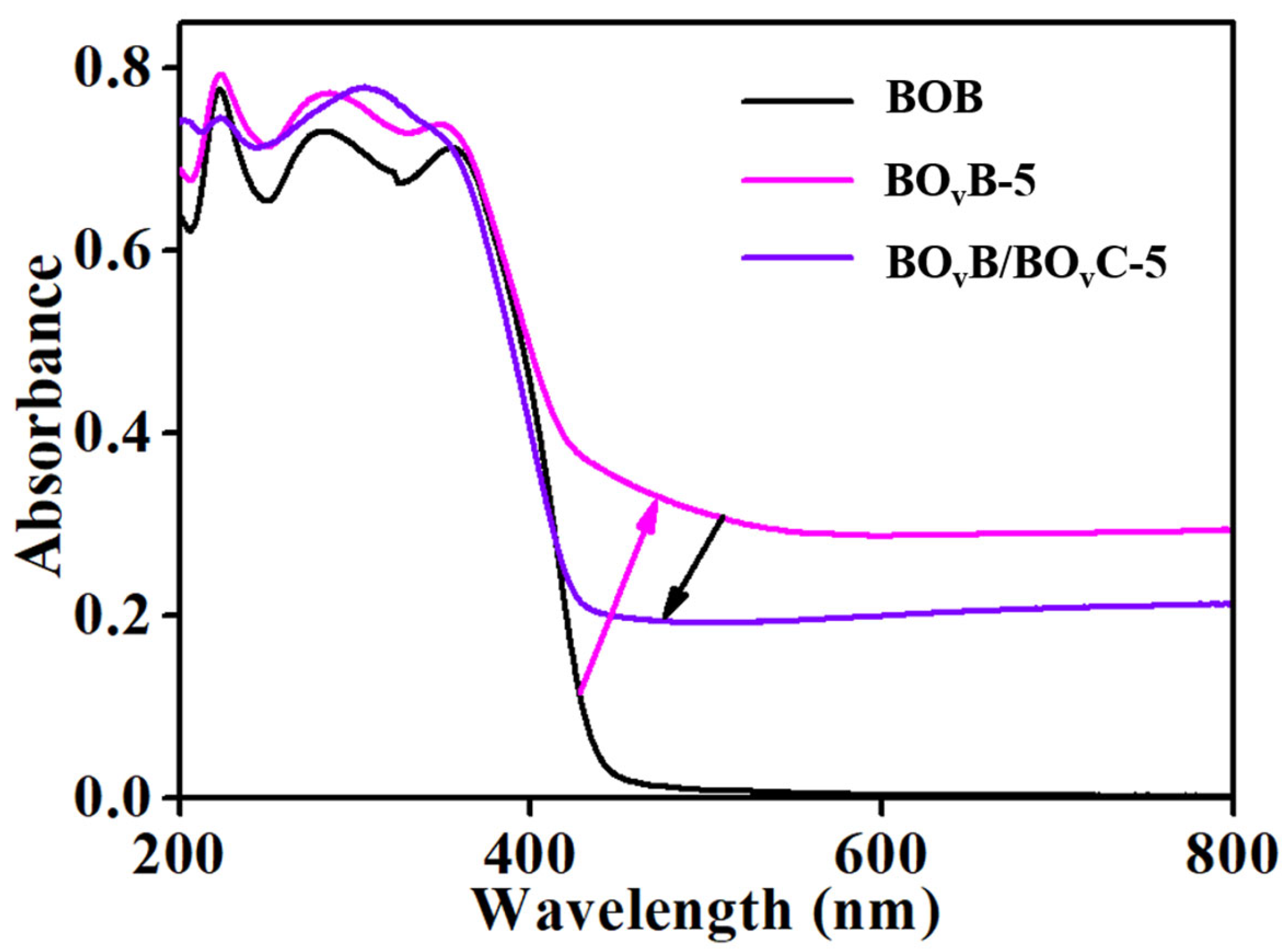
2.2. Analysis of UV-Vis Absorption Spectra
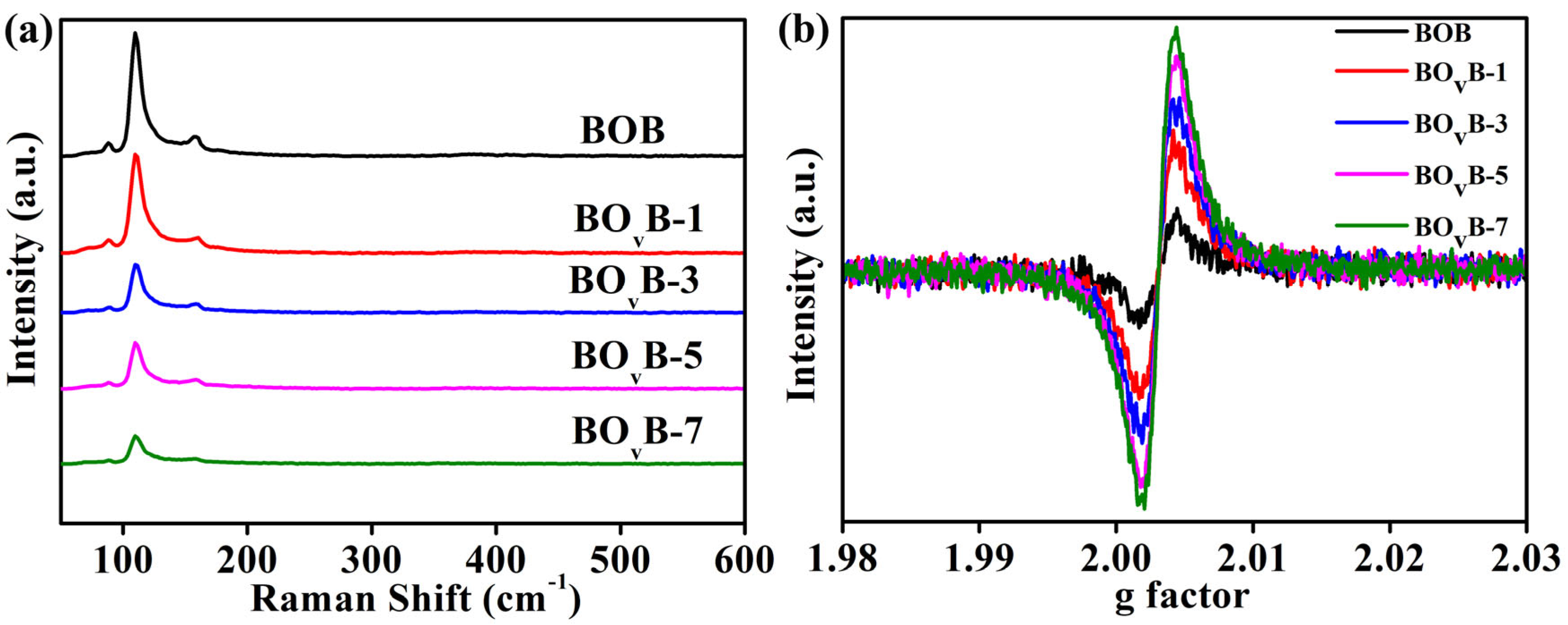
2.3. Raman and EPR Analyses
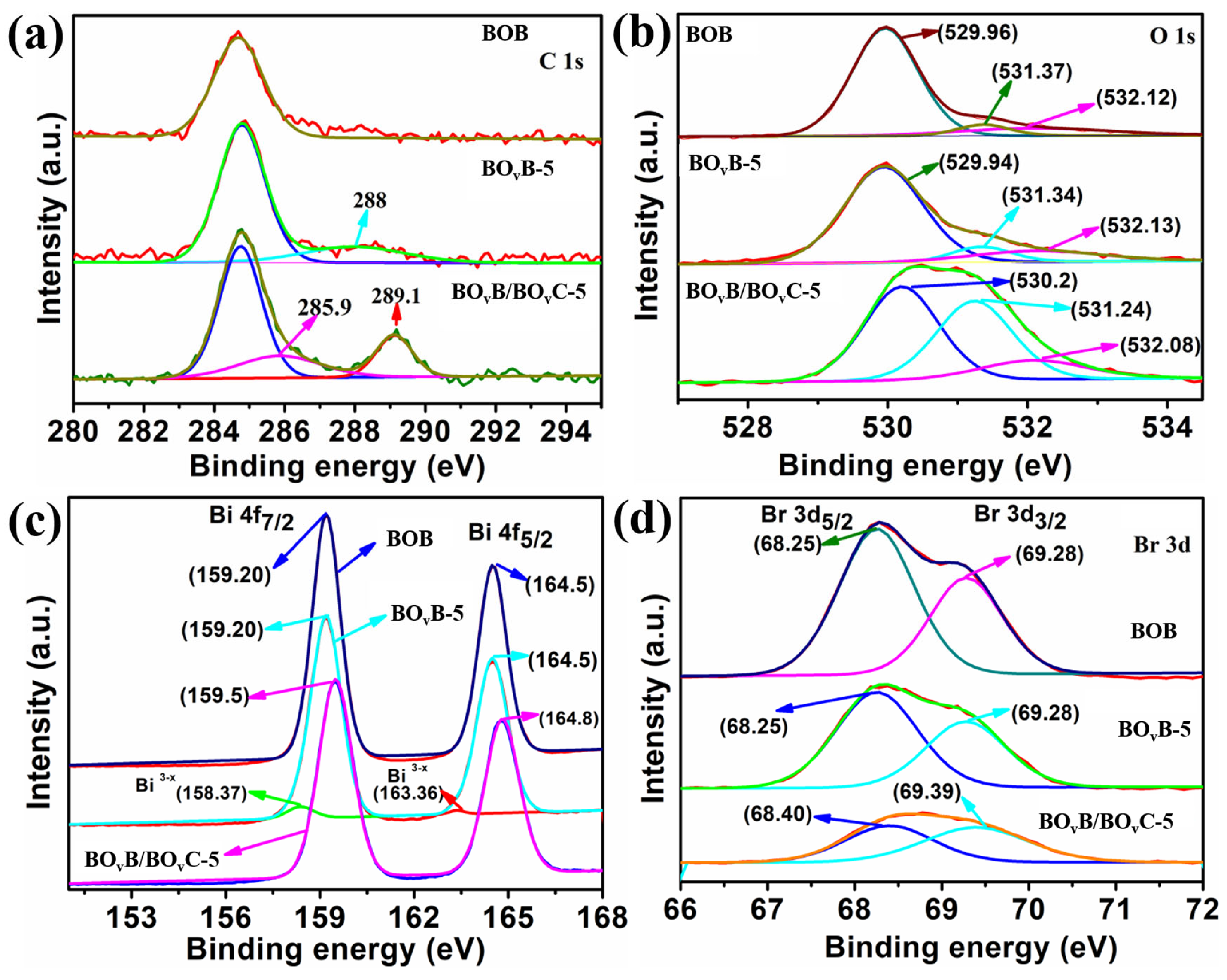
2.4. XPS Characterization
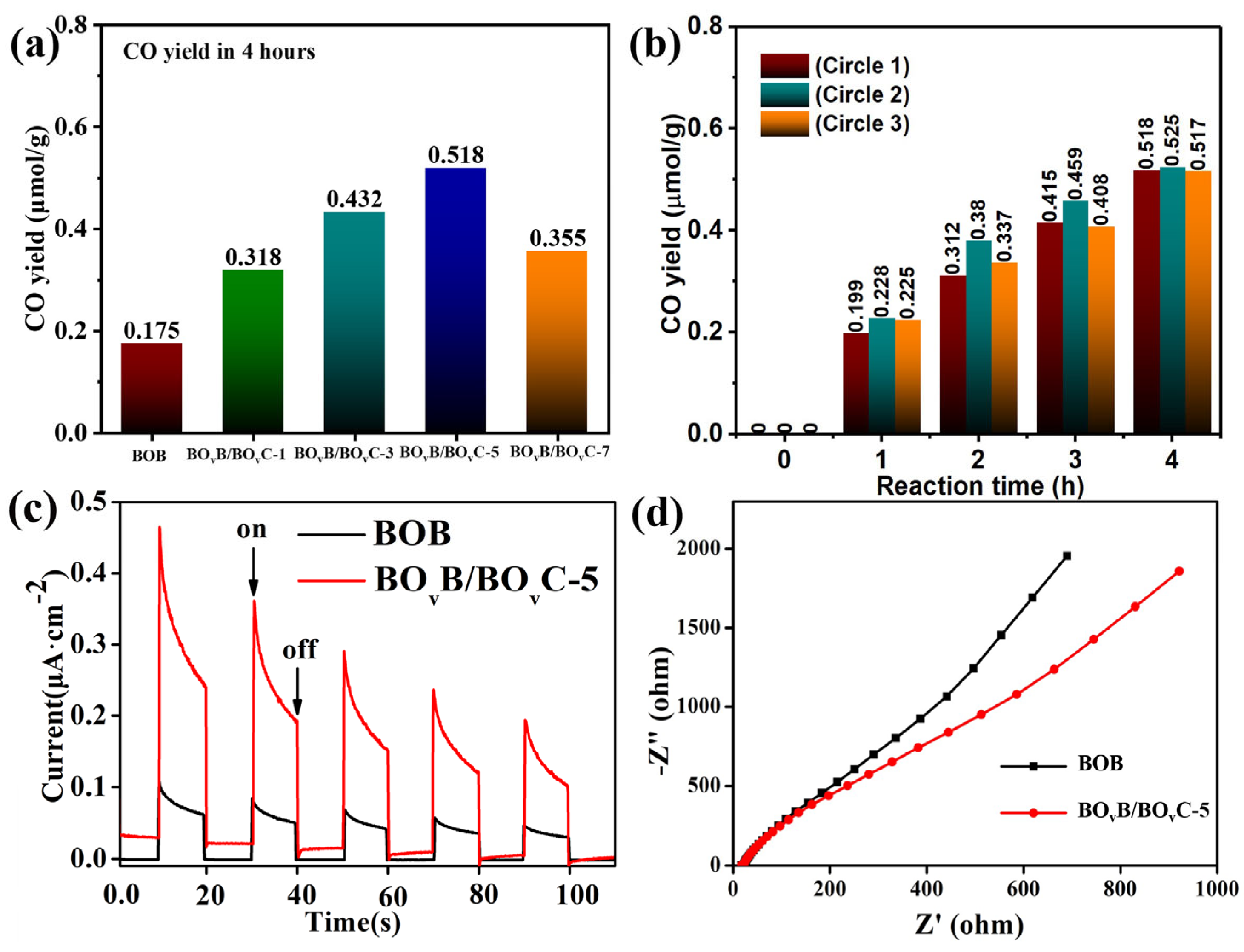
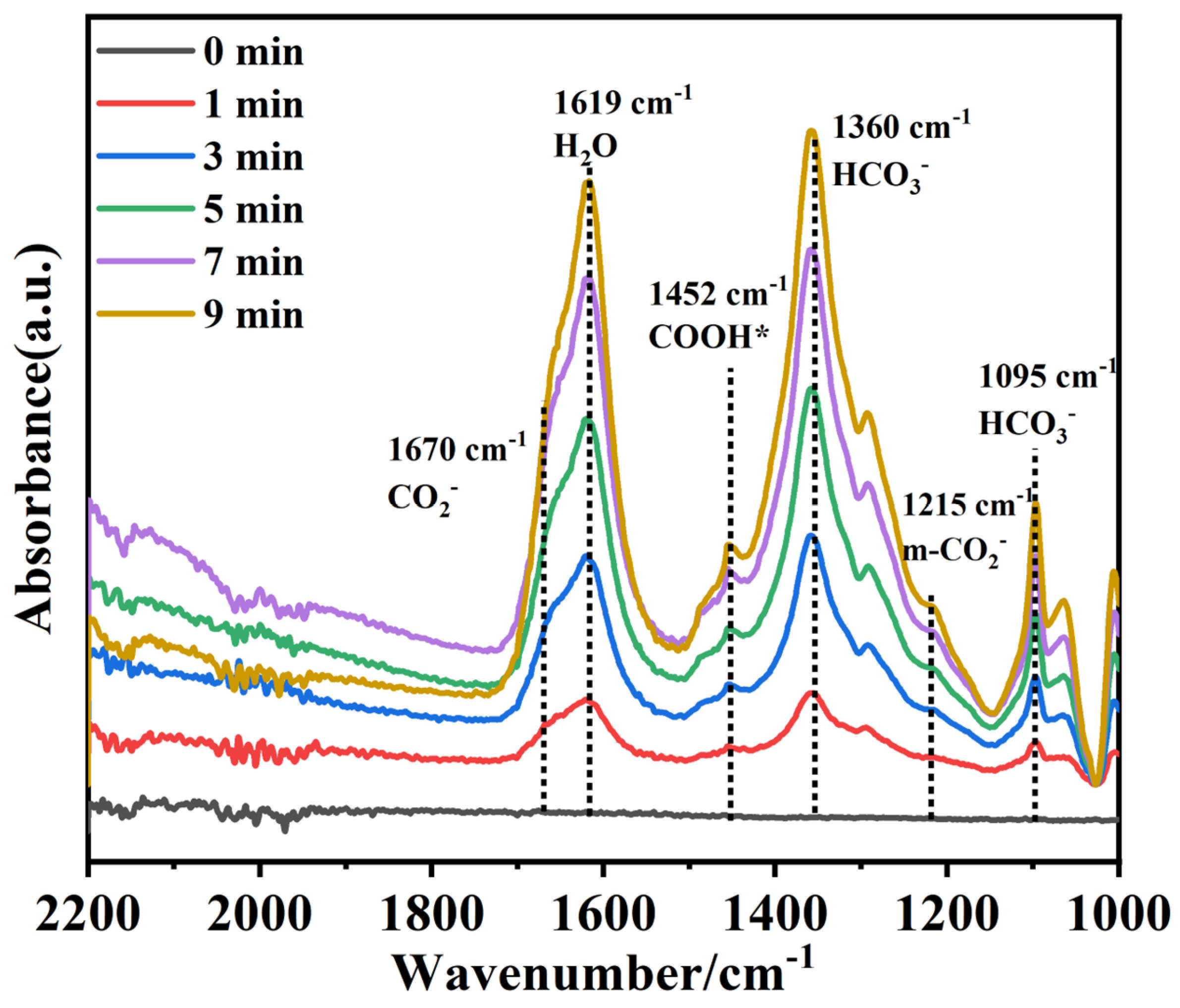
2.5. Researches on Photocatalytic Performance and CO2 Reaction Path
2.6. Mechanism
3. Experimental Sections
3.1. Materials
3.2. Synthesis of BiOBr and Defect-Rich BiOBr Photocatalysts
3.3. Characterization
3.4. Photocatalytic CO2 Reduction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, S.; Wang, H.; Kang, Z.; Jin, S.; Zhang, X.; Zheng, X.; Qi, Z.; Zhu, J.; Pan, B.; Xie, Y. Oxygen vacancy associated single-electron transfer for photofixation of CO2 to long-chain chemicals. Nat. Commun. 2019, 10, 788. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.-C.; Chen, L.-W.; Li, J.; Guo, Y.; Su, X.; Shu, M.; Zhang, Q.; Gao, W.-Y.; Li, S.; Yu, Z.-L.; et al. Metal-organic framework membranes with single-atomic centers for photocatalytic CO2 and O2 reduction. Nat. Commun. 2021, 12, 2682. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Low, J.; Long, R.; Kong, T.; Zhu, J.; Xiong, Y. Heterogeneous Single-Atom Photocatalysts: Fundamentals and Applications. Chem. Rev. 2020, 120, 12175–12216. [Google Scholar] [CrossRef]
- Chen, C.; Li, Y.; Yu, S.; Louisia, S.; Jin, J.; Li, M.; Ross, M.B.; Yang, P. Cu-Ag Tandem Catalysts for High-Rate CO2 Electrolysis toward Multicarbons. Joule 2020, 4, 1688–1699. [Google Scholar] [CrossRef]
- Ye, R.P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 5698. [Google Scholar] [CrossRef]
- Qian, C.; Sun, W.; Hung, D.L.; Qiu, C.; Makaremi, M.; Hari Kumar, S.G.; Wan, L.; Ghoussoub, M.; Wood, T.E.; Xia, M.; et al. Catalytic CO2 reduction by palladium-decorated silicon–hydride nanosheets. Nat. Catal. 2019, 2, 46–54. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Zhang, D.; Cheng, L.; Xiang, Q. Crystalline Carbon Nitride Supported Copper Single Atoms for Photocatalytic CO2 Reduction with Nearly 100% CO Selectivity. ACS Nano 2020, 14, 10552–10561. [Google Scholar] [CrossRef]
- Wang, X.; Lu, L.; Wang, B.; Xu, Z.; Xin, Z.; Yan, S.; Geng, Z.; Zou, Z. Frustrated Lewis Pairs Accelerating CO2 Reduction on Oxyhydroxide Photocatalysts with Surface Lattice Hydroxyls as a Solid-State Proton Donor. Adv. Funct. Mater. 2018, 28, 1804191. [Google Scholar] [CrossRef]
- Zhu, X.; Liang, X.; Wang, P.; Dai, Y.; Huang, B. Porous Ag-ZnO microspheres as efficient photocatalyst for methane and ethylene oxidation: Insight into the role of Ag particles. Appl. Surf. Sci. 2018, 456, 493–500. [Google Scholar] [CrossRef]
- Zhu, X.; Guan, Z.; Wang, P.; Zhang, Q.; Dai, Y.; Huang, B. Amorphous TiO2-modified CuBi2O4 Photocathode with enhanced photoelectrochemical hydrogen production activity. Chin. J. Catal. 2018, 39, 1704–1710. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Mao, C.; Liu, S.; Wang, X.; Liu, X.; Zhao, S.; Liu, X.; Huang, Y.; Zhang, L. Van Der Waals gap-rich BiOCl atomic layers realizing efficient, pure-water CO2-to-CO photocatalysis. Nat. Commun. 2021, 12, 5923. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.-P.; Huang, K.-K.; Liu, J.-H.; Wang, D.; Wang, Y.; Wang, X.; Li, Z.-D.; Wang, X.-Y.; Geng, Z.-B.; Hou, X.-Y.; et al. Magnetic-Field-Regulated TiO2 {100} Facets: A Strategy for C-C Coupling in CO2 Photocatalytic Conversion. Chem 2020, 6, 2335–2346. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, X.; Jiang, Z.; Zhang, W.; Ji, H.; Zhu, X.; Song, Y.; Mo, Z.; Li, H.; Xu, H. Construction of brown mesoporous carbon nitride with a wide spectral response for high performance photocatalytic H2 evolution. Inorg. Chem. Front. 2022, 9, 103–110. [Google Scholar] [CrossRef]
- Li, J.; Zheng, J.; Liu, X.; Yang, Y.; Han, X.; Huang, Z. Regulating the photoelectrocatalytic reduction efficiency of CO2 to syngas via SnO enhanced g-C3N4 based p-n heterojunction. Opt. Mater. 2023, 138, 113703. [Google Scholar] [CrossRef]
- Zuo, C.; Tai, X.; Su, Q. Ce3+ self-doping CeO2−x@CuO nanowires arrays on copper mesh at the gas-liquid interface: Enhanced performance of reducing CO2 to methanol under visible light. Opt. Mater. 2022, 133, 113038. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, J.; Dong, X.; Li, H. A Review and Recent Developments in Full-Spectrum Photocatalysis using ZnIn2S4-Based Photocatalysts. Energy Technol. 2021, 9, 2100033. [Google Scholar] [CrossRef]
- Bao, S.; Wang, Z.; Zhang, J.; Tian, B. Facet-Heterojunction-Based Z-Scheme BiVO4{010} Microplates Decorated with AgBr-Ag Nanoparticles for the Photocatalytic Inactivation of Bacteria and the Decomposition of Organic Contaminants. ACS Appl. Nano Mater. 2020, 3, 8604–8617. [Google Scholar] [CrossRef]
- Huang, H.; Cao, R.; Yu, S.; Xu, K.; Hao, W.; Wang, Y.; Dong, F.; Zhang, T.; Zhang, Y. Single-unit-cell layer established Bi2WO6 3D hierarchical architectures: Efficient adsorption, photocatalysis and dye-sensitized photoelectrochemical performance. Appl. Catal. B-Environ. 2017, 219, 526–537. [Google Scholar] [CrossRef]
- Yan, J.; Wang, C.; Ma, H.; Li, Y.; Liu, Y.; Suzuki, N.; Terashima, C.; Fujishima, A.; Zhang, X. Photothermal synergic enhancement of direct Z-scheme behavior of Bi4TaO8Cl/W18O49 heterostructure for CO2 reduction. Appl. Catal. B-Environ. 2020, 268, 118401. [Google Scholar] [CrossRef]
- Zhang, Y.; Shan, G.; Dong, F.; Wang, C.; Zhu, L. Glass fiber supported BiOI thin-film fixed-bed photocatalytic reactor for water decontamination under solar light irradiation. J. Environ. Sci. 2019, 80, 277–286. [Google Scholar] [CrossRef]
- Yin, W.; Sun, X.; Wang, W. Enhancement of photocatalytic efficiency by in situ fabrication of BiOBr/BiVO4 surface junctions. J. Environ. Sci. 2017, 60, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Miao, H.; Chen, J.; Zhu, X.; Yi, J.; Mo, Z.; Li, H.; Zheng, Z.; Huang, B.; Xu, H. Facet-dependent CdS/Bi4TaO8Cl Z-scheme heterojunction for enhanced photocatalytic tetracycline hydrochloride degradation and the carrier separation mechanism study via single-particle spectroscopy. Inorg. Chem. Front. 2022, 9, 2252–2263. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Cheng, M.; Wang, J.; Chen, F.; Zhou, C.; Xiong, W. BiOX (X = Cl, Br, I) photocatalytic nanomaterials: Applications for fuels and environmental management. Adv. Colloid Interface Sci. 2018, 254, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xie, Y.; Ling, Y.; Si, J.; Li, X.; Wang, J.; Ye, H.; Zhao, J.; Li, S.; Zhao, Q.; et al. One-step synthesis and Gd3+ decoration of BiOBr microspheres consisting of nanosheets toward improving photocatalytic reduction of CO2 into hydrocarbon fuel. Chem. Eng. J. 2020, 400, 125944. [Google Scholar] [CrossRef]
- Mi, Y.; Li, H.; Zhang, Y.; Du, N.; Hou, W. Synthesis and photocatalytic activity of BiOBr nanosheets with tunable crystal facets and sizes. Catal. Sci. Technol. 2018, 8, 2588–2597. [Google Scholar] [CrossRef]
- Ye, L.; Liu, J.; Jiang, Z.; Peng, T.; Zan, L. Facets coupling of BiOBr-g-C3N4 composite photocatalyst for enhanced visible-light-driven photocatalytic activity. Appl. Catal. B-Environ. 2013, 142–143, 1–7. [Google Scholar] [CrossRef]
- Xu, X.; Kou, S.; Guo, X.; Li, X.; Ma, X.; Mao, H. The Enhanced Photocatalytic Properties for Water Oxidation over Bi/BiVO4/V2O5 Composite. J. Phys. Chem. C 2017, 121, 16257–16265. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Shi, W.; Ling, P.; Sun, Y.; Jiao, X.; Gao, S.; Liang, L.; Xu, J.; Yan, W.; et al. Efficient Visible-Light-Driven CO2 Reduction Mediated by Defect-Engineered BiOBr Atomic Layers. Angew. Chem. Int. Ed. Engl. 2018, 57, 8719–8723. [Google Scholar] [CrossRef]
- Kibria, M.G.; Dinh, C.T.; Seifitokaldani, A.; De Luna, P.; Burdyny, T.; Quintero-Bermudez, R.; Ross, M.B.; Bushuyev, O.S.; Garcia de Arquer, F.P.; Yang, P.; et al. A Surface Reconstruction Route to High Productivity and Selectivity in CO2 Electroreduction toward C2+ Hydrocarbons. Adv. Mater. 2018, 30, 1804867. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.; Wang, N.; Tian, F.; Li, J. Surface reconstruction of BiSI nanorods for superb photocatalytic Cr(VI) reduction under near-infrared light irradiation. Chem. Eng. J. 2022, 435, 135152. [Google Scholar] [CrossRef]
- Guo, N.; Cao, Y.; Rong, Y.; Jia, D. Green synthesis of BiOBr modified Bi2O2CO3 nanocomposites with enhanced visible-responsive photocatalytic properties. RSC Adv. 2016, 6, 106046–106053. [Google Scholar] [CrossRef]
- Kong, X.Y.; Lee, W.P.C.; Ong, W.-J.; Chai, S.-P.; Mohamed, A.R. Oxygen-Deficient BiOBr as a Highly Stable Photocatalyst for Efficient CO2 Reduction into Renewable Carbon-Neutral Fuels. ChemCatChem 2016, 8, 3074–3081. [Google Scholar] [CrossRef]
- Wang, H.; Yong, D.; Chen, S.; Jiang, S.; Zhang, X.; Shao, W.; Zhang, Q.; Yan, W.; Pan, B.; Xie, Y. Oxygen-Vacancy-Mediated Exciton Dissociation in BiOBr for Boosting Charge-Carrier-Involved Molecular Oxygen Activation. J. Am. Chem. Soc. 2018, 140, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ma, Z.; Ye, L.; Ma, T.; Zhang, T.; Zhang, Y.; Huang, H. Macroscopic Spontaneous Polarization and Surface Oxygen Vacancies Collaboratively Boosting CO2 Photoreduction on BiOIO3 Single Crystals. Adv. Mater. 2020, 32, 1908350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Ma, Z. Facile Formation of Bi2O2CO3/Bi2MoO6 Nanosheets for Visible Light-Driven Photocatalysis. ACS Omega 2019, 4, 3871–3880. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.; Wang, J.; Dong, F.; Chu, P.K.; Zhang, T.; Zhang, Y. Anionic Group Self-Doping as a Promising Strategy: Band-Gap Engineering and Multi-Functional Applications of High-Performance CO32–-Doped Bi2O2CO3. ACS Catal. 2015, 5, 4094–4103. [Google Scholar] [CrossRef]
- Zhu, G.; Li, S.; Gao, J.; Zhang, F.; Liu, C.; Wang, Q.; Hojamberdiev, M. Constructing a 2D/2D Bi2O2CO3/Bi4O5Br2 heterostructure as a direct Z-scheme photocatalyst with enhanced photocatalytic activity for NOx removal. Appl. Surf. Sci. 2019, 493, 913–925. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, W.; Liu, G.; Chen, H.; Weng, Y.X.; Li, H.; Chu, P.K.; Xia, J. Excited Electron-Rich Bi(3−x)+ Sites: A Quantum Well-Like Structure for Highly-Promoted Selective Photocatalytic CO2 Reduction Performance. Adv. Funct. Mater. 2022, 32, 2202885. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, H.; Zhang, X.; Yang, W.; Cao, Y.; Yang, P. BiOI/Bi2O2CO3 Two-Dimensional Heteronanostructures with Boosting Charge Carrier Separation Behavior and Enhanced Visible-Light Photocatalytic Performance. J. Phys. Chem. C 2020, 124, 20294–20308. [Google Scholar] [CrossRef]
- Li, K.-L.; Lee, W.W.; Lu, C.-S.; Dai, Y.-M.; Chou, S.-Y.; Chen, H.-L.; Lin, H.-P.; Chen, C.-C. Synthesis of BiOBr, Bi3O4Br, and Bi12O17Br2 by controlled hydrothermal method and their photocatalytic properties. J. Taiwan Inst. Chem. Eng. 2014, 45, 2688–2697. [Google Scholar] [CrossRef]
- Su, N.; Zhu, D.; Zhang, P.; Fang, Y.; Chen, Y.; Fang, Z.; Zhou, X.; Li, C.; Dong, H. 3D/2D Heterojunction Fabricated from RuS2 Nanospheres Encapsulated in Polymeric Carbon Nitride Nanosheets for Selective Photocatalytic CO2 Reduction to CO. Inorg. Chem. 2022, 61, 15600–15606. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zong, H.; Viasus Pérez, C.J.; Miao, H.; Sun, W.; Yuan, Z.; Wang, S.; Zeng, G.; Xu, H.; Jiang, Z.; et al. Supercharged CO2 Photothermal catalytic Methanation: High Conversion, Rate, and Selectivity. Angew Chem. Int. Ed. Engl. 2023, e202218694. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.; Zhu, X.; Gao, Q.; Jiang, Z. Light Control-Induced Oxygen Vacancy Generation and In Situ Surface Heterojunction Reconstruction for Boosting CO2 Reduction. Molecules 2023, 28, 4057. https://doi.org/10.3390/molecules28104057
Yuan Z, Zhu X, Gao Q, Jiang Z. Light Control-Induced Oxygen Vacancy Generation and In Situ Surface Heterojunction Reconstruction for Boosting CO2 Reduction. Molecules. 2023; 28(10):4057. https://doi.org/10.3390/molecules28104057
Chicago/Turabian StyleYuan, Zhimin, Xianglin Zhu, Qichao Gao, and Zaiyong Jiang. 2023. "Light Control-Induced Oxygen Vacancy Generation and In Situ Surface Heterojunction Reconstruction for Boosting CO2 Reduction" Molecules 28, no. 10: 4057. https://doi.org/10.3390/molecules28104057
APA StyleYuan, Z., Zhu, X., Gao, Q., & Jiang, Z. (2023). Light Control-Induced Oxygen Vacancy Generation and In Situ Surface Heterojunction Reconstruction for Boosting CO2 Reduction. Molecules, 28(10), 4057. https://doi.org/10.3390/molecules28104057



