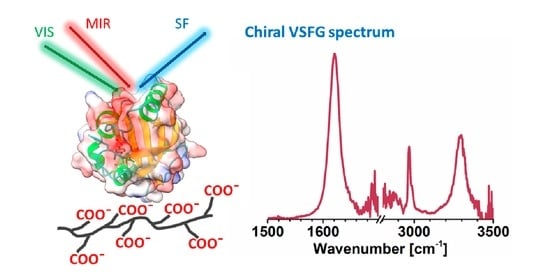
Photoactive Yellow Protein Adsorption at Hydrated Polyethyleneimine and Poly-l-Glutamic Acid Interfaces
Abstract
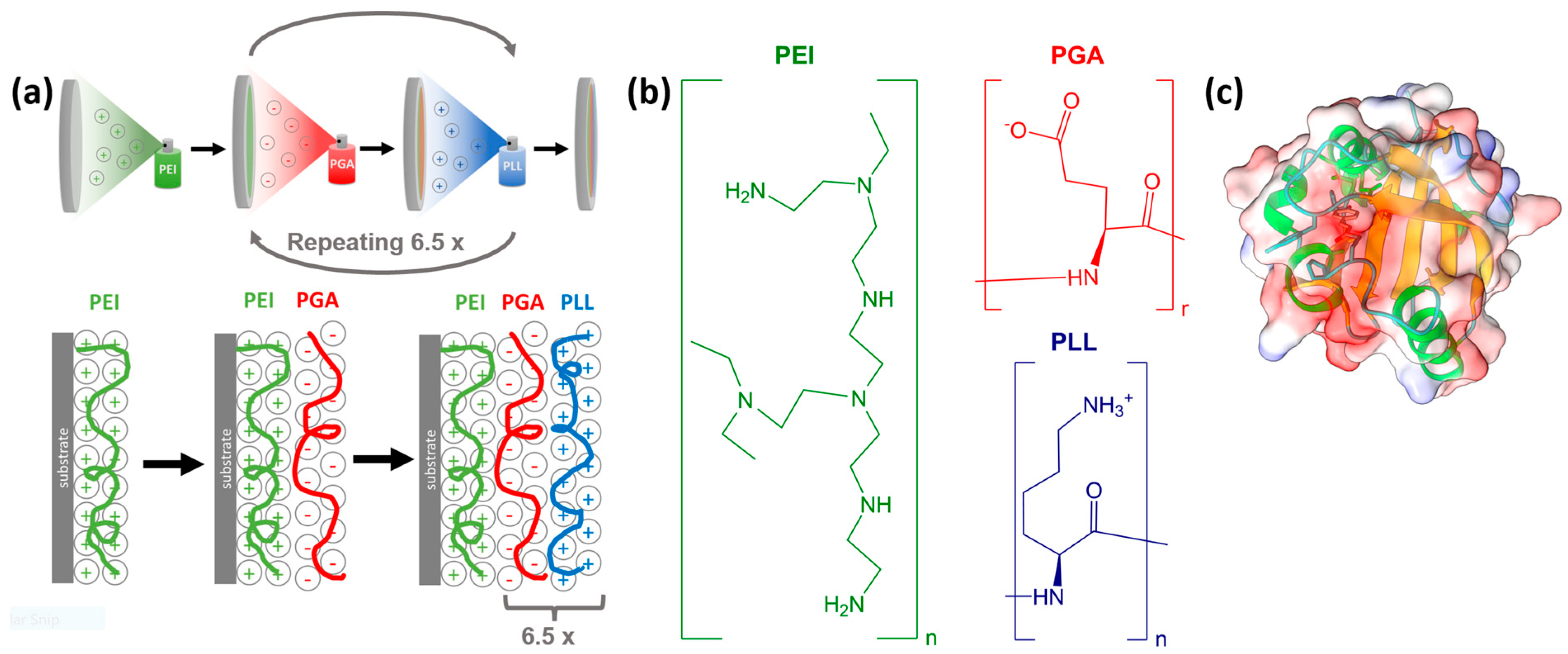
:1. Introduction
2. Results and Discussion
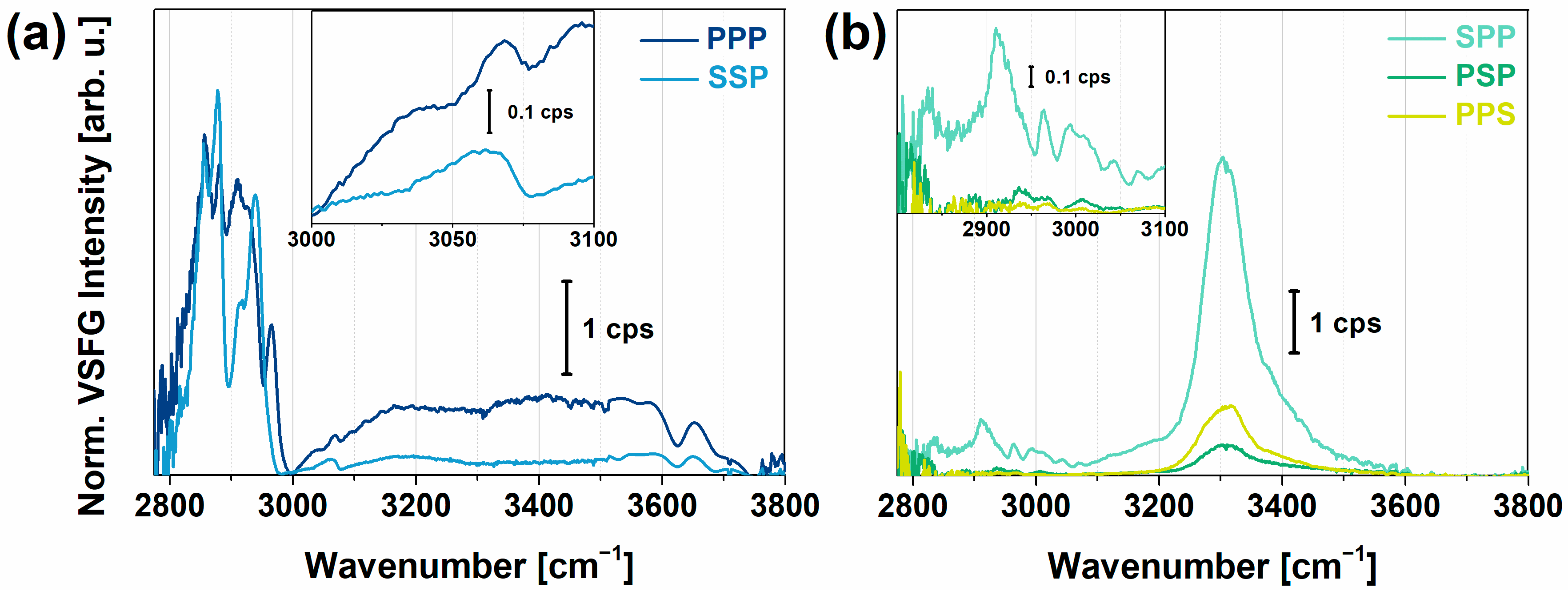
2.1. VSFG Spectra of Hydrated PYP Films
2.2. VSFG Spectra of Hydrated PEI and PEI+PGA at the Air-CaF2 Interface
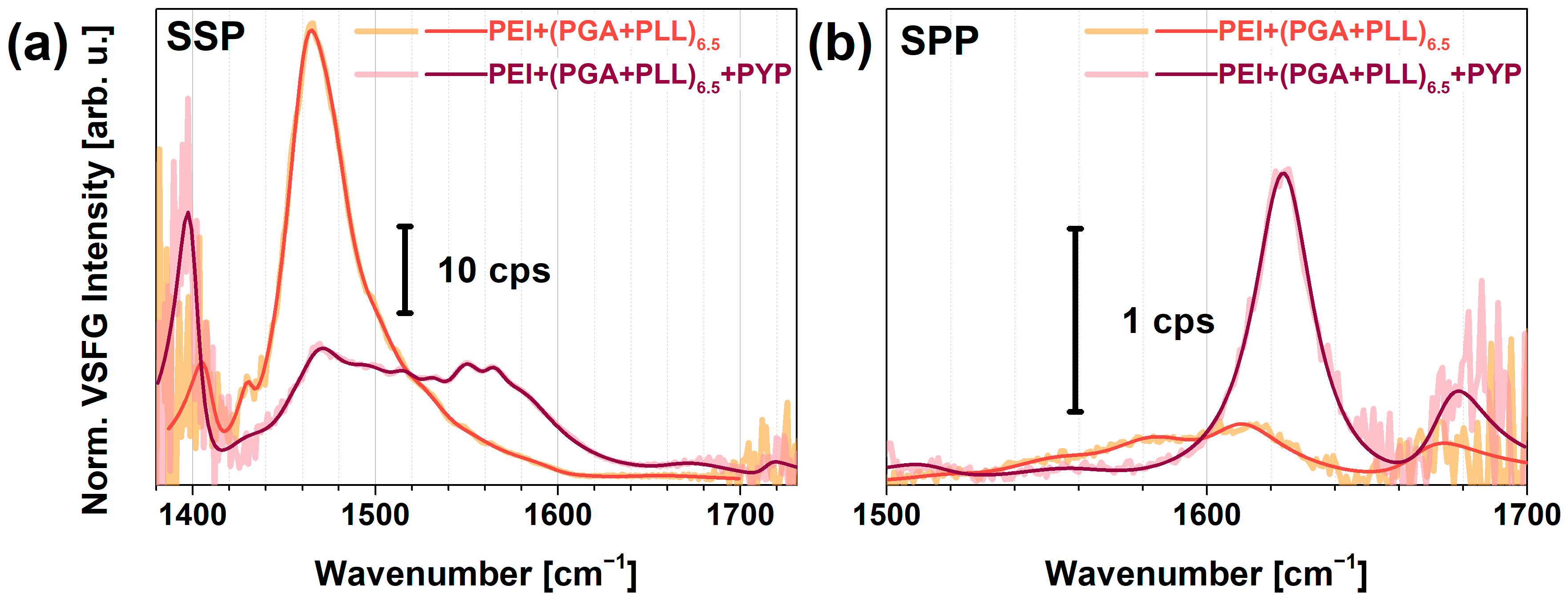
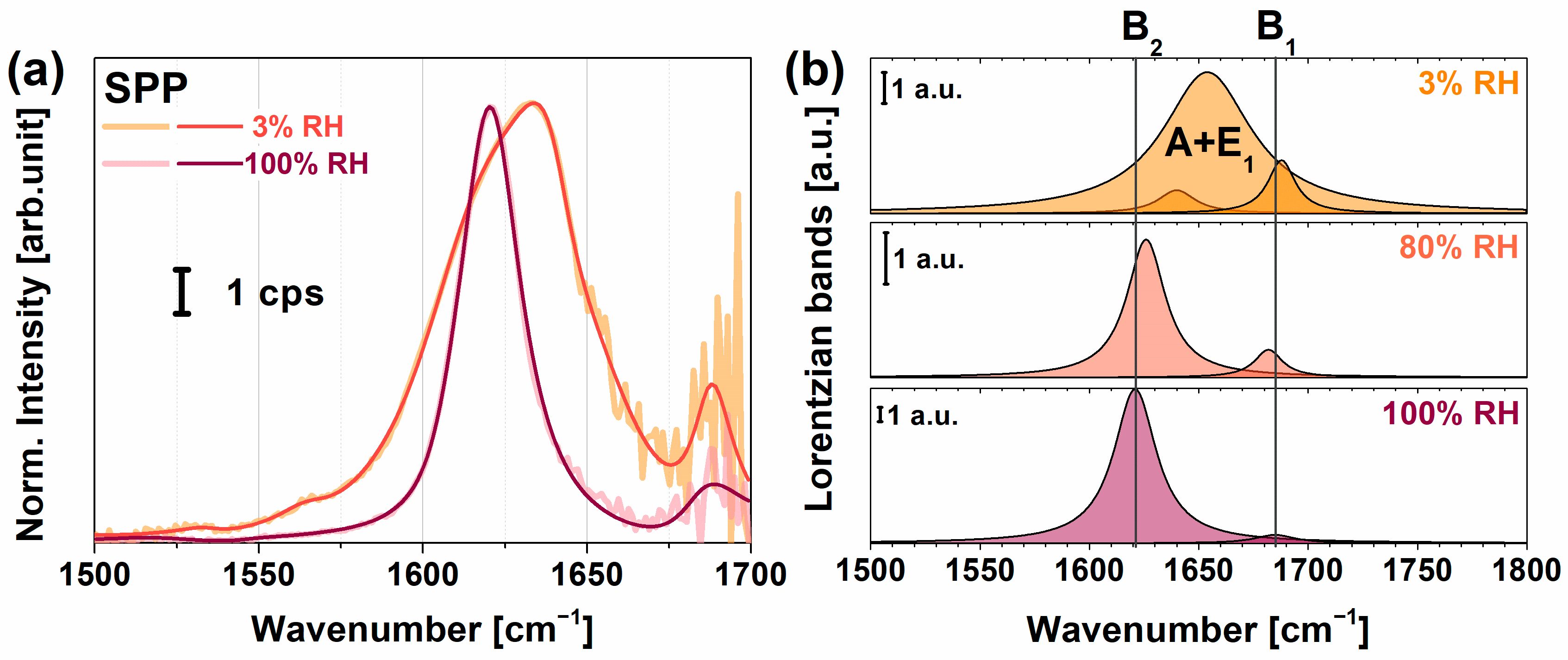
2.3. PYP Adsorption at Air-Polyelectrolyte Interfaces
3. Materials and Methods
3.1. Sample Preparation
3.2. Vibrational Sum-Frequency Generation (VSFG)
3.3. Atomic Force Microscopy (AFM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zimmerman, S.B.; Trach, S.O. Estimation of Macromolecule Concentrations and Excluded Volume Effects for the Cytoplasm of Escherichia Coli. J. Mol. Biol. 1991, 222, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Goodsell, D.S. Inside a Living Cell. Trends Biochem. Sci. 1991, 16, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J. Macromolecular Crowding: Obvious but Underappreciated. Trends Biochem. Sci. 2001, 26, 597–604. [Google Scholar] [CrossRef]
- Dér, A.; Kelemen, L.; Fábián, L.; Taneva, S.G.; Fodor, E.; Páli, T.; Cupane, A.; Cacace, M.G.; Ramsden, J.J. Interfacial Water Structure Controls Protein Conformation. J. Phys. Chem. B 2007, 111, 5344–5350. [Google Scholar] [CrossRef]
- Násztor, Z.; Bogár, F.; Dér, A. The Interfacial Tension Concept, as Revealed by Fluctuations. Curr. Opin. Colloid Interface Sci. 2016, 23, 29–40. [Google Scholar] [CrossRef]
- Bogár, F.; Bartha, F.; Násztor, Z.; Fábián, L.; Leitgeb, B.; Dér, A. On the Hofmeister Effect: Fluctuations at the Protein–Water Interface and the Surface Tension. J. Phys. Chem. B 2014, 118, 8496–8504. [Google Scholar] [CrossRef]
- Kincses, A.; Santa-Maria, A.R.; Walter, F.R.; Dér, L.; Horányi, N.; Lipka, D.V.; Valkai, S.; Deli, M.A.; Dér, A. A Chip Device to Determine Surface Charge Properties of Confluent Cell Monolayers by Measuring Streaming Potential. Lab Chip 2020, 20, 3792–3805. [Google Scholar] [CrossRef] [PubMed]
- Laaser, J.E.; Skoff, D.R.; Ho, J.-J.; Joo, Y.; Serrano, A.L.; Steinkruger, J.D.; Gopalan, P.; Gellman, S.H.; Zanni, M.T. Two-Dimensional Sum-Frequency Generation Reveals Structure and Dynamics of a Surface-Bound Peptide. J. Am. Chem. Soc. 2014, 136, 956–962. [Google Scholar] [CrossRef]
- Minton, A.P. The Influence of Macromolecular Crowding and Macromolecular Confinement on Biochemical Reactions in Physiological Media. J. Biol. Chem. 2001, 276, 10577–10580. [Google Scholar] [CrossRef]
- Ladam, G.; Gergely, C.; Senger, B.; Decher, G.; Voegel, J.-C.; Schaaf, P.; Cuisinier, F.J.G. Protein Interactions with Polyelectrolyte Multilayers: Interactions between Human Serum Albumin and Polystyrene Sulfonate/Polyallylamine Multilayers. Biomacromolecules 2000, 1, 674–687. [Google Scholar] [CrossRef]
- Mezzenga, R.; Fischer, P. The Self-Assembly, Aggregation and Phase Transitions of Food Protein Systems in One, Two and Three Dimensions. Reports Prog. Phys. 2013, 76, 046601. [Google Scholar] [CrossRef] [PubMed]
- Santa-Maria, A.R.; Walter, F.R.; Valkai, S.; Brás, A.R.; Mészáros, M.; Kincses, A.; Klepe, A.; Gaspar, D.; Castanho, M.A.R.B.; Zimányi, L.; et al. Lidocaine Turns the Surface Charge of Biological Membranes More Positive and Changes the Permeability of Blood-Brain Barrier Culture Models. Biochim. Biophys. Acta - Biomembr. 2019, 1861, 1579–1591. [Google Scholar] [CrossRef]
- Kovacs, B.; Saftics, A.; Biro, A.; Kurunczi, S.; Szalontai, B.; Kakasi, B.; Vonderviszt, F.; Der, A.; Horvath, R. Kinetics and Structure of Self-Assembled Flagellin Monolayers on Hydrophobic Surfaces in the Presence of Hofmeister Salts: Experimental Measurement of the Protein Interfacial Tension at the Nanometer Scale. J. Phys. Chem. C 2018, 122, 21375–21386. [Google Scholar] [CrossRef]
- Brash, J.L.; Horbett, T.A. Proteins at Interfaces; American Chemical Society: Washington, DC, USA, 1995; pp. 1–23. ISBN 0841233047. [Google Scholar]
- Lyklema, J.; Norde, W. Interfacial Behaviour of Biomacromolecules. In Interfaces, Surfactants and Colloids in Engineering; Jacobasch, H.-J., Ed.; Steinkopff: Darmstadt, Germany, 1996; Volume 101, pp. 9–17. ISBN 978-3-7985-1664-9. [Google Scholar]
- Stieger, K.R.; Ciornii, D.; Kölsch, A.; Hejazi, M.; Lokstein, H.; Feifel, S.C.; Zouni, A.; Lisdat, F. Engineering of Supramolecular Photoactive Protein Architectures: The Defined Co-Assembly of Photosystem i and Cytochrome: C Using a Nanoscaled DNA-Matrix. Nanoscale 2016, 8, 10695–10705. [Google Scholar] [CrossRef]
- Fábián, L.; Heiner, Z.; Mero, M.; Kiss, M.; Wolff, E.K.; Ormos, P.; Osvay, K.; Dér, A. Protein-Based Ultrafast Photonic Switching. Opt. Express 2011, 19, 18861. [Google Scholar] [CrossRef] [PubMed]
- Petrovszki, D.; Krekic, S.; Valkai, S.; Heiner, Z.; Dér, A. All-Optical Switching Demonstrated with Photoactive Yellow Protein Films. Biosensors 2021, 11, 432. [Google Scholar] [CrossRef]
- Krekic, S.; Mero, M.; Dér, A.; Heiner, Z. Ultrafast All-Optical Switching Using Doped Chromoprotein Films. J. Phys. Chem. C 2023, 127, 1499–1506. [Google Scholar] [CrossRef]
- Dhamelincourt, P.; Ramirez, F.J. Polarized Micro-Raman and FT-IR Spectra of L-Glutamine. Appl. Spectrosc. 1993, 47, 446–451. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, W.; Lin, K.; Hu, N.; Yu, Y.; Zhou, X.; Yuan, L.-F.; Hu, S.-M.; Luo, Y. Identification of Alcohol Conformers by Raman Spectra in the C–H Stretching Region. J. Phys. Chem. A 2015, 119, 3209–3217. [Google Scholar] [CrossRef]
- Szalontai, B.; Nagy, G.; Krumova, S.; Fodor, E.; Páli, T.; Taneva, S.G.; Garab, G.; Peters, J.; Dér, A. Hofmeister Ions Control Protein Dynamics. Biochim. Biophys. Acta - Gen. Subj. 2013, 1830, 4564–4572. [Google Scholar] [CrossRef]
- Zsiros, O.; Ünnep, R.; Nagy, G.; Almásy, L.; Patai, R.; Székely, N.K.; Kohlbrecher, J.; Garab, G.; Dér, A.; Kovács, L. Role of Protein-Water Interface in the Stacking Interactions of Granum Thylakoid Membranes—As Revealed by the Effects of Hofmeister Salts. Front. Plant Sci. 2020, 11, 1257. [Google Scholar] [CrossRef] [PubMed]
- Petrovszki, D.; Walter, F.R.; Vigh, J.P.; Kocsis, A.; Valkai, S.; Deli, M.A.; Dér, A. Penetration of the SARS-CoV-2 Spike Protein across the Blood–Brain Barrier, as Revealed by a Combination of a Human Cell Culture Model System and Optical Biosensing. Biomedicines 2022, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, J.J. OWLS: A Versatile Technique for Sensing with Bioarrays. Chimia (Aarau). 1999, 53, 67. [Google Scholar] [CrossRef]
- Horvath, R.; Cottier, K.; Pedersen, H.C.; Ramsden, J.J. Multidepth Screening of Living Cells Using Optical Waveguides. Biosens. Bioelectron. 2008, 24, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, D.; Azuma, T.; Noguchi, H.; Uosaki, K.; Takai, M. Role of Interfacial Water in Protein Adsorption onto Polymer Brushes as Studied by SFG Spectroscopy and QCM. J. Phys. Chem. C 2015, 119, 17193–17201. [Google Scholar] [CrossRef]
- Meister, K.; Paananen, A.; Speet, B.; Lienemann, M.; Bakker, H.J. Molecular Structure of Hydrophobins Studied with Site-Directed Mutagenesis and Vibrational Sum-Frequency Generation Spectroscopy. J. Phys. Chem. B 2017, 121, 9398–9402. [Google Scholar] [CrossRef] [PubMed]
- Strazdaite, S.; Meister, K.; Bakker, H.J. Orientation of Polar Molecules near Charged Protein Interfaces. Phys. Chem. Chem. Phys. 2016, 18, 7414–7418. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Roeters, S.J.; Bonn, M.; Peukert, W.; Woutersen, S.; Weidner, T. Structure and Dynamics of Interfacial Peptides and Proteins from Vibrational Sum-Frequency Generation Spectroscopy. Chem. Rev. 2020, 120, 3420–3465. [Google Scholar] [CrossRef]
- Yesudas, F.; Mero, M.; Kneipp, J.; Heiner, Z. High-Resolution and High-Repetition-Rate Vibrational Sum-Frequency Generation Spectroscopy of One- and Two-Component Phosphatidylcholine Monolayers. Anal. Bioanal. Chem. 2019, 411, 4861–4871. [Google Scholar] [CrossRef]
- Hu, X.-H.; Fu, L.; Hou, J.; Zhang, Y.-N.; Zhang, Z.; Wang, H.-F. N–H Chirality in Folded Peptide LK7β Is Governed by the Cα–H Chirality. J. Phys. Chem. Lett. 2020, 11, 1282–1290. [Google Scholar] [CrossRef]
- Okuno, M.; Ishibashi, T.A. Heterodyne-Detected Achiral and Chiral Vibrational Sum Frequency Generation of Proteins at Air/Water Interface. J. Phys. Chem. C 2015, 119, 9947–9954. [Google Scholar] [CrossRef]
- Okuno, M.; Ishibashi, T. Chirality Discriminated by Heterodyne-Detected Vibrational Sum Frequency Generation. J. Phys. Chem. Lett. 2014, 5, 2874–2878. [Google Scholar] [CrossRef] [PubMed]
- Yan, E.C.Y.; Fu, L.; Wang, Z.; Liu, W. Biological Macromolecules at Interfaces Probed by Chiral Vibrational Sum Frequency Generation Spectroscopy. Chem. Rev. 2014, 114, 8471–8498. [Google Scholar] [CrossRef]
- Guo, W.; Lu, T.; Gandhi, Z.; Chen, Z. Probing Orientations and Conformations of Peptides and Proteins at Buried Interfaces. J. Phys. Chem. Lett. 2021, 12, 10144–10155. [Google Scholar] [CrossRef]
- Stephens, P.J. Theory of Vibrational Circular Dichroism. J. Phys. Chem. 1985, 89, 748–752. [Google Scholar] [CrossRef]
- Keiderling, T.A. Structure of Condensed Phase Peptides: Insights from Vibrational Circular Dichroism and Raman Optical Activity Techniques. Chem. Rev. 2020, 120, 3381–3419. [Google Scholar] [CrossRef] [PubMed]
- Barron, L.D.; Zhu, F.; Hecht, L.; Tranter, G.E.; Isaacs, N.W. Raman Optical Activity: An Incisive Probe of Molecular Chirality and Biomolecular Structure. J. Mol. Struct. 2007, 834–836, 7–16. [Google Scholar] [CrossRef]
- Blanch, E. Vibrational Raman Optical Activity of Proteins, Nucleic Acids, and Viruses. Methods 2003, 29, 196–209. [Google Scholar] [CrossRef]
- Zajac, G.; Kaczor, A.; Pallares Zazo, A.; Mlynarski, J.; Dudek, M.; Baranska, M. Aggregation-Induced Resonance Raman Optical Activity (AIRROA): A New Mechanism for Chirality Enhancement. J. Phys. Chem. B 2016, 120, 4028–4033. [Google Scholar] [CrossRef]
- Meyer, T.E. Isolation and Characterization of Soluble Cytochromes, Ferredoxins and Other Chromophoric Proteins from the Halophilic Phototrophic Bacterium Ectothiorhodospira Halophila. Biochim. Biophys. Acta - Bioenerg. 1985, 806, 175–183. [Google Scholar] [CrossRef]
- Meyer, T.E.; Yakali, E.; Cusanovich, M.A.; Tollin, G. Properties of a Water-Soluble, Yellow Protein Isolated from a Halophilic Phototrophic Bacterium That Has Photochemical Activity Analogous to Sensory Rhodopsin. Biochemistry 1987, 26, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Hellingwerf, K.J.; Hendriks, J.; Gensch, T. Photoactive Yellow Protein, A New Type of Photoreceptor Protein: Will This “Yellow Lab” Bring Us Where We Want to Go? J. Phys. Chem. A 2003, 107, 1082–1094. [Google Scholar] [CrossRef]
- Krekic, S.; Zakar, T.; Gombos, Z.; Valkai, S.; Mero, M.; Zimányi, L.; Heiner, Z.; Dér, A. Nonlinear Optical Investigation of Microbial Chromoproteins. Front. Plant Sci. 2020, 11, 1567. [Google Scholar] [CrossRef] [PubMed]
- Khoroshyy, P.; Dér, A.; Zimányi, L. Effect of Hofmeister Cosolutes on the Photocycle of Photoactive Yellow Protein at Moderately Alkaline PH. J. Photochem. Photobiol. B Biol. 2013, 120, 111–119. [Google Scholar] [CrossRef]
- Krekic, S.; Nagy, D.; Taneva, S.G.; Fábián, L.; Zimányi, L.; Dér, A. Spectrokinetic Characterization of Photoactive Yellow Protein Films for Integrated Optical Applications. Eur. Biophys. J. 2019, 48, 465–473. [Google Scholar] [CrossRef]
- Pellequer, J.-L.; Wager-Smith, K.A.; Kay, S.A.; Getzoff, E.D. Photoactive Yellow Protein: A Structural Prototype for the Three-Dimensional Fold of the PAS Domain Superfamily. Proc. Natl. Acad. Sci. USA 1998, 95, 5884–5890. [Google Scholar] [CrossRef]
- Van Beeumen, J.J.; Devreese, B.V.; Van Bun, S.M.; Hoff, W.D.; Hellingwerf, K.J.; Meyer, T.E.; Cusanovich, M.A.; Mcree, D.E. Primary Structure of a Photoactive Yellow Protein from the Phototrophic Bacterium Ectothiorhodospira Halophila, with Evidence for the Mass and the Binding Site of the Chromophore. Protein Sci. 1993, 2, 1114–1125. [Google Scholar] [CrossRef]
- Hoff, W.D.; van Stokkum, I.H.; van Ramesdonk, H.J.; van Brederode, M.E.; Brouwer, A.M.; Fitch, J.C.; Meyer, T.E.; van Grondelle, R.; Hellingwerf, K.J. Measurement and Global Analysis of the Absorbance Changes in the Photocycle of the Photoactive Yellow Protein from Ectothiorhodospira Halophila. Biophys. J. 1994, 67, 1691–1705. [Google Scholar] [CrossRef]
- Baca, M.; Borgstahl, G.E.O.; Boissinot, M.; Burke, P.M.; Williams, D.R.; Slater, K.A.; Getzoff, E.D. Complete Chemical Structure of Photoactive Yellow Protein: Novel Thioester-Linked 4-Hydroxycinnamyl Chromophore and Photocycle Chemistry. Biochemistry 1994, 33, 14369–14377. [Google Scholar] [CrossRef]
- Graham, D.; Phillips, M. Proteins at Liquid Interfaces. J. Colloid Interface Sci. 1979, 70, 415–426. [Google Scholar] [CrossRef]
- Heiner, Z.; Petrov, V.; Mero, M. Compact, High-Repetition-Rate Source for Broadband Sum-Frequency Generation Spectroscopy. APL Photonics 2017, 2, 066102. [Google Scholar] [CrossRef]
- Heiner, Z.; Wang, L.; Petrov, V.; Mero, M. Broadband Vibrational Sum-Frequency Generation Spectrometer at 100 KHz in the 950-1750 cm−1 Spectral Range Utilizing a LiGaS 2 Optical Parametric Amplifier. Opt. Express 2019, 27, 15289. [Google Scholar] [CrossRef]
- Debreczeny, M.; Ball, V.; Boulmedais, F.; Szalontai, B.; Voegel, J.-C.; Schaaf, P. Multilayers Built from Two Component Polyanions and Single Component Polycation Solutions: A Way To Engineer Films with Desired Secondary Structure. J. Phys. Chem. B 2003, 107, 12734–12739. [Google Scholar] [CrossRef]
- Kim, J.; Somorjai, G.A. Molecular Packing of Lysozyme, Fibrinogen, and Bovine Serum Albumin on Hydrophilic and Hydrophobic Surfaces Studied by Infrared−Visible Sum Frequency Generation and Fluorescence Microscopy. J. Am. Chem. Soc. 2003, 125, 3150–3158. [Google Scholar] [CrossRef] [PubMed]
- Perets, E.A.; Videla, P.E.; Yan, E.C.Y.; Batista, V.S. Chiral Inversion of Amino Acids in Antiparallel β-Sheets at Interfaces Probed by Vibrational Sum Frequency Generation Spectroscopy. J. Phys. Chem. B 2019, 123, 5769–5781. [Google Scholar] [CrossRef] [PubMed]
- Perets, E.A.; Konstantinovsky, D.; Fu, L.; Chen, J.; Wang, H.-F.; Hammes-Schiffer, S.; Yan, E.C.Y. Mirror-Image Antiparallel β-Sheets Organize Water Molecules into Superstructures of Opposite Chirality. Proc. Natl. Acad. Sci. USA 2020, 117, 32902–32909. [Google Scholar] [CrossRef]
- Sovago, M.; Vartiainen, E.; Bonn, M. Determining Absolute Molecular Orientation at Interfaces: A Phase Retrieval Approach for Sum Frequency Generation Spectroscopy. J. Phys. Chem. C 2009, 113, 6100–6106. [Google Scholar] [CrossRef]
- de Beer, A.G.F.; Samson, J.-S.; Hua, W.; Huang, Z.; Chen, X.; Allen, H.C.; Roke, S. Direct Comparison of Phase-Sensitive Vibrational Sum Frequency Generation with Maximum Entropy Method: Case Study of Water. J. Chem. Phys. 2011, 135, 224701. [Google Scholar] [CrossRef]
- York, R.L.; Holinga, G.J.; Somorjai, G.A. An Investigation of the Influence of Chain Length on the Interfacial Ordering of L-Lysine and l-Proline and Their Homopeptides at Hydrophobic and Hydrophilic Interfaces Studied by Sum Frequency Generation and Quartz Crystal Microbalance. Langmuir 2009, 25, 9369–9374. [Google Scholar] [CrossRef]
- Lott, G.A.; King, M.D.; Hill, M.W.; Scatena, L.F. Effects of Relative Humidity on the Surface and Bulk Structures of Linear Polyethylenimine Thin Films. J. Phys. Chem. C 2014, 118, 17686–17698. [Google Scholar] [CrossRef]
- Hashida, T.; Tashiro, K. Structural Investigation on Water-Induced Phase Transitions of Poly(Ethylene Imine), Part IV: Changes of Intra- and Intermolecular Hydrogen Bonds in the Hydration Processes as Revealed by Time-Resolved Raman Spectral Measurements. Polymer 2007, 48, 7614–7622. [Google Scholar] [CrossRef]
- Navarrete, J.T.L.; Hernández, V.; Ramírez, F.J. Vibrational Study of Aspartic Acid and Glutamic Acid Dipeptides. J. Mol. Struct. 1995, 348, 249–252. [Google Scholar] [CrossRef]
- Livingstone, R.A.; Zhang, Z.; Piatkowski, L.; Bakker, H.J.; Hunger, J.; Bonn, M.; Backus, E.H.G. Water in Contact with a Cationic Lipid Exhibits Bulklike Vibrational Dynamics. J. Phys. Chem. B 2016, 120, 10069–10078. [Google Scholar] [CrossRef]
- Panayotov, I.V.; Collart-Dutilleul, P.-Y.; Salehi, H.; Martin, M.; Végh, A.; Yachouh, J.; Vladimirov, B.; Sipos, P.; Szalontai, B.; Gergely, C.; et al. Sprayed Cells and Polyelectrolyte Films for Biomaterial Functionalization: The Influence of Physical PLL-PGA Film Treatments on Dental Pulp Cell Behavior. Macromol. Biosci. 2014, 14, 1771–1782. [Google Scholar] [CrossRef]
- Picart, C.; Ladam, G.; Senger, B.; Voegel, J.C.; Schaaf, P.; Cuisinier, F.J.G.; Gergely, C. Determination of Structural Parameters Characterizing Thin Films by Optical Methods: A Comparison between Scanning Angle Reflectometry and Optical Waveguide Lightmode Spectroscopy. J. Chem. Phys. 2001, 115, 1086–1094. [Google Scholar] [CrossRef]
- Michel, M.; Toniazzo, V.; Ruch, D.; Ball, V. Deposition Mechanisms in Layer-by-Layer or Step-by-Step Deposition Methods: From Elastic and Impermeable Films to Soft Membranes with Ion Exchange Properties. ISRN Mater. Sci. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Barth, A. The Infrared Absorption of Amino Acid Side Chains. Prog. Biophys. Mol. Biol. 2000, 74, 141–173. [Google Scholar] [CrossRef]
- Fulara, A.; Dzwolak, W. Bifurcated Hydrogen Bonds Stabilize Fibrils of Poly(l -Glutamic) Acid. J. Phys. Chem. B 2010, 114, 8278–8283. [Google Scholar] [CrossRef]
- Xie, A.; Kelemen, L.; Hendriks, J.; White, B.J.; Hellingwerf, K.J.; Hoff, W.D. Formation of a New Buried Charge Drives a Large-Amplitude Protein Quake in Photoreceptor Activation. Biochemistry 2001, 40, 1510–1517. [Google Scholar] [CrossRef]
- McColl, I.H.; Blanch, E.W.; Gill, A.C.; Rhie, A.G.O.; Ritchie, M.A.; Hecht, L.; Nielsen, K.; Barron, L.D. A New Perspective on β-Sheet Structures Using Vibrational Raman Optical Activity: From Poly(L-Lysine) to the Prion Protein. J. Am. Chem. Soc. 2003, 125, 10019–10026. [Google Scholar] [CrossRef]
- Tobias, F.; Keiderling, T.A. Role of Side Chains in β-Sheet Self-Assembly into Peptide Fibrils. IR and VCD Spectroscopic Studies of Glutamic Acid-Containing Peptides. Langmuir 2016, 32, 4653–4661. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krekic, S.; Mero, M.; Kuhl, M.; Balasubramanian, K.; Dér, A.; Heiner, Z. Photoactive Yellow Protein Adsorption at Hydrated Polyethyleneimine and Poly-l-Glutamic Acid Interfaces. Molecules 2023, 28, 4077. https://doi.org/10.3390/molecules28104077
Krekic S, Mero M, Kuhl M, Balasubramanian K, Dér A, Heiner Z. Photoactive Yellow Protein Adsorption at Hydrated Polyethyleneimine and Poly-l-Glutamic Acid Interfaces. Molecules. 2023; 28(10):4077. https://doi.org/10.3390/molecules28104077
Chicago/Turabian StyleKrekic, Szilvia, Mark Mero, Michel Kuhl, Kannan Balasubramanian, András Dér, and Zsuzsanna Heiner. 2023. "Photoactive Yellow Protein Adsorption at Hydrated Polyethyleneimine and Poly-l-Glutamic Acid Interfaces" Molecules 28, no. 10: 4077. https://doi.org/10.3390/molecules28104077
APA StyleKrekic, S., Mero, M., Kuhl, M., Balasubramanian, K., Dér, A., & Heiner, Z. (2023). Photoactive Yellow Protein Adsorption at Hydrated Polyethyleneimine and Poly-l-Glutamic Acid Interfaces. Molecules, 28(10), 4077. https://doi.org/10.3390/molecules28104077