Effects of Taraxerol on Oxidative and Inflammatory Mediators in Isoproterenol-Induced Cardiotoxicity in an Animal Model
Abstract
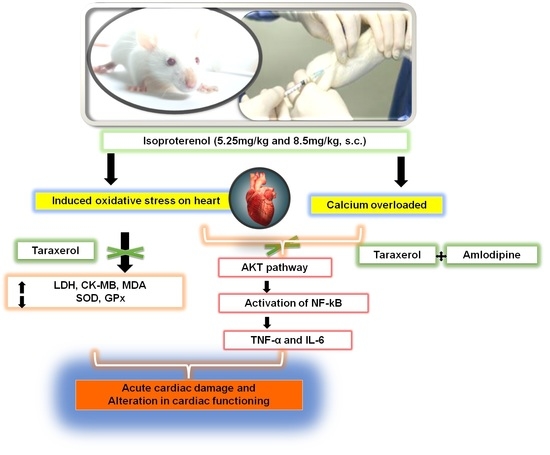
:1. Introduction
2. Results
2.1. Effect of Taraxerol Pretreatment on Heart Rate, Heart Weight, and Blood Pressure
2.2. Effect of Taraxerol Pretreatment on Cardiac Biomarker Levels
2.3. Effect of Taraxerol Pretreatment on Antioxidant Levels
2.4. Effect of Taraxerol Pretreatment on Inflammatory Mediator Levels in the Serum
2.5. Effect of Taraxerol Pretreatment on Heart Histology
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Animals
4.3. Experimental Protocols
4.4. Measurement of Inflammatory Cytokines
4.5. Histology of Cardiac Tissue
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Barbalho, S.M.; Flato, U.A.P.; Tofano, R.J.; de Goulart, R.A.; Guiguer, E.L.; Detregiachi, C.R.P.; Buchaim, D.V.; Araújo, A.C.; Buchain, R.L.; Reina, F.T.R.; et al. Physical exercise and myokines: Relationships with sarcopenia and cardiovascular complications. Int. J. Mol. Sci. 2020, 21, 3607. [Google Scholar] [CrossRef] [PubMed]
- Akter, H.; Mamunur Rashid, M.; Shahidul Islam, M.; Amjad Hossen, M.; Atiar Rahman, M.; Algheshairy, R.M.; Almujaydil, M.S.; Alharbi, H.F.; Alnajeebi, A.M. Biometabolites of Tamarindus indica play a remarkable cardioprotective role as a functional food in doxorubicin-induced cardiotoxicity models. J. Funct. Foods 2022, 96, 105212. [Google Scholar] [CrossRef]
- Kivimäki, M.; Steptoe, A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 2018, 15, 215–229. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; De Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics-2015 update: A report from the American Heart Association. Circulation 2015, 131, 152. [Google Scholar] [CrossRef]
- Du, X.; Su, X.; Zhang, W.; Yi, S.; Zhang, G.; Jiang, S.; Li, H.; Li, S.; Xia, F. Progress, Opportunities, and Challenges of Troponin Analysis in the Early Diagnosis of Cardiovascular Diseases. Anal. Chem. 2022, 94, 442–463. [Google Scholar] [CrossRef] [PubMed]
- Elasoru, S.E.; Rhana, P.; de Oliveira Barreto, T.; Naves de Souza, D.L.; Menezes-Filho, J.E.R.; Souza, D.S.; Loes Moreira, M.V.; Gomes Campos, M.T.; Adedosu, O.T.; Roman-Campos, D.; et al. Andrographolide protects against isoproterenol-induced myocardial infarction in rats through inhibition of L-type Ca2+ and increase of cardiac transient outward K+ currents. Eur. J. Pharmacol. 2021, 906, 174194. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, M.J.; Hsue, P.Y.; Benjamin, L.A.; Bloomfield, G.S.; Currier, J.S.; Freiberg, M.S.; Grinspoon, S.K.; Levin, J.; Longenecker, C.T.; Post, W.S. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living with HIV: A Scientific Statement from the American Heart Association. Circulation 2019, 140, 695. [Google Scholar] [CrossRef] [PubMed]
- Chiong, M.; Wang, Z.V.; Pedrozo, Z.; Cao, D.J.; Troncoso, R.; Ibacache, M.; Criollo, A.; Nemchenko, A.; Hill, J.A.; Lavandero, S. Cardiomyocyte death: Mechanisms and translational implications. Cell Death Dis. 2011, 2, e244. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.B.; Hernández-Reséndiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef]
- Rahnavard, M.; Hassanpour, M.; Ahmadi, M.; Heidarzadeh, M.; Amini, H.; Javanmard, M.Z.; Nouri, M.; Rahbarghazi, R.; Safaie, N. Curcumin ameliorated myocardial infarction by inhibition of cardiotoxicity in the rat model. J. Cell Biochem. 2019, 120, 28480. [Google Scholar] [CrossRef]
- Krijnen, P.A.J.; Nijmeijer, R.; Meijer, C.J.L.M.; Visser, C.A.; Hack, C.E.; Niessen, H.W.M. Apoptosis in myocardial ischaemia and infarction. J. Clin. Pathol. 2002, 55, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Yusufoglu, H.; Foudah, A.; Alam, A.; Soliman, G. Cardioprotective and nephroprotective activities of methanolic extracts from Pulicaria somalensis herbs against carbon tetrachloride induced toxicity in rats. Planta Med. 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Yusufoglu, H.S.; Soliman, G.A.; Foudah, A.I.; Abdulkader, M.S.; El-Banna, H.A.; Alam, A.; Salkini, M.A. Protective effect of arnebia hispidissima against carbon tetrachloride-induced heart and kidney injury in rats. Int. J. Pharmacol. 2018, 14, 1019. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, L.; Ding, M.; Li, M. Protective effect of vasicine against myocardial infarction in rats via modulation of oxidative stress, inflammation, and the PI3K/AKT pathway. Drug Des. Dev. Ther. 2019, 13, 3773–3784. [Google Scholar] [CrossRef] [PubMed]
- Mus, A.A.; Goh, L.P.W.; Marbawi, H.; Gansau, J.A. The Biosynthesis and Medicinal Properties of Taraxerol. Biomedicines 2022, 10, 807. [Google Scholar] [CrossRef]
- Swain, S.S.; Rout, K.K.; Chand, P.K. Production of triterpenoid anti-cancer compound taraxerol in Agrobacterium-transformed root cultures of butterfly pea (Clitoria ternatea L.). Appl. Biochem. Biotechnol. 2012, 168, 487–503. [Google Scholar] [CrossRef]
- Chunhakant, S.; Chaicharoenpong, C. Antityrosinase, Antioxidant, and Cytotoxic Activities of Phytochemical Constituents from Manilkara zapota L. Bark. Molecules 2019, 24, 2798. [Google Scholar] [CrossRef]
- Gupta, G.K.; Chahal, J.; Bhatia, M. Clitoria ternatea (L.): Old and new aspects. J. Pharm. Res. 2015, 3, 2610–2614. [Google Scholar]
- Biswas, M.; Biswas, K.; Ghosh, A.K.; Haldar, P.K. A pentacyclic triterpenoid possessing anti-inflammatory activity from the fruits of Dregea volubilis. Pharmacogn. Mag. 2009, 5, 64. [Google Scholar] [CrossRef]
- Yao, X.; Lu, B.; Lü, C.; Bai, Q.; Yan, D.; Xu, H. Taraxerol induces cell apoptosis through a mitochondria-mediated pathway in hela cells. Cell J. 2017, 19, 4543. [Google Scholar] [CrossRef]
- Ranjith, D.; Viswanath, S. In silico antidiabetic activity of bioactive compounds in Ipomoea mauritiana Jacq. Pharma Innov. J. 2019, 8, 5–11. [Google Scholar]
- Zingare, M.L.; Zingare, P.L.; Dubey, A.K.; Ansari, A. Clitoria ternatea (Aparajita): A review of the antioxidant, antidiabetic and hepatoprotective potentials. Int. J. Pharm. Biol. Sci. 2013, 3, 203–213. [Google Scholar]
- Rosli, N.; Khairuddean, M.; Jamain, Z. Phytochemical and Biological Activity Studies of the Leaves of Garcinia hombroniana Pierre. Malaysian J. Chem. 2020, 22, 81–102. [Google Scholar]
- Khanra, R.; Dewanjee, S.; Dua, T.K.; Bhattacharjee, N. Taraxerol, a pentacyclic triterpene from Abroma augusta leaf, attenuates acute inflammation via inhibition of NF-κB signaling. Biomed. Pharmacother. 2017, 88, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Ur Rahman, U.; Ali, S.; Ubaidullah; Khan, I.; Khan, M.A.; Arif, S.; ur Wazir, S.R. Anti-inflammatory activity of taraxerol acetate. J. Med. Sci. 2016, 24, 216–219. [Google Scholar]
- Wexler, B.C. Myocardial infarction in young vs old male rats: Pathophysiologic changes. Am. Heart J. 1978, 96, 70–80. [Google Scholar] [CrossRef]
- Li, H.; Xie, Y.H.; Yang, Q.; Wang, S.W.; Le Zhang, B.; Wang, J.B.; Cao, W.; Bi, L.L.; Sun, J.Y.; Miao, S.; et al. Cardioprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats. PLoS ONE 2012, 7, e48872. [Google Scholar] [CrossRef]
- Amran, A.Z.; Jantan, I.; Dianita, R.; Buang, F. Protective effects of the standardized extract of Zingiber officinale on myocardium against isoproterenol-induced biochemical and histopathological alterations in rats. Pharm. Biol. 2015, 53, 1795–1802. [Google Scholar] [CrossRef]
- Dhivya, V.; Priya, L.B.; Chirayil, H.T.; Sathiskumar, S.; Huang, C.Y.; Padma, V.V. Piperine modulates isoproterenol induced myocardial ischemia through antioxidant and anti-dyslipidemic effect in male Wistar rats. Biomed. Pharmacother. 2017, 87, 705–713. [Google Scholar] [CrossRef]
- Panda, S. Butanolic fraction of Moringa oleifera Lam. (moringaceae) attenuates isoprotrenol–induced cardiac necrosis and oxidative stress in rats: An EPR study. EXCLI J. 2015, 14, 64. [Google Scholar] [CrossRef]
- Jiki, Z.; Lecour, S.; Nduhirabandi, F. Cardiovascular benefits of dietary melatonin: A myth or a reality? Front. Physiol. 2018, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, K.S.; Prince, P.S.M. Caffeic acid protects rat heart mitochondria against isoproterenol-induced oxidative damage. Cell Stress Chaperones 2010, 15, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Kamat, P.K.; Kalani, A.; Familtseva, A.; Tyagi, S.C. High Methionine Diet Poses Cardiac Threat: A Molecular Insight. J. Cell Physiol. 2016, 231, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chuang, C.C.; Zuo, L. Molecular Characterization of Reactive Oxygen Species in Myocardial Ischemia-Reperfusion Injury. BioMed Res. Int. 2015, 2015, 864946. [Google Scholar] [CrossRef]
- Ganapathy, R.; Ramachandran, A.; Shivalingaiah, S.B.; Bishir, M.; Bhojaraj, S.; Sridhar, S.; Mohan, S.K.; Veeraraghavan, V.P.; Chidambaram, S.B.; Essa, M.M.; et al. Cardioprotective potential of polyphenols rich Thraatchathi Chooranam against isoproterenol induced myocardial necrosis in experimental rats. BMC Complement. Med. Ther. 2020, 20, 356. [Google Scholar] [CrossRef]
- Priscilla, D.H.; Prince, P.S.M. Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem. Biol. Interact. 2009, 179, 118–124. [Google Scholar] [CrossRef]
- Farvin, K.H.S.; Anandan, R.; Kumar, S.H.S.; Shiny, K.S.; Mathew, S.; Sankar, T.V.; Nair, P.G.V. Cardioprotective effect of squalene on lipid profile in isoprenaline-induced myocardial infarction in rats. J. Med. Food 2006, 9, 531–536. [Google Scholar] [CrossRef]
- Kakar, M.; Chakarborty, P.; Behl, T.; Singh, S.; Sharma, N.; Sachdeva, M. Insight into the role of inflammation in progression of diabetes associated neuropathy. Res. J. Pharm. Technol. 2020, 13, 5477–5483. [Google Scholar]
- Devika, P.T.; Stanely Mainzen Prince, P. Protective effect of (-)-epigallocatechin-gallate (EGCG) on lipid peroxide metabolism in isoproterenol induced myocardial infarction in male Wistar rats: A histopathological study. Biomed. Pharmacother. 2008, 62, 701–708. [Google Scholar] [CrossRef]
- Madhesh, M.; Vaiyapuri, M. Effect of luteolin on lipid peroxidation and antioxidants in acute and chronic periods of isoproterenol induced myocardial infarction in rats. J. Acute Med. 2012, 2, 70–76. [Google Scholar] [CrossRef]
- Shaik, A.H.; Rasool, S.N.; Vikram Kumar Reddy, A.; Abdul Kareem, M.; Saayi Krushna, G.; Lakshmi Devi, K. Cardioprotective effect of HPLC standardized ethanolic extract of Terminalia pallida fruits against isoproterenol-induced myocardial infarction in albino rats. J. Ethnopharmacol. 2012, 141, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A.; Nameni, G.; Hajiluian, G.; Mesgari-Abbasi, M. Cardiac tissue oxidative stress and inflammation after vitamin D administrations in high fat- diet induced obese rats. BMC Cardiovasc. Disord. 2017, 17, 161. [Google Scholar] [CrossRef]
- Krushna, G.S.S.; Kareem, M.A.; Reddy, V.D.; Padmavathi, P.; Hussain, S.A.; Kodidhela, L.D. Aegle marmelos fruit extract attenuates isoproterenol-induced oxidative stress in rats. J. Clin. Biochem. Nutr. 2012, 50, 199–204. [Google Scholar] [CrossRef]
- Gupta, A.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Yadav, S.; Anwer, K.; La Cruz, C.V.D.; Chigurupati, S.; Farasani, A.; et al. Elucidating the Neuroprotective Effect of Tecoma stans Leaf Extract in STZ-Induced Diabetic Neuropathy. Evid.-Based Complement. Altern. Med. 2022, 2022, 3833392. [Google Scholar] [CrossRef]
- Ušaj, M.; Moretto, L.; Månsson, A. Critical Evaluation of Current Hypotheses for the Pathogenesis of Hypertrophic Cardiomyopathy. Int. J. Mol. Sci. 2022, 23, 2195. [Google Scholar] [CrossRef]
- Yao, X.; Li, G.; Bai, Q.; Xu, H.; Lü, C. Taraxerol inhibits LPS-induced inflammatory responses through suppression of TAK1 and Akt activation. Int. Immunopharmacol. 2022, 23, 2195. [Google Scholar] [CrossRef]
- Alkholifi, F.K.; Devi, S.; Yusufoglu, H.S.; Alam, A. The Cardioprotective Effect of Corosolic Acid in the Diabetic Rats: A Possible Mechanism of the PPAR-γ Pathway. Molecules 2023, 28, 929. [Google Scholar] [CrossRef]
- Harhaj, E.W.; Dixit, V.M. Regulation of NF-κB by deubiquitinases. Immunol. Rev. 2012, 246, 107–124. [Google Scholar] [CrossRef]
- Skaug, B.; Jiang, X.; Chen, Z.J. The role of ubiquitin in NF-κB regulatory pathways. Annu. Rev. Biochem. 2009, 78, 769–796. [Google Scholar] [CrossRef]
- Macías-Rubalcava, M.L.; Hernández-Bautista, B.E.; Jiménez-Estrada, M.; Cruz-Ortega, R.; Anaya, A.L. Pentacyclic triterpenes with selective bioactivity from Sebastiania adenophora leaves, euphorbiaceae. J. Chem. Ecol. 2007, 33, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Babbar, R.; Agarwal, S.; Kotwani, A.; Fahim, M. Mechanistic clues in the cardioprotective effect of terminalia arjuna bark extract in isoproterenol-induced chronic heart failure in rats. Cardiovasc. Toxicol. 2011, 11, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, M.; Song, J.; Shi, Y.; Qin, X.; Gao, Z.; Lv, Y.; Du, G. The cardioprotective effects of the new crystal form of puerarin in isoproterenol-induced myocardial ischemia rats based on metabolomics. Sci. Rep. 2020, 10, 17787. [Google Scholar] [CrossRef] [PubMed]





| Treatment | MDA (nmol/g of Serum) | GPx (Units/mg of Protein) | SOD (Units/mg of Protein) |
|---|---|---|---|
| Normal control | 1.05 ± 0.67 | 4.12 ± 0.27 | 5.54 ± 0.63 |
| ISO control | 5.35 ± 1.07 * | 1.13 ± 0.18 * | 0.22 ± 0.09 * |
| Amlodipine | 2.50 ± 0.86 # | 3.85 ± 0.32 # | 4.08 ± 0.82 # |
| Taraxerol 20 mg/kg | 3.28 ± 0.89 * | 1.52 ± 0.28 * | 1.18 ± 0.52 # |
| Taraxerol 40 mg/kg | 2.88 ± 0.93 # | 2.89 ± 0.55 # | 2.88 ± 0.75 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aodah, A.H.; Devi, S.; Alkholifi, F.K.; Yusufoglu, H.S.; Foudah, A.I.; Alam, A. Effects of Taraxerol on Oxidative and Inflammatory Mediators in Isoproterenol-Induced Cardiotoxicity in an Animal Model. Molecules 2023, 28, 4089. https://doi.org/10.3390/molecules28104089
Aodah AH, Devi S, Alkholifi FK, Yusufoglu HS, Foudah AI, Alam A. Effects of Taraxerol on Oxidative and Inflammatory Mediators in Isoproterenol-Induced Cardiotoxicity in an Animal Model. Molecules. 2023; 28(10):4089. https://doi.org/10.3390/molecules28104089
Chicago/Turabian StyleAodah, Alhussain H., Sushma Devi, Faisal K. Alkholifi, Hasan S. Yusufoglu, Ahmed I. Foudah, and Aftab Alam. 2023. "Effects of Taraxerol on Oxidative and Inflammatory Mediators in Isoproterenol-Induced Cardiotoxicity in an Animal Model" Molecules 28, no. 10: 4089. https://doi.org/10.3390/molecules28104089
APA StyleAodah, A. H., Devi, S., Alkholifi, F. K., Yusufoglu, H. S., Foudah, A. I., & Alam, A. (2023). Effects of Taraxerol on Oxidative and Inflammatory Mediators in Isoproterenol-Induced Cardiotoxicity in an Animal Model. Molecules, 28(10), 4089. https://doi.org/10.3390/molecules28104089