The Synergistic Effect of Triazine and Phosphaphenanthrene Units on the Physico-Chemical Behavior of Polyimides
Abstract
:1. Introduction
2. Results and Discussions
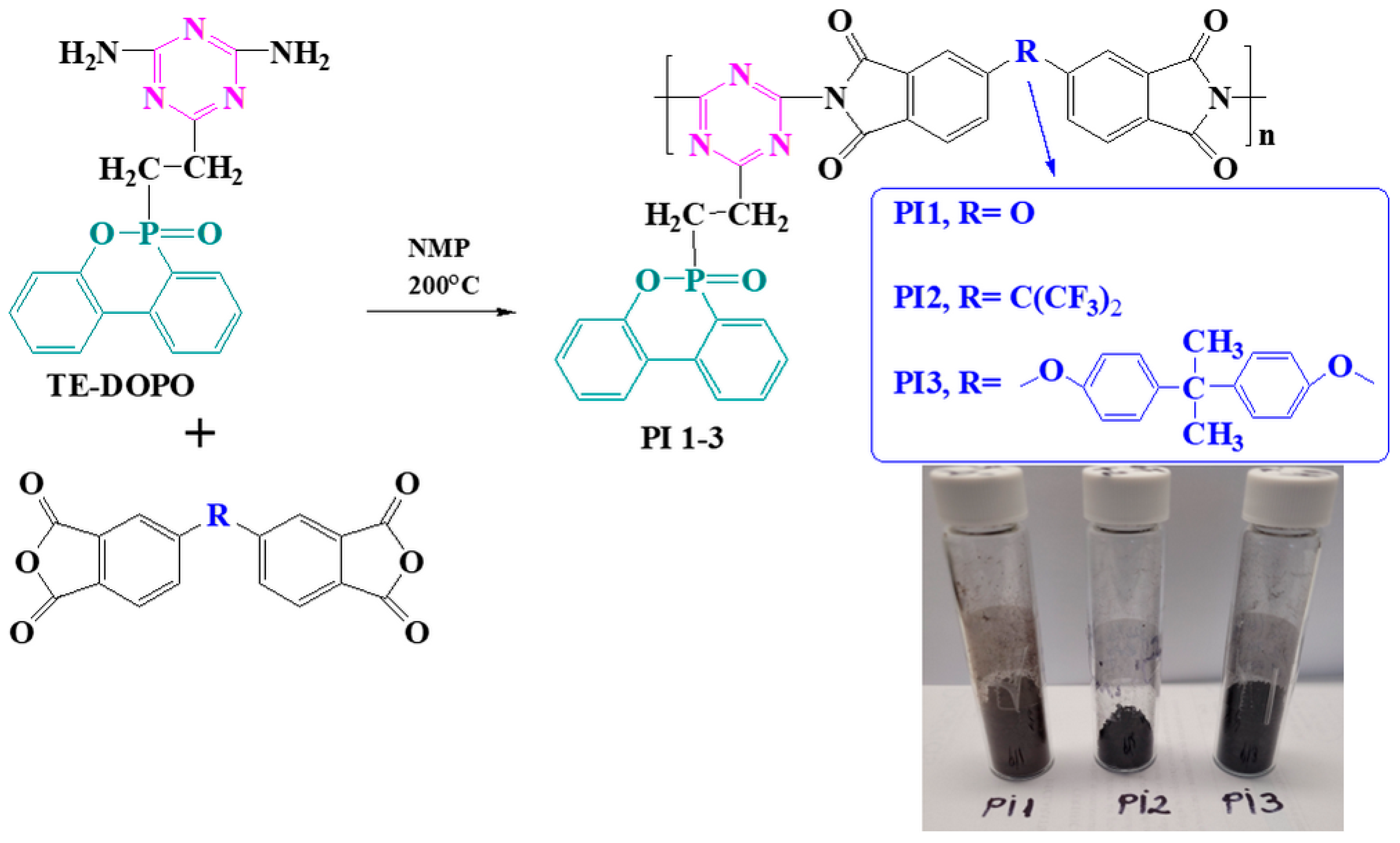
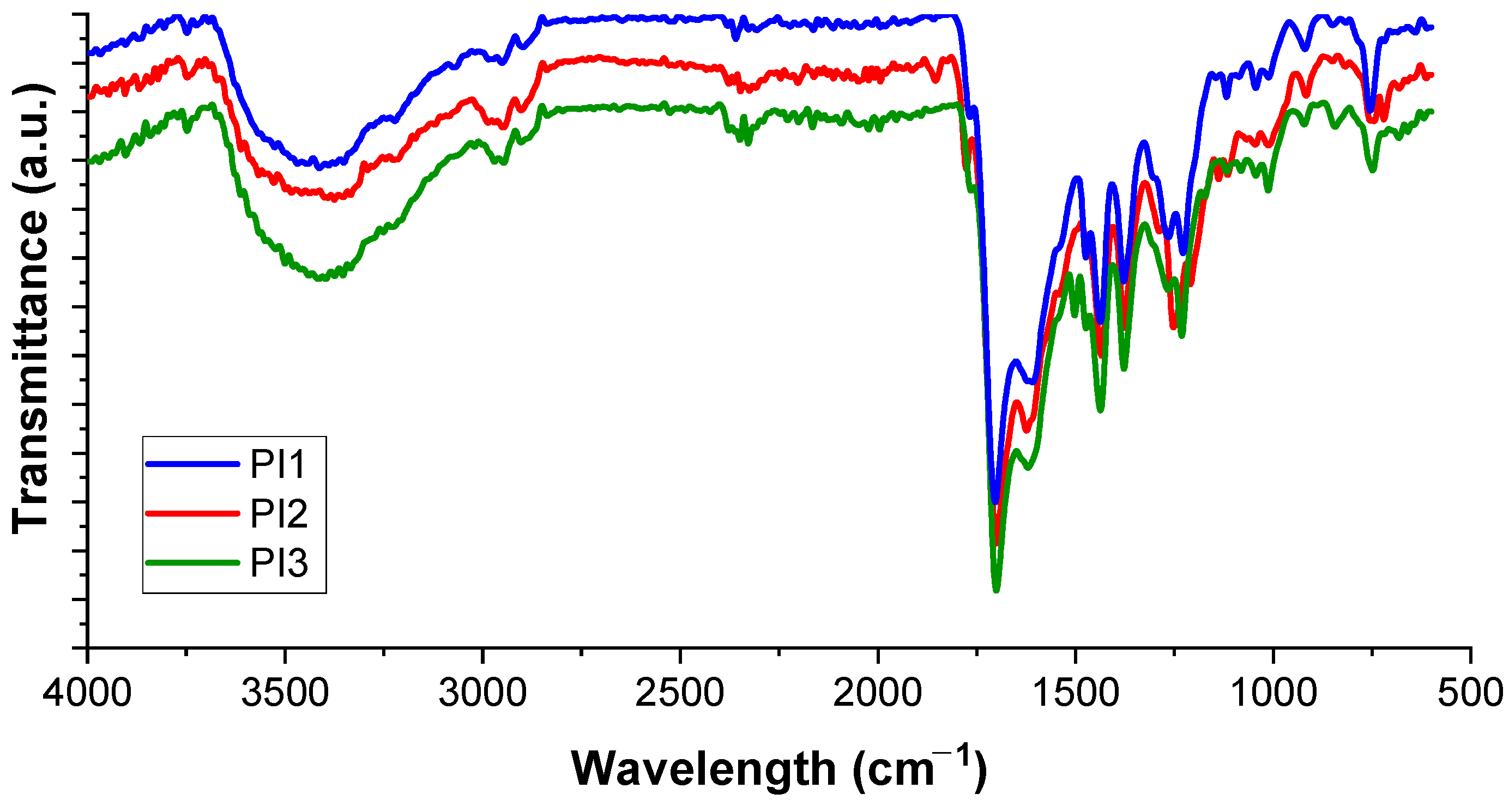
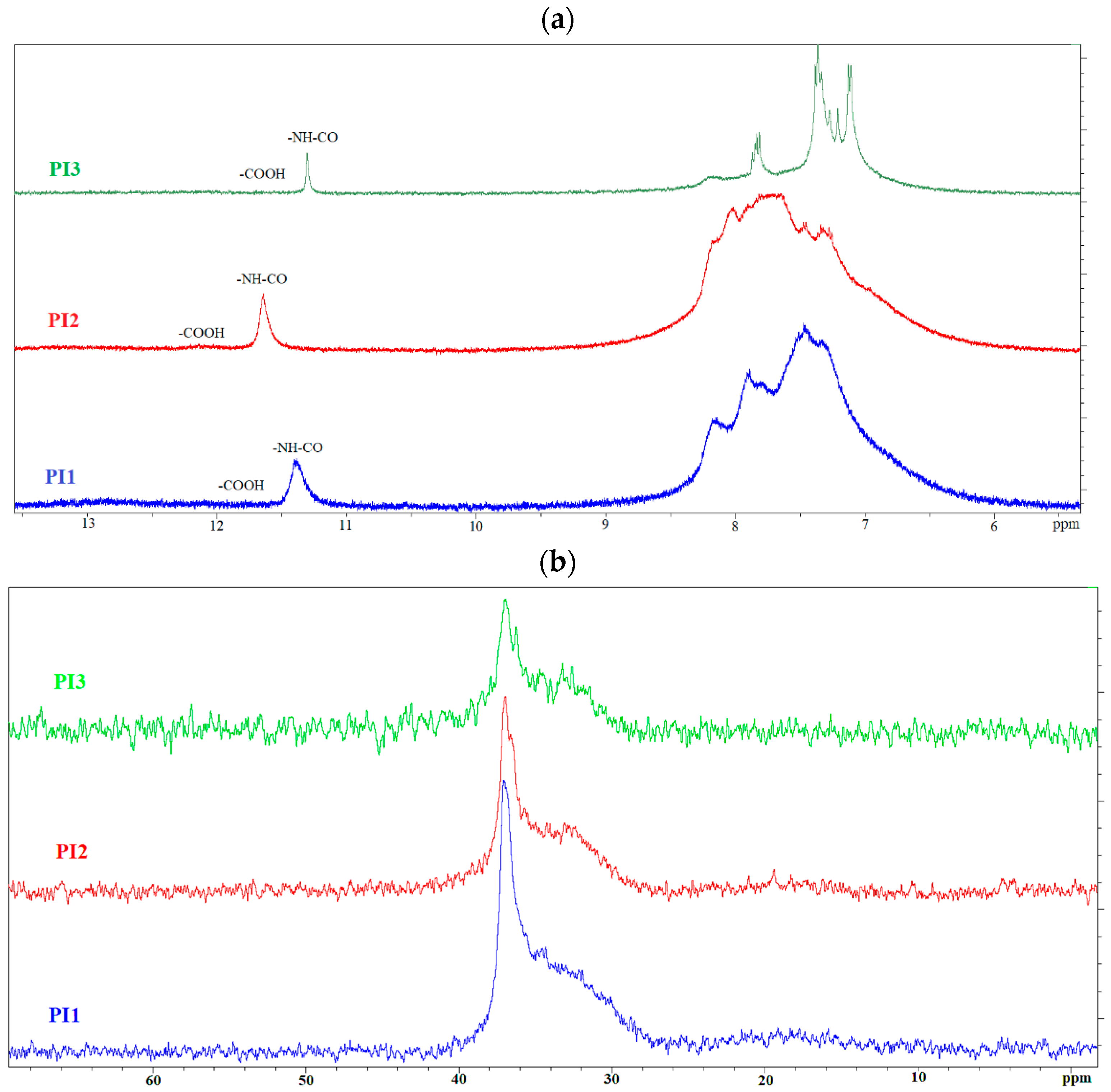
2.1. Structural Characterization
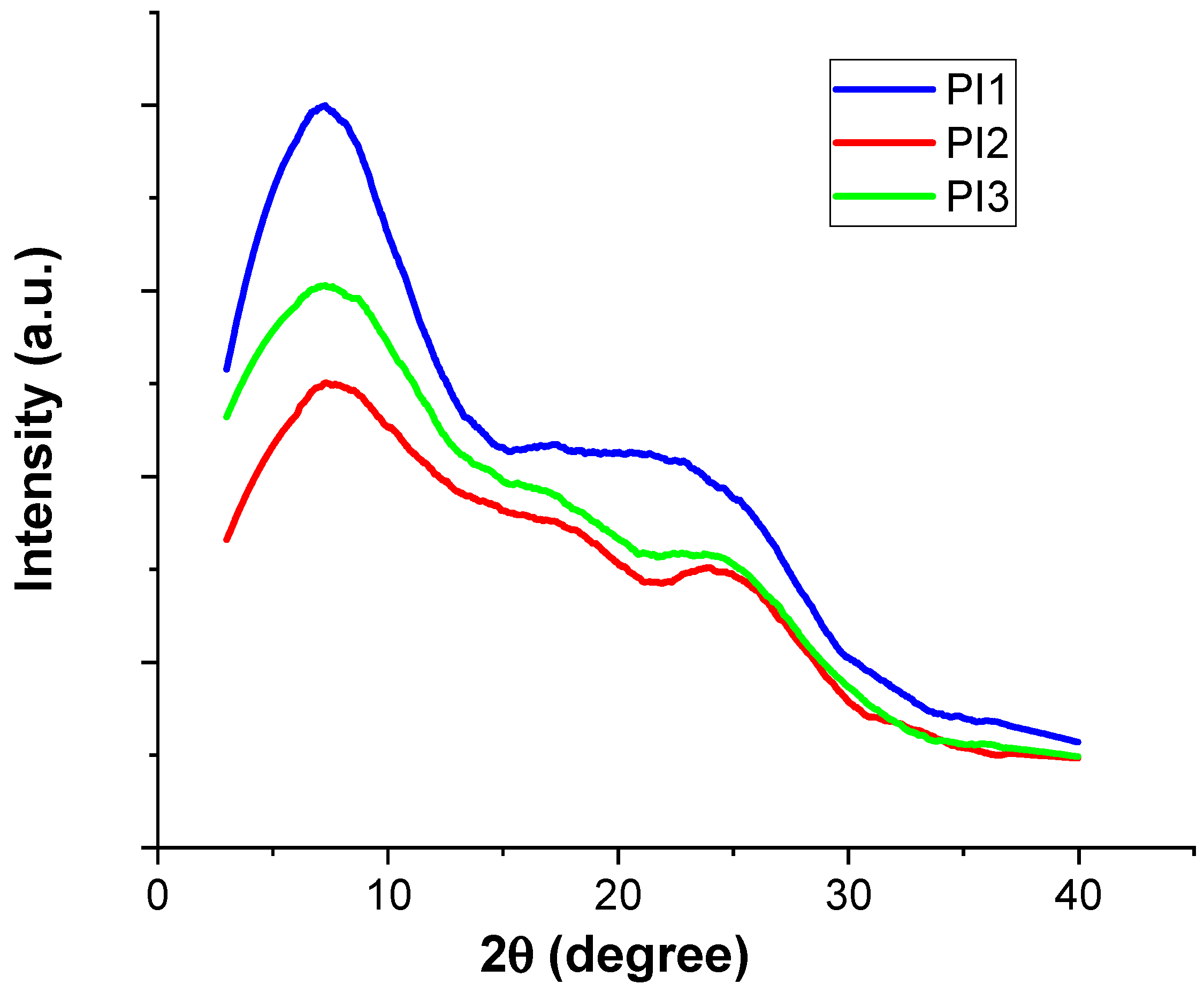
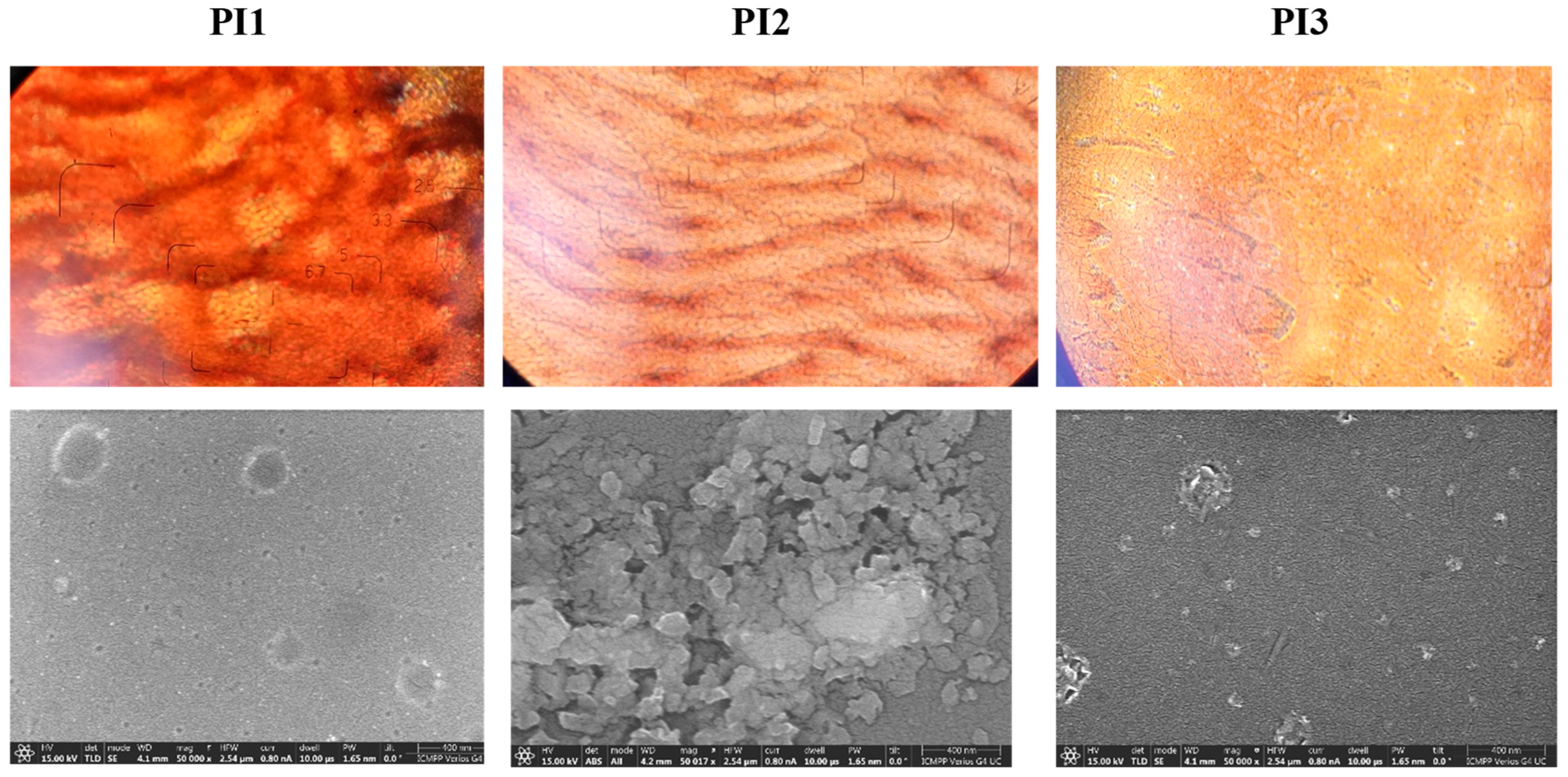
2.2. XRD and SEM Studies
2.3. Thermal Properties
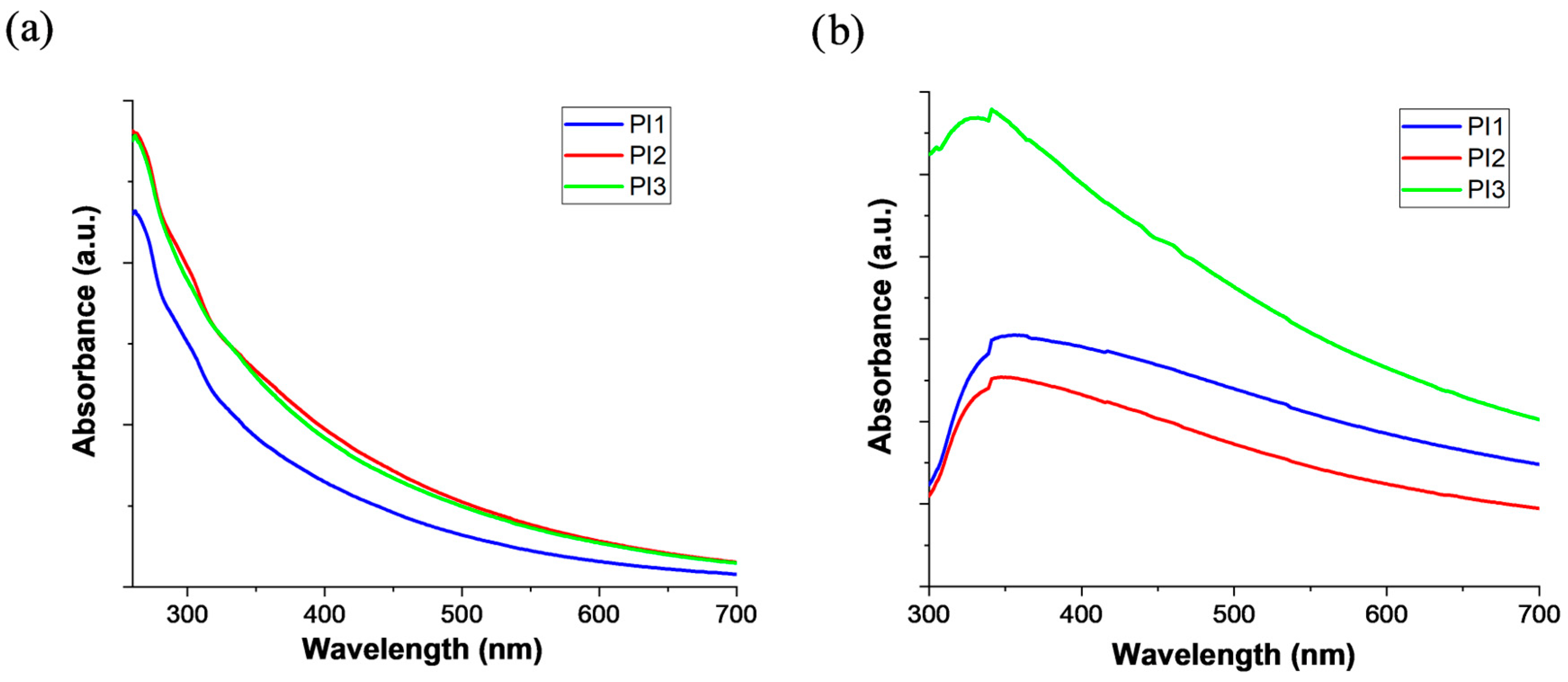
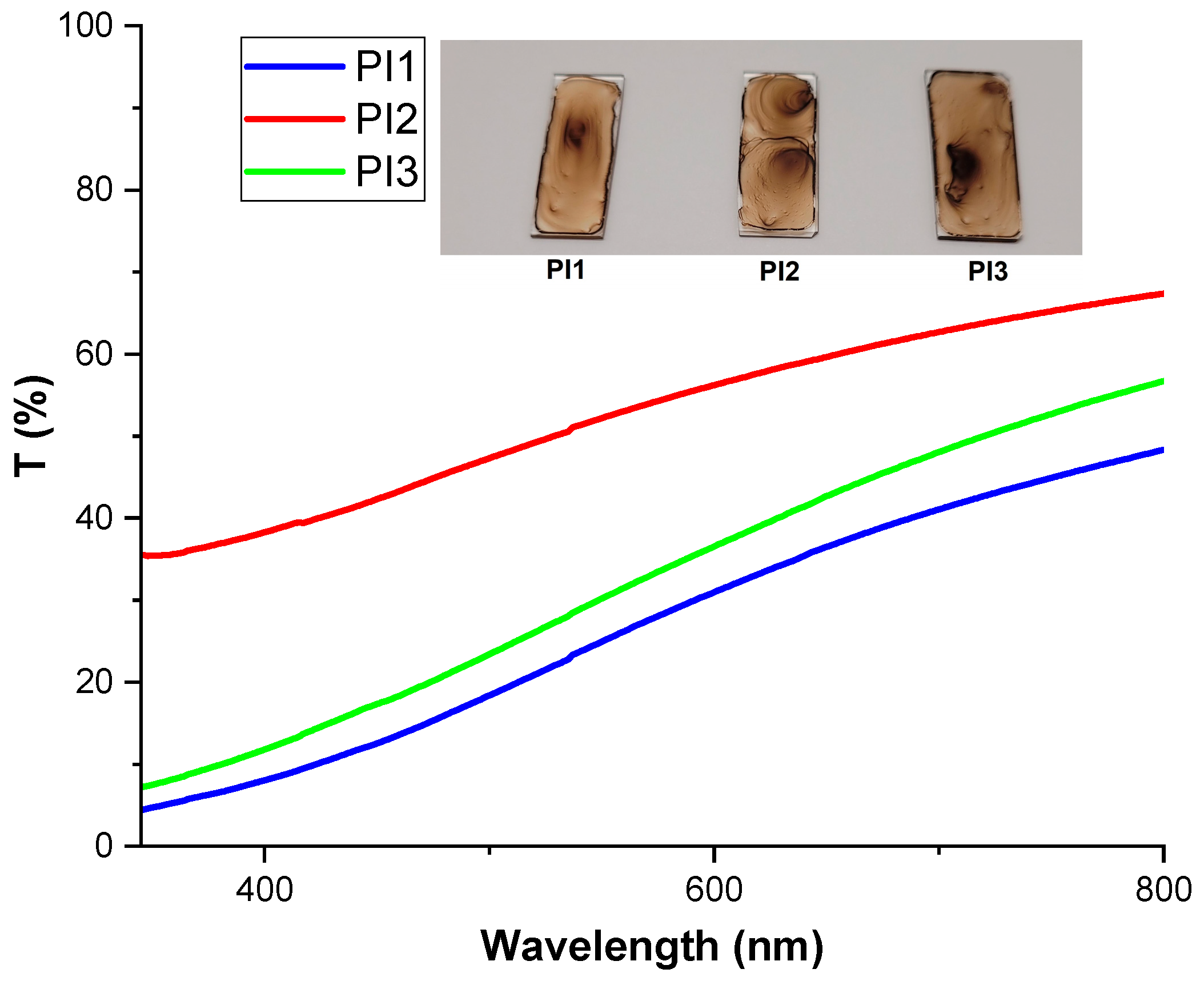
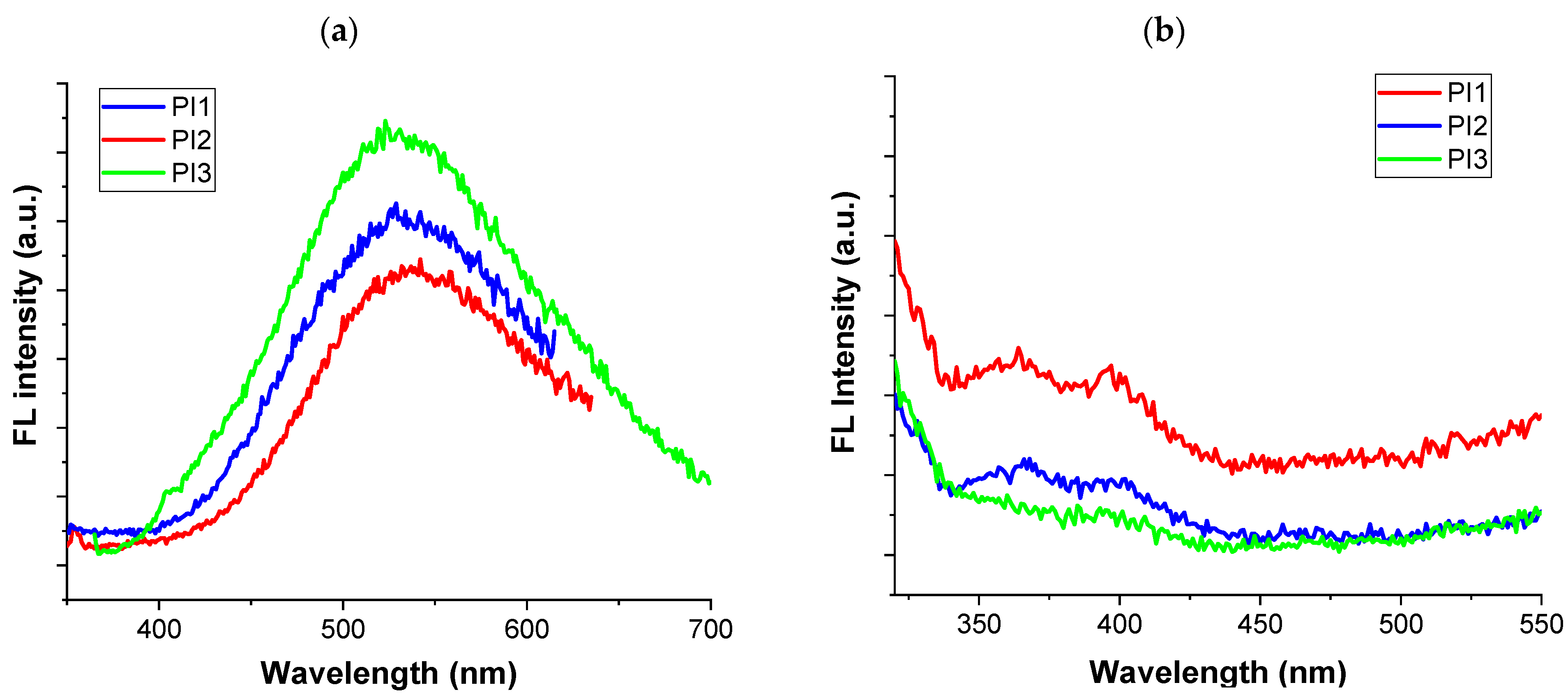
2.4. Photo-Optical Characteristics
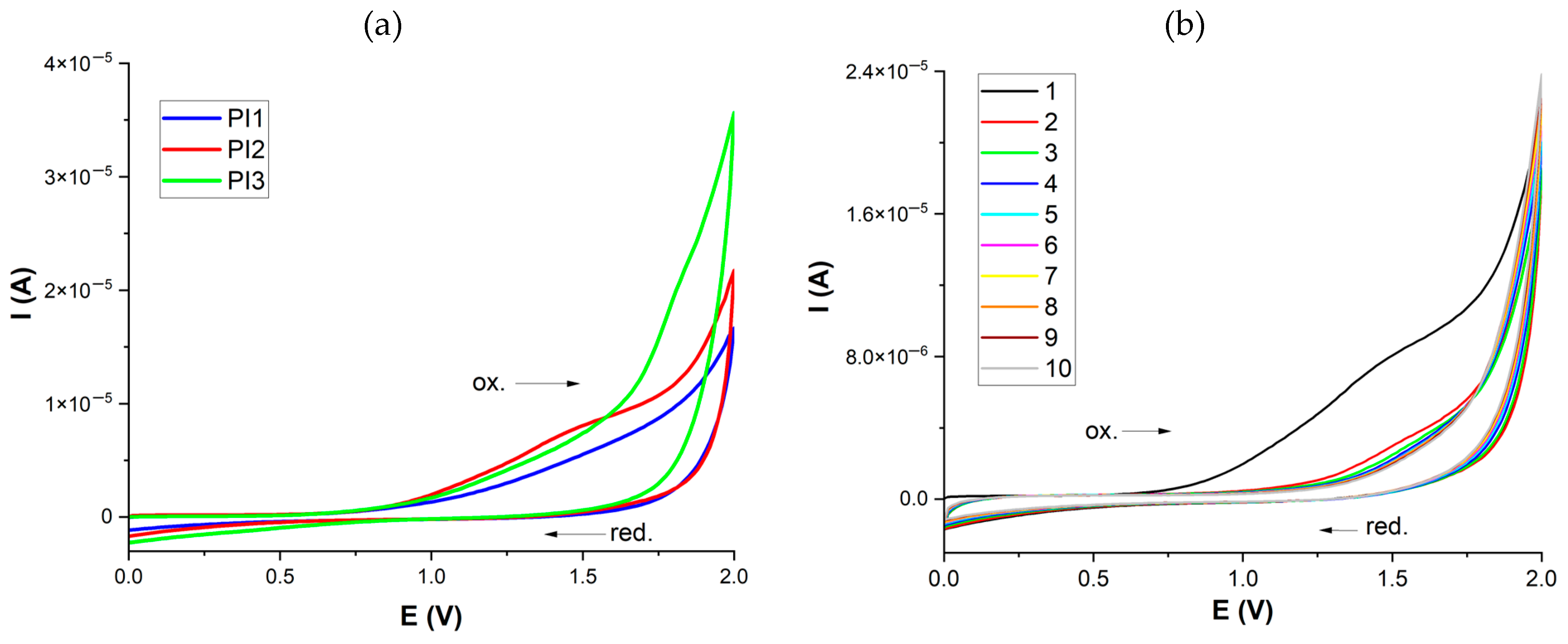
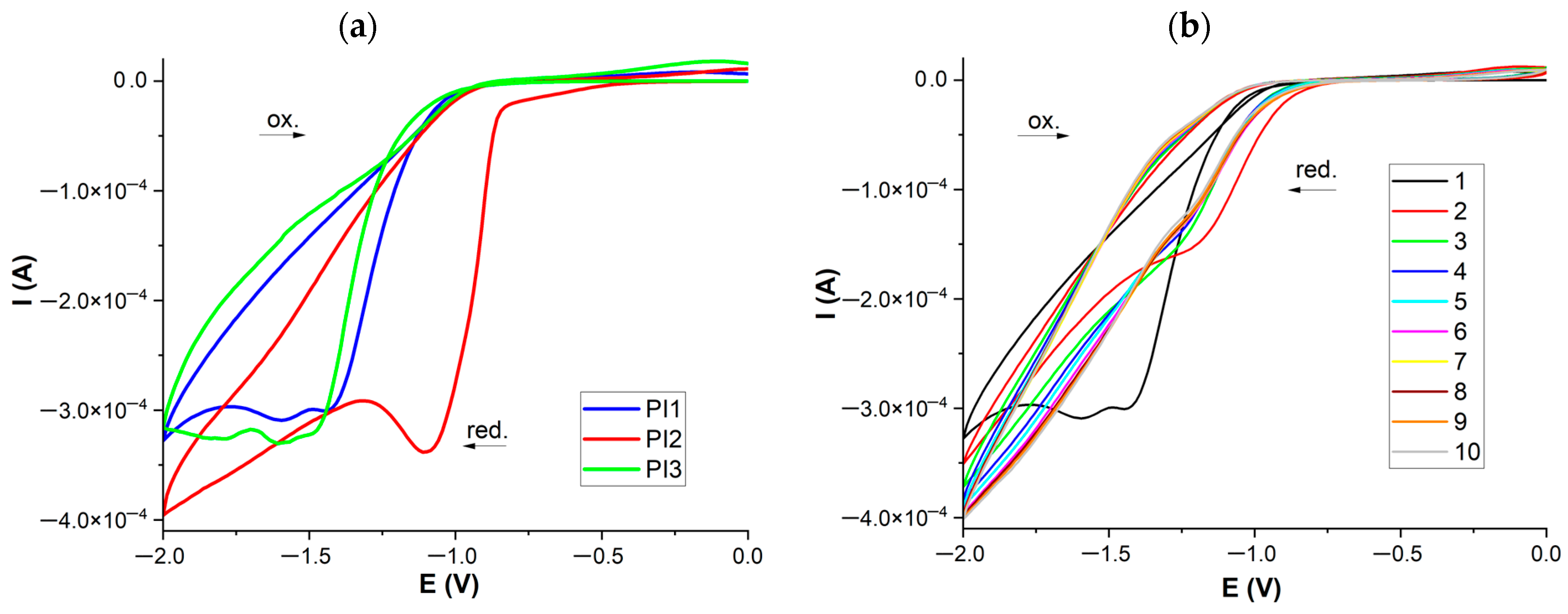
2.5. Cyclic Voltammetry (CV) Measurements
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Film Preparation
3.4. Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Ni, H.J.; Liu, J.G.; Wang, Z.H.; Yang, S.Y. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Ding, Y.; Hou, H.; Zhao, Y.; Zhu, Z.; Fong, H. Electrospun polyimide nanofibers and their applications. Prog. Polym. Sci. 2016, 61, 67–103. [Google Scholar] [CrossRef]
- Yi, L.; Huang, W.; Yan, D. Polyimides with Side Groups: Synthesis and effects of side groups on their properties. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 533–559. [Google Scholar] [CrossRef]
- Zhuang, Y.; Seong, J.G.; Lee, Y.M. Polyimides containing aliphatic/alicyclic segments in the main chains. Progr. Polym. Sci. 2019, 92, 35–88. [Google Scholar] [CrossRef]
- Xu, Z.; Croft, Z.L.; Guo, D.; Cao, K.; Liu, G. Recent development of polyimides: Synthesis, processing, and application in gas separation. J. Polym. Sci. 2021, 59, 943–962. [Google Scholar] [CrossRef]
- Wu, Z.; He, J.; Yang, H.; Yang, S. Progress in aromatic polyimide films for electronic applications: Preparation, structure and properties. Polymers 2022, 14, 1269. [Google Scholar] [CrossRef]
- Yi, C.; Li, W.; Shi, S.; He, K.; Ma, P.; Chen, M.; Yang, C. High-temperature-resistant and colorless polyimide: Preparations, properties, and applications. Solar Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- Fu, M.C.; Higashihara, T.; Ueda, M. Recent progress in thermally stable and photosensitive polymers. Polym. J. 2017, 50, 57–76. [Google Scholar] [CrossRef]
- Damaceanu, M.D.; Constantin, C.P.; Bejan, A.E.; Mihaila, M.; Kusko, M.; Diaconu, C.; Mihalache, I.; Pascu, R. Heteroatom-mediated performance of dye-sensitized solar cells based on T-shaped molecules. Dyes Pigm. 2019, 166, 15–31. [Google Scholar] [CrossRef]
- Constantin, C.P.; Lisa, G.; Damaceanu, M.D. Assessing the electrical characteristics of p-n heterojunction prototype diodes realized with n-type polyimide materials. Macromolecules 2021, 54, 941–957. [Google Scholar] [CrossRef]
- Butnaru, I.; Constantin, C.P.; Damaceanu, M.D. Optimization of triphenylamine-based polyimide structure towards molecular sensors for selective detection of heavy/transition metal ions. J. Photochem. Photobiol. A-Chem. 2023, 435, 114271. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, S.; Liu, X.; Wang, Z.; Ma, L.; Hou, K.; Yang, Z.; Wang, J. Polyimide-based lubricating coatings synergistically enhanced by MoS2@HCNF hybrid. Compos. Part A Appl. Sci. Manuf. 2017, 102, 9–17. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, X.; Li, J. Graphene lubrication. Appl. Mater. Today 2020, 20, 100662. [Google Scholar] [CrossRef]
- Bruma, M.; Damaceanu, M.D.; Muller, P. Comparative study of polyimides containing oxadiazole and ether groups. High Perform. Polym. 2009, 21, 522–534. [Google Scholar] [CrossRef]
- Butnaru, I.; Varganici, C.-D.; Pinteala, M.; Lehner, S.; Bruma, M.; Gaan, S. Thermal decomposition of polyimides containing phosphine-oxide units. J. Anal. Appl. Pyrolys. 2018, 134, 254–264. [Google Scholar] [CrossRef]
- Butnaru, I.; Sava, I.; Damaceanu, M.-D. Exploring the impact of triphenylmethane incorporation on physical properties of polyimides with emphasis on optical and halochromic behaviour. Polymer 2020, 200, 122621. [Google Scholar] [CrossRef]
- Butnaru, I.; Chiriac, A.P.; Asandulesa, M.; Sava, I.; Lisa, G.; Damaceanu, M.D. Tailoring poly(ether-imide) films features towards high performance flexible substrates. J. Ind. Eng. Chem. 2021, 93, 436–447. [Google Scholar] [CrossRef]
- Damaceanu, M.-D.; Constantin, C.-P.; Bruma, M.; Begunov, R.S. The photo-optical and electrochemical activity promoted by trifluoromethyl-substituted and ortho-catenated triphenylamine core in poly(ether-imide)s. Polymer 2018, 151, 34–46. [Google Scholar] [CrossRef]
- Guerrini, M.; Delgado Aznar, E.; Cocchi, C. Electronic and optical properties of protonated triazine derivatives. J. Phys. Chem. C 2020, 124, 27801–27810. [Google Scholar] [CrossRef]
- Prokhorov, A.M.; Kozhevnikov, D.N. Chapter 6.3—Triazines, Tetrazines, and Fused Ring Polyaza Systems, in Progress in Heterocyclic Chemistry; Gribble, G.W., Joule, J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 24, pp. 421–441. [Google Scholar]
- Omer, K.M.; Ku, S.; Chen, Y.; Wong, K.; Bard, A.J. Electrochemical behavior and electrogenerated chemiluminescence of star-shaped D−A compounds with a 1,3,5-triazine core and substituted fluorene arms. J. Am. Chem. Soc. 2010, 132, 10944–10952. [Google Scholar] [CrossRef]
- Zhong, H.; Lai, H.; Fang, Q. New conjugated triazine based molecular materials for application in optoelectronic devices: Design, synthesis, and properties. J. Phys. Chem. C 2011, 115, 2423–2427. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, R.; Zhu, X.; Li, Y.; Chang, J.; Zhu, H.; Ma, L.; Lv, W.; Guo, J. Synthesis and luminescent properties of star-burst D-π-A compounds based on 1,3,5 triazine core and carbazole end-capped phenylene ethynylene arms. J. Lumin. 2014, 156, 130–136. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Moradi, R.; Lashgari, N.; Kruger, H.G. Metal-Free Synthetic Organic Dyes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 197–217. [Google Scholar]
- Li, Z.T.; Zhou, J.Y.; Xu, R.F.; Liu, S.P.; Wang, Y.K.; Li, P.; Wu, W.T.; Wu, M.B. Synthesis of three dimensional extended conjugated polyimide and application as sodium-ion battery anode. Chem. Eng. J. 2016, 287, 516–522. [Google Scholar] [CrossRef]
- Mokhtari, N.; Dinari, M.; Rahmanian, O. Novel porous organic triazine-based polyimide with high nitrogen levels for highly efficient removal of Ni(II) from aqueous solution. Polym. Int. 2019, 68, 1178–1185. [Google Scholar] [CrossRef]
- Bugel, S.; Hoang, Q.D.; Spiess, A.; Sun, Y.Y.; Xing, S.H.; Janiak, C. Biphenyl-based covalent triazine framework/Matrimid(R) mixed-matrix membranes for CO2/CH4 separation. Membranes 2021, 11, 795. [Google Scholar] [CrossRef]
- Zhao, G.F.; Li, H.N.; Gao, Z.H.; Xu, L.F.; Mei, Z.Y.; Cai, S.; Liu, T.T.; Yang, X.F.; Guo, H.; Sun, X.L. Dual-active-center of polyimide and triazine modified atomic-layer covalent organic frameworks for high-performance Li storage. Adv. Funct. Mater. 2021, 31, 2101019. [Google Scholar] [CrossRef]
- Dai, H.C.; Zou, J.C.; Gao, Y.B.; Li, Z.Y.; Zhang, C.Y.; Zhang, G.Q.; Wang, X.B.; Wang, C.L. A novel conjugated porous polymer based on triazine and imide as cathodes for sodium storage. J. Polym. Sci. 2022, 60, 992–1001. [Google Scholar] [CrossRef]
- Chang, C.W.; Lin, C.H.; Cheng, P.W.; Hwang, H.J.; Dai, S.A. Facile and efficient preparation of phosphinate-functionalized aromatic diamines and their high-Tg polyimides. J. Polym. Sci. Part. A Polym. Chem. 2009, 47, 2486–2499. [Google Scholar] [CrossRef]
- Jian, S.J.; Ding, C.H.; Yang, T.; Zhang, C.W.; Hou, H.Q. Effect of trace diphenyl phosphate on mechanical and thermal performance of polyimide composite films. Compos. Com. 2018, 7, 42–46. [Google Scholar] [CrossRef]
- Wang, D.; Yu, J.J.; Duan, G.G.; Liu, K.M.; Hou, H. Electrospun polyimide nonwovens with enhanced mechanical and thermal properties by addition of trace plasticizer. J. Mater. Sci. 2020, 55, 5667–5679. [Google Scholar] [CrossRef]
- Wu, L.; Wu, X.; Qi, H.R.; An, Y.C.; Jia, Y.J.; Zhang, Y.; Zhi, X.X.; Liu, J.G. Colorless and transparent semi-alicyclic polyimide films with intrinsic flame retardancy based on alicyclic dianhydrides and aromatic phosphorous-containing diamine: Preparation and properties. Polym. Adv. Technol. 2021, 32, 1061–1074. [Google Scholar] [CrossRef]
- Hamciuc, C.; Hamciuc, E.; Serbezeanu, D.; Vlad-Bubulac, T.; Cazacu, M. Phosphorus-containing poly(ester-imide)-polydimethylsiloxane copolymers. Polym. Int. 2011, 60, 312–321. [Google Scholar] [CrossRef]
- Agrawal, S.; Narula, A.K. Facile synthesis of new thermally stable and organo-soluble polyamide-imides from phosphorus-containing aromatic amines and various dianhydrides. J. Polym. Eng. 2013, 33, 509–520. [Google Scholar] [CrossRef]
- Cakir, M.; Karatas, S.; Menceloglu, Y.; Kayaman-Apohan, N.; Gungor, A. Phosphorus-containing sulfonated polyimides for proton exchange membranes. Macromol. Chem. Phys. 2008, 209, 919–929. [Google Scholar] [CrossRef]
- Ghorai, A.; Banerjee, S. Phosphorus-containing aromatic polymers: Synthesis, structure, properties and membrane-based applications. Progr. Polym. Sci. 2023, 138, 101646. [Google Scholar] [CrossRef]
- Song, G.H.; Li, X.S.; Jiang, Q.Y.; Mu, J.X.; Jiang, Z.H. A novel structural polyimide material with synergistic phosphorus and POSS for atomic oxygen resistance. RSC Adv. 2015, 5, 11980–11988. [Google Scholar] [CrossRef]
- Wu, B.H.; Zhang, Y.; Yang, D.Y.; Yang, Y.B.; Yu, Q.; Che, L.; Liu, J.G. Self-healing anti-atomic-oxygen phosphorus-containing polyimide film via molecular level incorporation of nanocage trisilanolphenyl POSS: Preparation and characterization. Polymers 2019, 11, 1013. [Google Scholar] [CrossRef]
- Guan, Y.; Sun, Y.; Shang, D.J. Amine-functionalized POSS cross-linked the poly(imide siloxane) block copolymers nanocomposites: Preparation, thermal properties, against atomic oxygen erosion. J. Macromol. Sci. Part A Pure Appl. Chem. 2022, 59, 241–248. [Google Scholar] [CrossRef]
- Butnaru, I.; Fernandez-Ronco, M.; Czech-Polak, J.; Heneczkowski, M.; Bruma, M.; Gaan, S. Effect of meltable triazine-DOPO additive on rheological, mechanical, and flammability properties of PA6. Polymers 2015, 7, 1541–1563. [Google Scholar] [CrossRef]
- Chatterjee, R.; Bisoi, S.; Kumar, A.G.; Padmanabhan, V.; Banerjee, S. Polyimides containing phosphaphenanthrene skeleton: Gas-transport properties and molecular dynamics simulations. ACS Omega 2018, 3, 13510–13523. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, S.L.; Cheng, P.W. Dietheramine from an alkaline-stable phosphinated bisphenol for soluble polyetherimides. Polymer 2011, 52, 1249–1255. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Hsu, C.-Y.; Wu, C.-S. Polyimides possessing bulky phosphorus groups: Synthesis and characterization. J. Appl. Polym. Sci. 2003, 89, 791–796. [Google Scholar] [CrossRef]
- Homocianu, M.; Serbezeanu, D.; Lisa, G.; Brebu, M.; Vlad-Bubulac, T. Optical and flame-retardant properties of a series of polyimides containing side chained bulky phosphaphenanthrene units. Int. J. Mol. Sci. 2022, 23, 13174. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Yang, Q.; Zhu, R.; Gu, Y. The study on imidization degree of polyamic acid in solution and ordering degree of its polyimide film. J. Mater. Sci. 2007, 43, 338–344. [Google Scholar] [CrossRef]
- Uchida, S.; Ishige, R.; Takeichi, T.; Ando, S. Promotion of thermal imidization of semi-aliphatic polyimide precursors by incorporation of polyethylene glycol and their modified solid structures. J. Photopolym. Sci. Technol. 2017, 30, 139–146. [Google Scholar] [CrossRef]
- Rahnamoun, A.; Engelhart, D.P.; Humagain, S.; Koerner, H.; Plis, E.; Kennedy, W.J.; Cooper, R.; Greenbaum, S.G.; Hoffmann, R.; van Duin, A.C.T. Chemical dynamics characteristics of Kapton polyimide damaged by electron beam irradiation. Polymer 2019, 176, 135–145. [Google Scholar] [CrossRef]
- Wakita, J.; Jin, S.; Shin, T.J.; Ree, M.; Ando, S. Analysis of molecular aggregation structures of fully aromatic and semialiphatic polyimide films with synchrotron grazing incidence wide-angle X-ray scattering. Macromolecules 2010, 43, 1930–1941. [Google Scholar] [CrossRef]
- Hajibeygi, M.; Habibnejad, N.; Shabanian, M.; Khonakdar, H.A. Fabrication and study of thermal and combustion resistance of DOPO-functionalized polyamide reinforced with organo-modified Mg(OH)2 nanoparticles. Polym. Int. 2021, 70, 317–330. [Google Scholar] [CrossRef]
- Damaceanu, M.-D.; Bacosca, I.; Bruma, M.; Robison, J.; Rusanov, A. Heterocyclic polyimides containing siloxane groups in the main chain. Polym. Int. 2009, 58, 1041–1050. [Google Scholar] [CrossRef]
- Zhan, Y.; Guan, X.; Ren, E.; Lin, S.; Lan, J. Fabrication of zeolitic imidazolate framework-8 functional polyacrylonitrile nanofibrous mats for dye removal. J. Polym. Res. 2019, 26, 171. [Google Scholar] [CrossRef]
- Sun, D.; Si, C.; Wang, T.; Zysman-Colman, E. 1,3,5-Triazine-functionalized thermally activated delayed fluorescence emitters for organic light-emitting diodes. Adv. Photonics Res. 2022, 3, 2200203. [Google Scholar] [CrossRef]
- Li, Z.; Kou, K.; Zhang, J.; Zhang, Y.; Wang, Y.; Pan, C. Solubility, electrochemical behavior and thermal stability of polyimides synthesized from 1,3,5-triazine-based diamine. J. Mater. Sci. Mater. Electron. 2017, 28, 6079–6087. [Google Scholar] [CrossRef]

| Polyimide | Solvent | |||||||
|---|---|---|---|---|---|---|---|---|
| NMP | DMAc | DMSO | DMF | EtOH | CHCl3 | DCM | Acetone | |
| PI1 | ++ | ++ | ++ | ++ | − | − | − | − |
| PI2 | ++ | ++ | ++ | ++ | − | − | − | − |
| PI3 | ++ | ++ | ++ | ++ | − | − | − | − |
| Polyimide | Mn (g/mol) | Mw (g/mol) | Mw/Mn | T5 (°C) | Tmax (°C) | W800 (%) |
|---|---|---|---|---|---|---|
| PI1 | 151,700 | 354,500 | 2.34 | 336 | 490 | 46.44 |
| PI2 | 301,700 | 693,900 | 2.30 | 352 | 516/691 | 27.18 |
| PI3 | 274,200 | 599,000 | 2.19 | 339 | 499 | 53.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butnaru, I.; Damaceanu, M.-D. The Synergistic Effect of Triazine and Phosphaphenanthrene Units on the Physico-Chemical Behavior of Polyimides. Molecules 2023, 28, 4072. https://doi.org/10.3390/molecules28104072
Butnaru I, Damaceanu M-D. The Synergistic Effect of Triazine and Phosphaphenanthrene Units on the Physico-Chemical Behavior of Polyimides. Molecules. 2023; 28(10):4072. https://doi.org/10.3390/molecules28104072
Chicago/Turabian StyleButnaru, Irina, and Mariana-Dana Damaceanu. 2023. "The Synergistic Effect of Triazine and Phosphaphenanthrene Units on the Physico-Chemical Behavior of Polyimides" Molecules 28, no. 10: 4072. https://doi.org/10.3390/molecules28104072
APA StyleButnaru, I., & Damaceanu, M. -D. (2023). The Synergistic Effect of Triazine and Phosphaphenanthrene Units on the Physico-Chemical Behavior of Polyimides. Molecules, 28(10), 4072. https://doi.org/10.3390/molecules28104072






