Improving Crystallization and Stability of Perovskite Solar Cells Using a Low-Temperature Treated A-Site Cation Solution in the Sequential Deposition
Abstract
1. Introduction
2. Results and Discussion
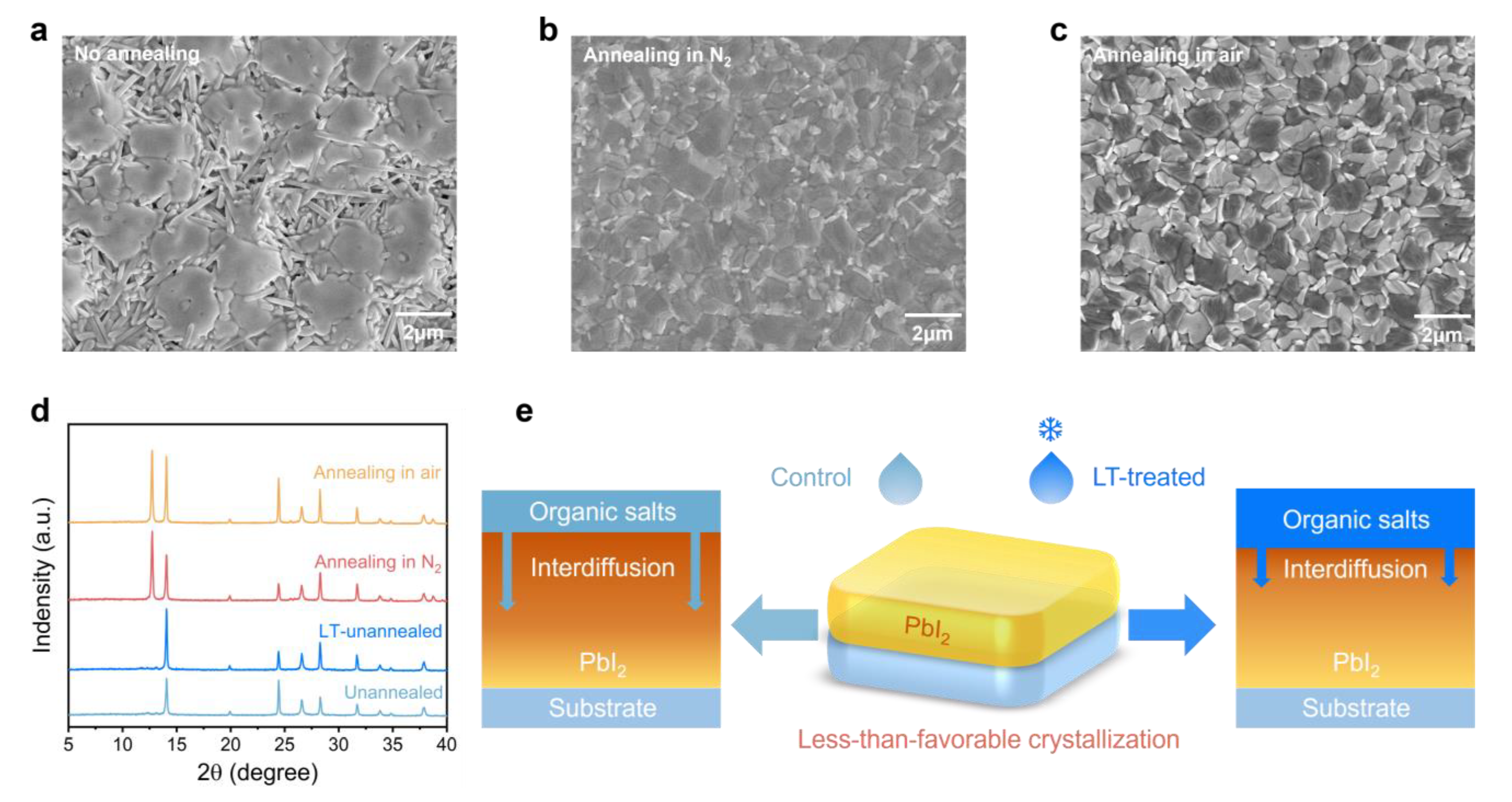
2.1. Mechanisms of LT Treatment
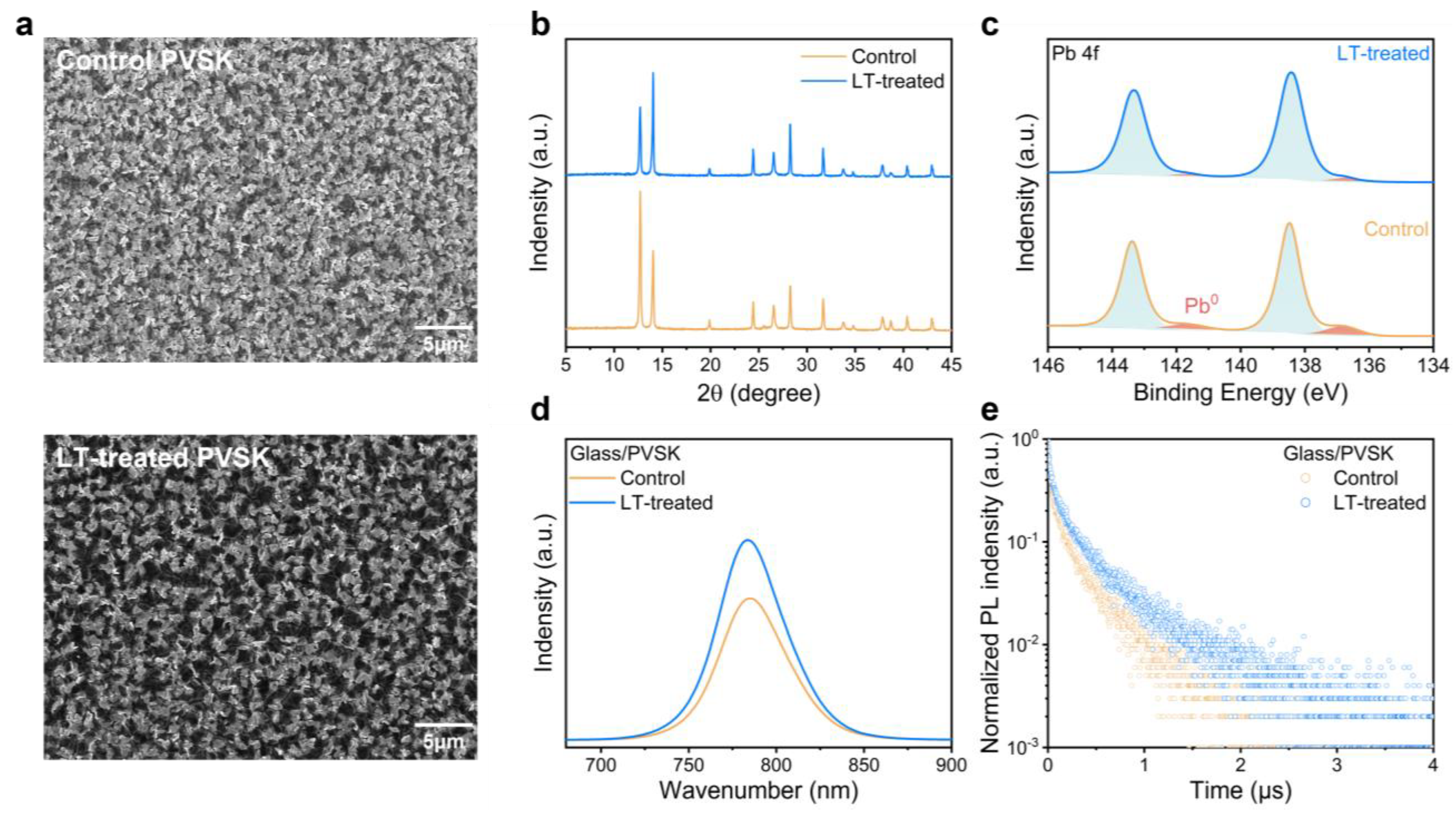
2.2. Film Characterization
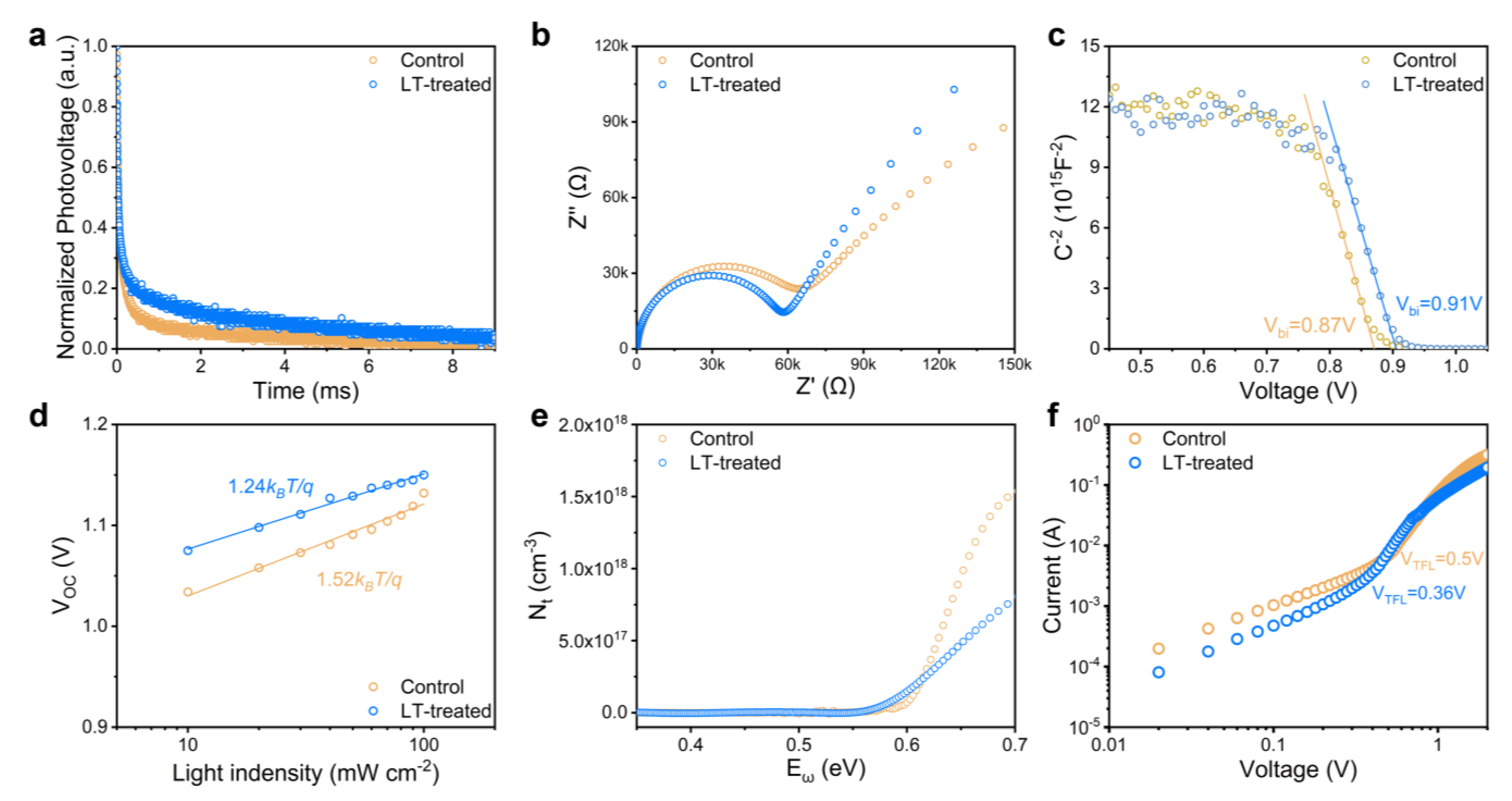
2.3. Carrier Dynamic Characterization
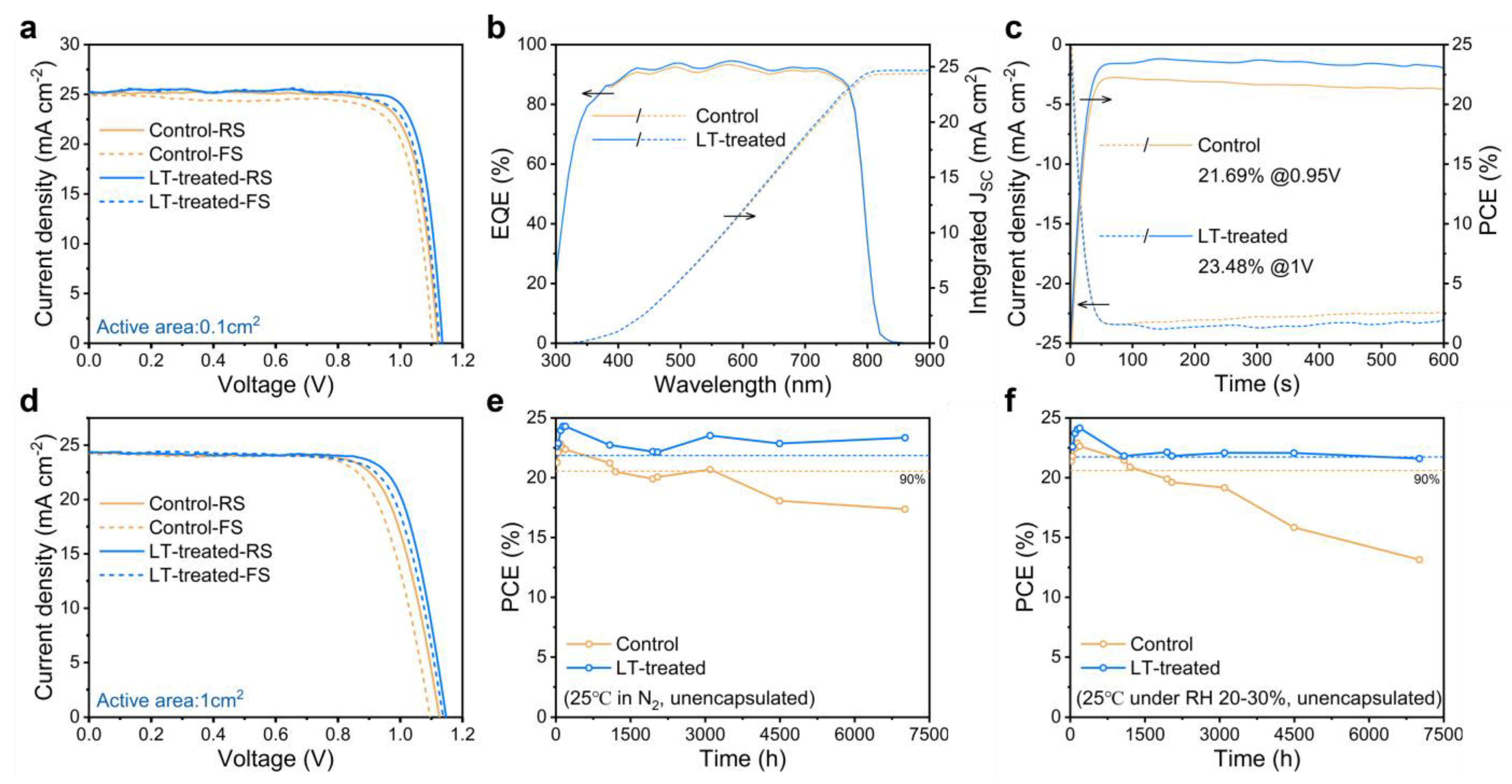
2.4. Photovoltaic Performance and Stability of PSCs
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation of Precursor Solutions
3.3. Fabrication of the Devices
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, P.; Zhang, X.; Zhou, Y.; Jiang, Q.; Ye, Q.; Chu, Z.; Li, X.; Yang, X.; Yin, Z.; You, J. Solvent-Controlled Growth of Inorganic Perovskite Films in Dry Environment for Efficient and Stable Solar Cells. Nat. Commun. 2018, 9, 2225. [Google Scholar] [CrossRef] [PubMed]
- Al-Ashouri, A.; Köhnen, E.; Li, B.; Magomedov, A.; Hempel, H.; Caprioglio, P.; Márquez, J.A.; Morales Vilches, A.B.; Kasparavicius, E.; Smith, J.A.; et al. Monolithic Perovskite/Silicon Tandem Solar Cell with >29% Efficiency by Enhanced Hole Extraction. Science 2020, 370, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jeong, J.; Lu, H.; Lee, T.K.; Eickemeyer, F.T.; Liu, Y.; Choi, I.W.; Choi, S.J.; Jo, Y.; Kim, H.-B.; et al. Conformal Quantum Dot–SnO2 Layers as Electron Transporters for Efficient Perovskite Solar Cells. Science 2022, 375, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, F.; Qu, Z.; Yu, S.; Shen, T.; Deng, H.-X.; Chu, X.; Peng, X.; Yuan, Y.; Zhang, X.; et al. Inactive (PbI2)2RbCl Stabilizes Perovskite Films for Efficient Solar Cells. Science 2022, 377, 531–534. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Yun, H.-S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled Growth of Perovskite Layers with Volatile Alkylammonium Chlorides. Nature 2023, 616, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Grätzel, M.; Nazeeruddin, M.K. Organohalide Lead Perovskites for Photovoltaic Applications. Energy Environ. Sci. 2014, 7, 2448–2463. [Google Scholar] [CrossRef]
- Ivaniuk, K.; Cherpak, V.; Stakhira, P.; Baryshnikov, G.; Minaev, B.; Hotra, Z.; Turyk, P.; Zhydachevskii, Y.; Volyniuk, D.; Aksimentyeva, O.; et al. BaZrO3 Perovskite Nanoparticles as Emissive Material for Organic/Inorganic Hybrid Light-Emitting Diodes. Dye. Pigment. 2017, 145, 399–403. [Google Scholar] [CrossRef]
- Burlingame, Q.; Huang, X.; Liu, X.; Jeong, C.; Coburn, C.; Forrest, S.R. Intrinsically Stable Organic Solar Cells under High-Intensity Illumination. Nature 2019, 573, 394–397. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, D.; Suo, J.; Cao, Y.; Eickemeyer, F.T.; Vlachopoulos, N.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Hydroxamic Acid Pre-Adsorption Raises the Efficiency of Cosensitized Solar Cells. Nature 2023, 613, 60–65. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Enhanced Electron Extraction Using SnO2 for High-Efficiency Planar-Structure HC(NH2)2 PbI3-Based Perovskite Solar Cells. Nat. Energy 2017, 2, 16177. [Google Scholar] [CrossRef]
- Jiang, Q.; Chu, Z.; Wang, P.; Yang, X.; Liu, H.; Wang, Y.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Planar-Structure Perovskite Solar Cells with Efficiency beyond 21%. Adv. Mater. 2017, 29, 1703852. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Wang, C.; Zhou, Q.; Wang, L.; Wang, X.; Yang, L.; Ding, J.; Chen, C.; Wu, J.; Li, X.; et al. Rear Interface Engineering to Suppress Migration of Iodide Ions for Efficient Perovskite Solar Cells with Minimized Hysteresis. Adv. Funct. Mater. 2022, 32, 2107823. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Y.; Li, Z.; Qiao, L.; Xiong, Q.; Deng, L.; Zhang, Z.; Long, R.; Zhou, Q.; Du, Y.; et al. Marked Passivation Effect of Naphthalene-1,8-Dicarboximides in High-Performance Perovskite Solar Cells. Adv. Mater. 2021, 33, 2008405. [Google Scholar] [CrossRef]
- Ahlawat, P.; Hinderhofer, A.; Alharbi, E.A.; Lu, H.; Ummadisingu, A.; Niu, H.; Invernizzi, M.; Zakeeruddin, S.M.; Dar, M.I.; Schreiber, F.; et al. A Combined Molecular Dynamics and Experimental Study Oftwo-Step Process Enabling Low-Temperature Formation of Phase-Pure α-FAPbI3. Sci. Adv. 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Chen, C.; Lou, Y.; Wang, K.; Su, Z.; Dong, C.; Chen, J.; Shi, Y.; Gao, X.; Wang, Z. Ternary Two-Step Sequential Deposition Induced Perovskite Orientational Crystallization for High-Performance Photovoltaic Devices. Adv. Energy Mater. 2021, 11, 2101538. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, X.; Chen, B.; Kelly, L.L.; Zhao, J.; Lin, Y.; Toney, M.F.; Huang, J. Crystallization in One-Step Solution Deposition of Perovskite Films: Upward or Downward? Sci. Adv. 2021, 7, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Jang, I.H.; Pellet, N.; Grätzel, M.; Park, N.G. Growth of CH3 NH3 PbI3 Cuboids with Controlled Size for High-Efficiency Perovskite Solar Cells. Nat. Nanotechnol. 2014, 9, 927–932. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, Y.; Xie, H.; Lyu, L.; Liu, X.; Gao, Y.; Huang, J. Qualifying Composition Dependent p and n Self-Doping in CH3NH3PbI3. Appl. Phys. Lett. 2014, 105, 163508. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Z.; Chen, J.; Gao, P. Surface Passivation of Organic-Inorganic Hybrid Perovskites with Methylhydrazine Iodide for Enhanced Photovoltaic Device Performance. Inorganics 2023, 11, 168. [Google Scholar] [CrossRef]
- Xiao, Z.; Bi, C.; Shao, Y.; Dong, Q.; Wang, Q.; Yuan, Y.; Wang, C.; Gao, Y.; Huang, J. Efficient, High Yield Perovskite Photovoltaic Devices Grown by Interdiffusion of Solution-Processed Precursor Stacking Layers. Energy Environ. Sci. 2014, 7, 2619–2623. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential Deposition as a Route to High-Performance Perovskite-Sensitized Solar Cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, H.; Hong, Z.; Luo, S.; Duan, H.S.; Wang, H.H.; Liu, Y.; Li, G.; Yang, Y. Planar Heterojunction Perovskite Solar Cells via Vapor-Assisted Solution Process. J. Am. Chem. Soc. 2014, 136, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Zhang, C.; Zhuang, Q.; Li, H.; Yang, H.; Chen, J.; Zang, Z. Stabilizing Buried Interface via Synergistic Effect of Fluorine and Sulfonyl Functional Groups Toward Efficient and Stable Perovskite Solar Cells. Nano-Micro Lett. 2023, 15, 17. [Google Scholar] [CrossRef]
- Ummadisingu, A.; Grätzel, M. Revealing the Detailed Path of Sequential Deposition for Metal Halide Perovskite Formation. Sci. Adv. 2018, 4, e1701402. [Google Scholar] [CrossRef]
- Qin, Z.; Chen, Y.; Wang, X.; Wei, N.; Liu, X.; Chen, H.; Miao, Y.; Zhao, Y. Zwitterion-Functionalized SnO2 Substrate Induced Sequential Deposition of Black-Phase FAPbI3 with Rearranged PbI2 Residue. Adv. Mater. 2022, 34, 2203143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Xu, Z.; Wang, R.; Dong, X.; Fu, Q.; Liu, Y. Crystal Growth Regulation of 2D/3D Perovskite Films for Solar Cells with Both High Efficiency and Stability. Adv. Mater. 2022, 34, 2200705. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, S.; Li, Q.; Cheng, Q.; He, B.; Hu, Z.; Shen, Y.; Chen, H.; Xu, G.; Ou, X.; et al. High-Polarizability Organic Ferroelectric Materials Doping for Enhancing the Built-In Electric Field of Perovskite Solar Cells Realizing Efficiency over 24%. Adv. Mater. 2022, 34, 2110482. [Google Scholar] [CrossRef]
- Duan, X.; Li, X.; Tan, L.; Huang, Z.; Yang, J.; Liu, G.; Lin, Z.; Chen, Y. Controlling Crystal Growth via an Autonomously Longitudinal Scaffold for Planar Perovskite Solar Cells. Adv. Mater. 2020, 32, 2000617. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, K.; Zhu, Y.; Wang, M.; Li, L. Iodine Induced PbI2 Porous Morphology Manipulation for High-Performance Planar Perovskite Solar Cells. Sol. RRL 2018, 2, 1800230. [Google Scholar] [CrossRef]
- Luo, C.; Zheng, G.; Gao, F.; Wang, X.; Zhao, Y.; Gao, X.; Zhao, Q. Facet Orientation Tailoring via 2D-Seed-Induced Growth Enables Highly Efficient and Stable Perovskite Solar Cells. Joule 2022, 6, 240–257. [Google Scholar] [CrossRef]
- He, J.; Sheng, W.; Yang, J.; Zhong, Y.; Su, Y.; Tan, L.; Chen, Y. Omnidirectional Diffusion of Organic Amine Salts Assisted by Ordered Arrays in Porous Lead Iodide for Two-Step Deposited Large-Area Perovskite Solar Cells. Energy Environ. Sci. 2023, 16, 629–640. [Google Scholar] [CrossRef]
- Pham, N.D.; Tiong, V.T.; Yao, D.; Martens, W.; Guerrero, A.; Bisquert, J.; Wang, H. Guanidinium Thiocyanate Selective Ostwald Ripening Induced Large Grain for High Performance Perovskite Solar Cells. Nano Energy 2017, 41, 476–487. [Google Scholar] [CrossRef]
- Fujihara, T.; Terakawa, S.; Matsushima, T.; Qin, C.; Yahiro, M.; Adachi, C. Fabrication of High Coverage MASnI3 Perovskite Films for Stable, Planar Heterojunction Solar Cells. J. Mater. Chem. C 2017, 5, 1121–1127. [Google Scholar] [CrossRef]
- Bai, D.; Bian, H.; Jin, Z.; Wang, H.; Meng, L.; Wang, Q.; Liu, S.F. Temperature-Assisted Crystallization for Inorganic CsPbI2Br Perovskite Solar Cells to Attain High Stabilized Efficiency 14.81%. Nano Energy 2018, 52, 408–415. [Google Scholar] [CrossRef]
- Ding, B.; Li, Y.; Huang, S.Y.; Chu, Q.Q.; Li, C.X.; Li, C.J.; Yang, G.J. Material Nucleation/Growth Competition Tuning towards Highly Reproducible Planar Perovskite Solar Cells with Efficiency Exceeding 20%. J. Mater. Chem. A 2017, 5, 6840–6848. [Google Scholar] [CrossRef]
- Cui, D.; Liu, X.; Wu, T.; Lin, X.; Luo, X.; Wu, Y.; Segawa, H.; Yang, X.; Zhang, Y.; Wang, Y.; et al. Making Room for Growing Oriented FASnI3 with Large Grains via Cold Precursor Solution. Adv. Funct. Mater. 2021, 31, 2100931. [Google Scholar] [CrossRef]
- Jang, G.; Kwon, H.; Ma, S.; Yun, S.; Yang, H.; Moon, J. Cold Antisolvent Bathing Derived Highly Efficient Large-Area Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1901719. [Google Scholar] [CrossRef]
- Taherianfard, H.; Kim, G.W.; Byranvand, M.M.; Choi, K.; Kang, G.; Choi, H.; Tajabadi, F.; Taghavinia, N.; Park, T. Effective Management of Nucleation and Crystallization Processes in Perovskite Formation via Facile Control of Antisolvent Temperature. ACS Appl. Energy Mater. 2020, 3, 1506–1514. [Google Scholar] [CrossRef]
- Lohmann, K.B.; Patel, J.B.; Rothmann, M.U.; Xia, C.Q.; Oliver, R.D.J.; Herz, L.M.; Snaith, H.J.; Johnston, M.B. Control over Crystal Size in Vapor Deposited Metal-Halide Perovskite Films. ACS Energy Lett. 2020, 5, 710–717. [Google Scholar] [CrossRef]
- Jang, G.; Ma, S.; Kwon, H.C.; Goh, S.; Ban, H.; Kim, J.S.; Kim, J.H.; Moon, J. Elucidation of the Formation Mechanism of Highly Oriented Multiphase Ruddlesden-Popper Perovskite Solar Cells. ACS Energy Lett. 2021, 6, 249–260. [Google Scholar] [CrossRef]
- You, J.; Hong, Z.; Song, T.-B.; Meng, L.; Liu, Y.; Jiang, C.; Zhou, H.; Chang, W.H.; Li, G.; Yang, Y. Moisture Assisted Perovskite Film Growth for High Performance Solar Cells. Appl. Phys. Lett. 2014, 105, 183902. [Google Scholar] [CrossRef]
- Anderson, P.J.; Morgan, P.L. Effects of Water Vapour on Sintering of MgO. Trans. Faraday Soc. 1964, 60, 930–937. [Google Scholar] [CrossRef]
- Urai, J.L.; Spiers, C.J.; Zwart, H.J.; Lister, G.S. Weakening of Rock Salt by Water during Long-Term Creep. Nature 1986, 324, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Janos, L. Ural. Water Assisted Dynamic Recrystallization and Weakening in Polycrystalline Bischofite. Tectonophysics 1983, 96, 125–157. [Google Scholar] [CrossRef]
- Bi, C.; Shao, Y.; Yuan, Y.; Xiao, Z.; Wang, C.; Gao, Y.; Huang, J. Understanding the Formation and Evolution of Interdiffusion Grown Organolead Halide Perovskite Thin Films by Thermal Annealing. J. Mater. Chem. A 2014, 2, 18508–18514. [Google Scholar] [CrossRef]
- Zhou, Y.; Game, O.S.; Pang, S.; Padture, N.P. Microstructures of Organometal Trihalide Perovskites for Solar Cells: Their Evolution from Solutions and Characterization. J. Phys. Chem. Lett. 2015, 6, 4827–4839. [Google Scholar] [CrossRef]
- Liang, J.; Hu, X.; Wang, C.; Liang, C.; Chen, C.; Xiao, M.; Li, J.; Tao, C.; Xing, G.; Yu, R.; et al. Origins and Influences of Metallic Lead in Perovskite Solar Cells. Joule 2022, 6, 816–833. [Google Scholar] [CrossRef]
- Mai, C.L.; Zhou, Q.; Xiong, Q.; Chen, C.C.; Xu, J.; Zhang, Z.; Lee, H.W.; Yeh, C.Y.; Gao, P. Donor–π–Acceptor Type Porphyrin Derivatives Assisted Defect Passivation for Efficient Hybrid Perovskite Solar Cells. Adv. Funct. Mater. 2021, 31, 2007762. [Google Scholar] [CrossRef]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva-Acuña, C. Multi-Phase Microstructures Drive Exciton Dissociation in Neat Semicrystalline Polymeric Semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef]
- Khelifi, S.; Decock, K.; Lauwaert, J.; Vrielinck, H.; Spoltore, D.; Piersimoni, F.; Manca, J.; Belghachi, A.; Burgelman, M. Investigation of Defects by Admittance Spectroscopy Measurements in Poly (3-Hexylthiophene):(6,6)-Phenyl C61-Butyric Acid Methyl Ester Organic Solar Cells Degraded under Air Exposure. J. Appl. Phys. 2011, 110, 094509. [Google Scholar] [CrossRef]
- Shao, Y.; Xiao, Z.; Bi, C.; Yuan, Y.; Huang, J. Origin and Elimination of Photocurrent Hysteresis by Fullerene Passivation in CH3NH3PbI3 Planar Heterojunction Solar Cells. Nat. Commun. 2014, 5, 5784. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, J.; Yang, Z.; Yang, D.; Ren, X.; Xu, H.; Yang, Z.; Liu, S.F. 20-Mm-Large Single-Crystalline Formamidinium-Perovskite Wafer for Mass Production of Integrated Photodetectors. Adv. Opt. Mater. 2016, 4, 1829–1837. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Xiong, Q.; Hu, C.; Wang, C.; Zhang, N.; Lien, S.-Y.; Gao, P. Improving Crystallization and Stability of Perovskite Solar Cells Using a Low-Temperature Treated A-Site Cation Solution in the Sequential Deposition. Molecules 2023, 28, 4103. https://doi.org/10.3390/molecules28104103
Li T, Xiong Q, Hu C, Wang C, Zhang N, Lien S-Y, Gao P. Improving Crystallization and Stability of Perovskite Solar Cells Using a Low-Temperature Treated A-Site Cation Solution in the Sequential Deposition. Molecules. 2023; 28(10):4103. https://doi.org/10.3390/molecules28104103
Chicago/Turabian StyleLi, Tinghao, Qiu Xiong, Chongzhu Hu, Can Wang, Ni Zhang, Shui-Yang Lien, and Peng Gao. 2023. "Improving Crystallization and Stability of Perovskite Solar Cells Using a Low-Temperature Treated A-Site Cation Solution in the Sequential Deposition" Molecules 28, no. 10: 4103. https://doi.org/10.3390/molecules28104103
APA StyleLi, T., Xiong, Q., Hu, C., Wang, C., Zhang, N., Lien, S.-Y., & Gao, P. (2023). Improving Crystallization and Stability of Perovskite Solar Cells Using a Low-Temperature Treated A-Site Cation Solution in the Sequential Deposition. Molecules, 28(10), 4103. https://doi.org/10.3390/molecules28104103








