Nanocomposites Based on Spin-Crossover Nanoparticles and Silica-Coated Gold Nanorods: A Nonlinear Optical Study
Abstract
:1. Introduction
2. Results and Discussion
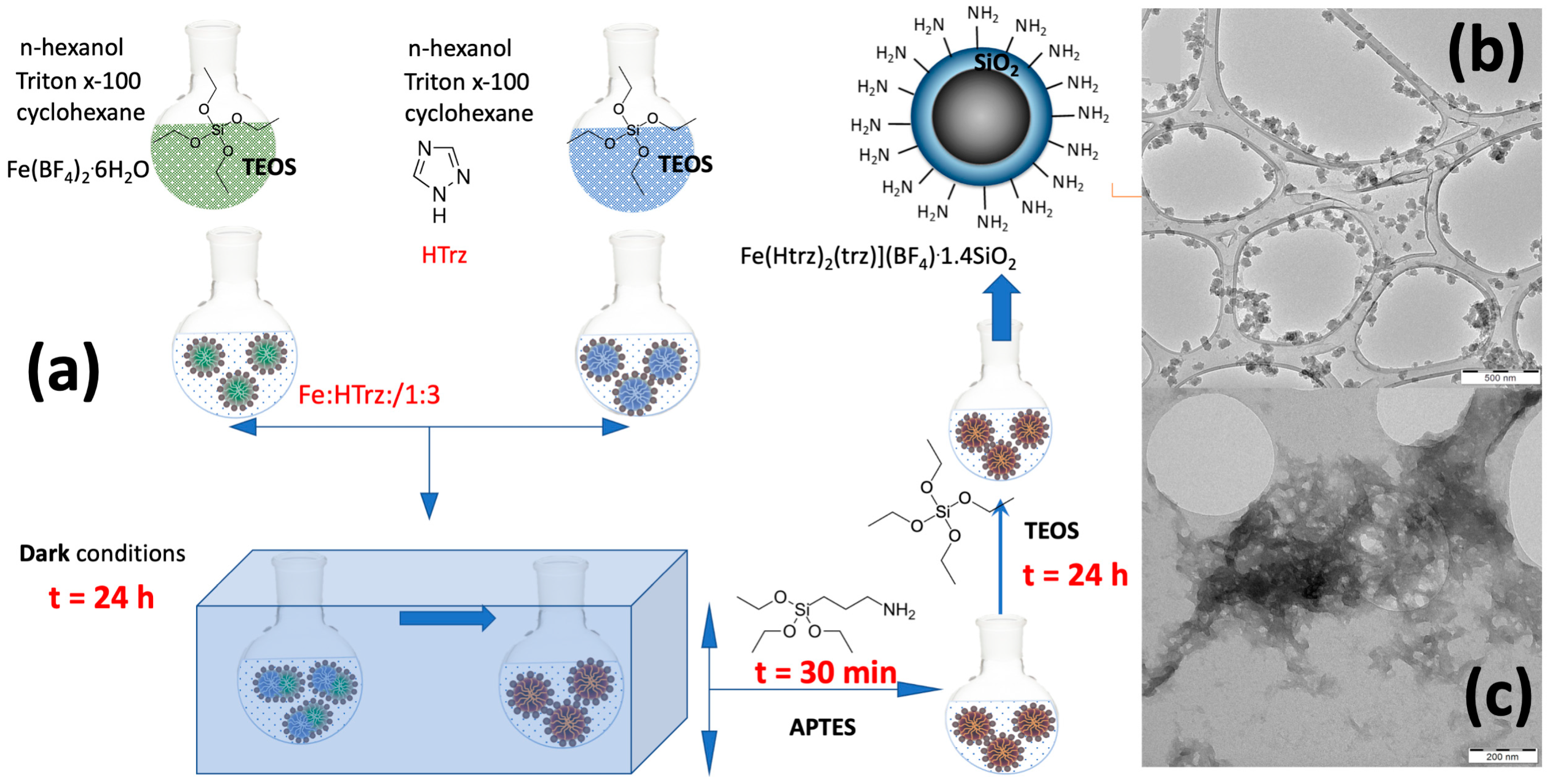
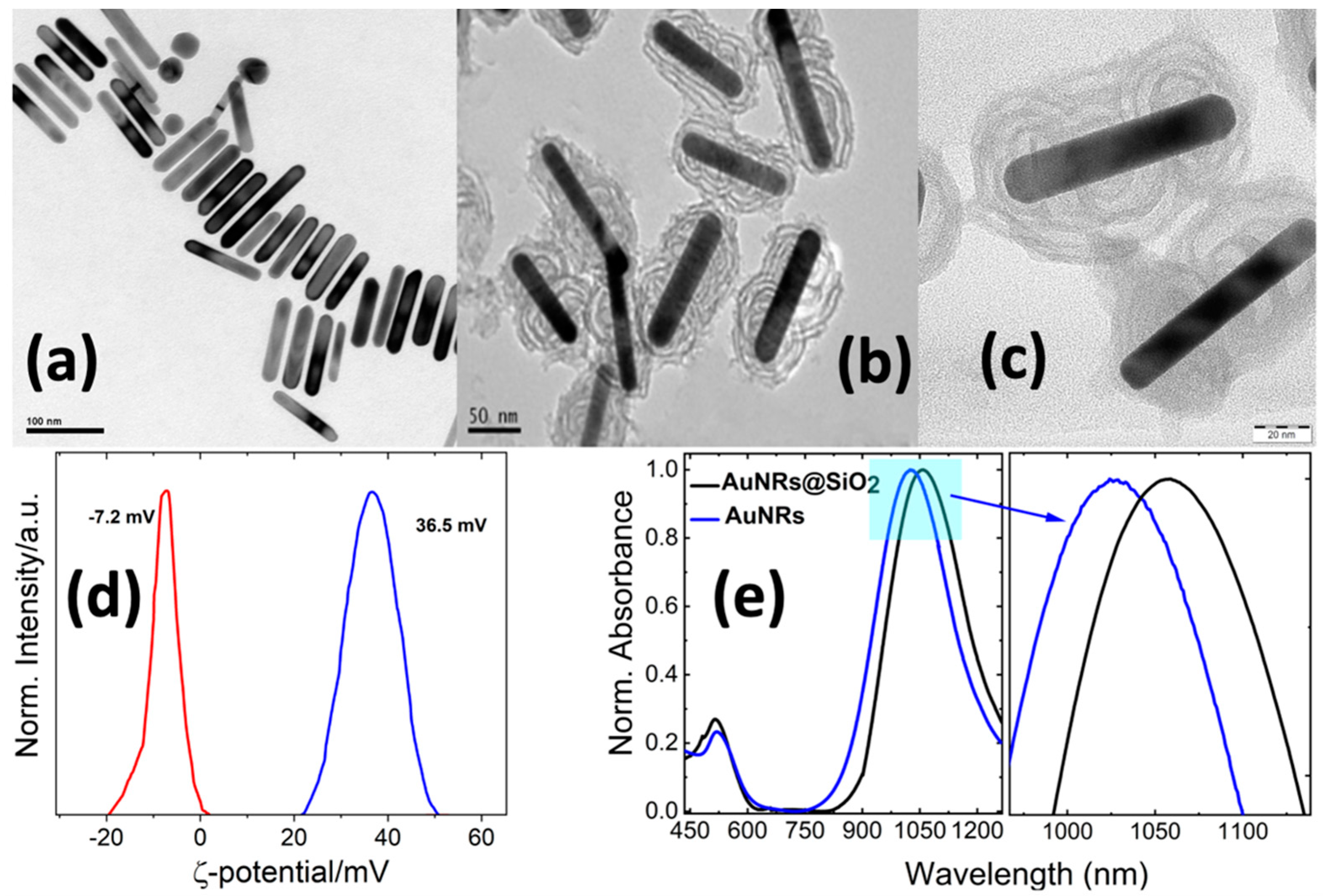
2.1. General Synthetic Aspects
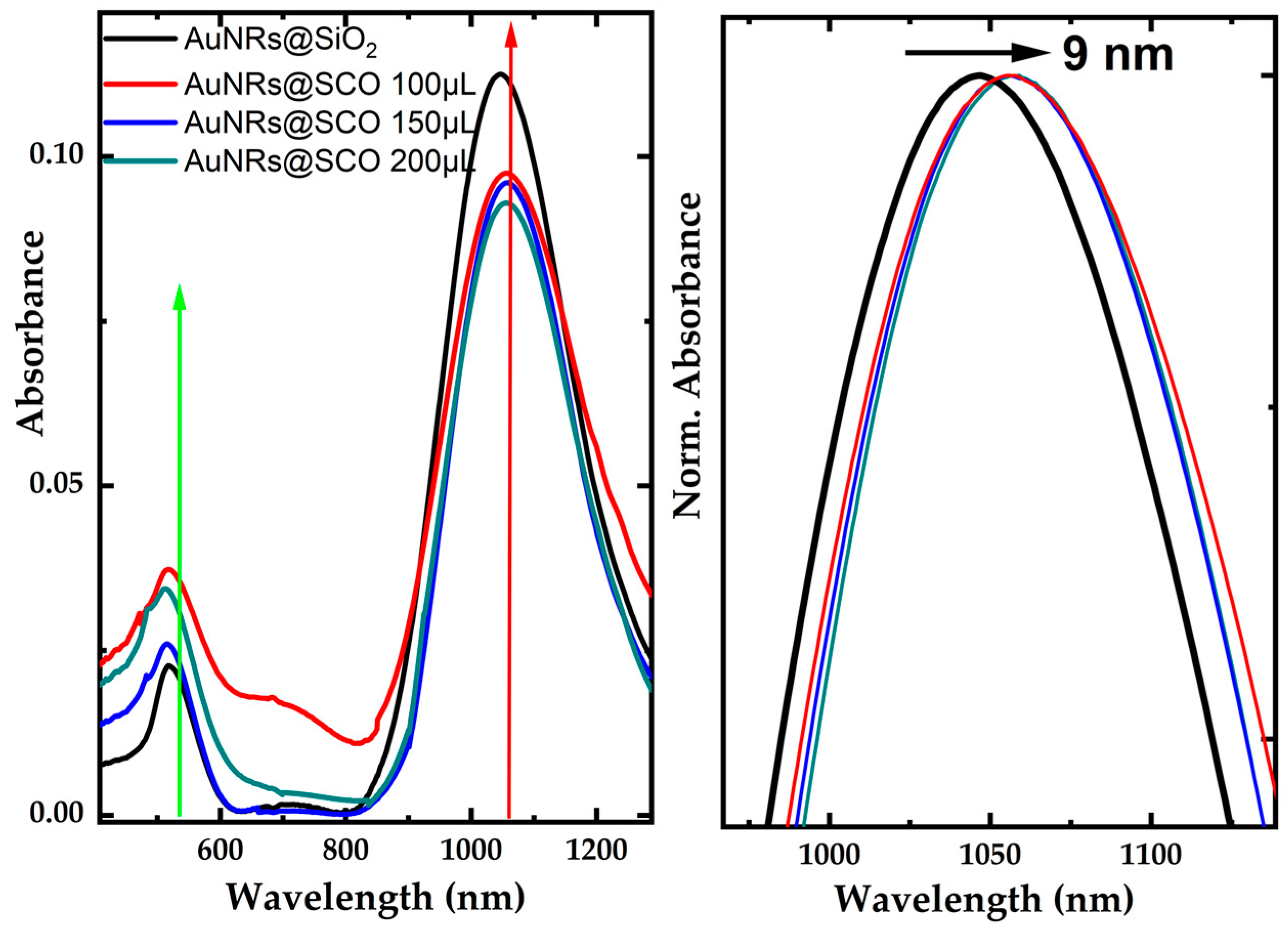
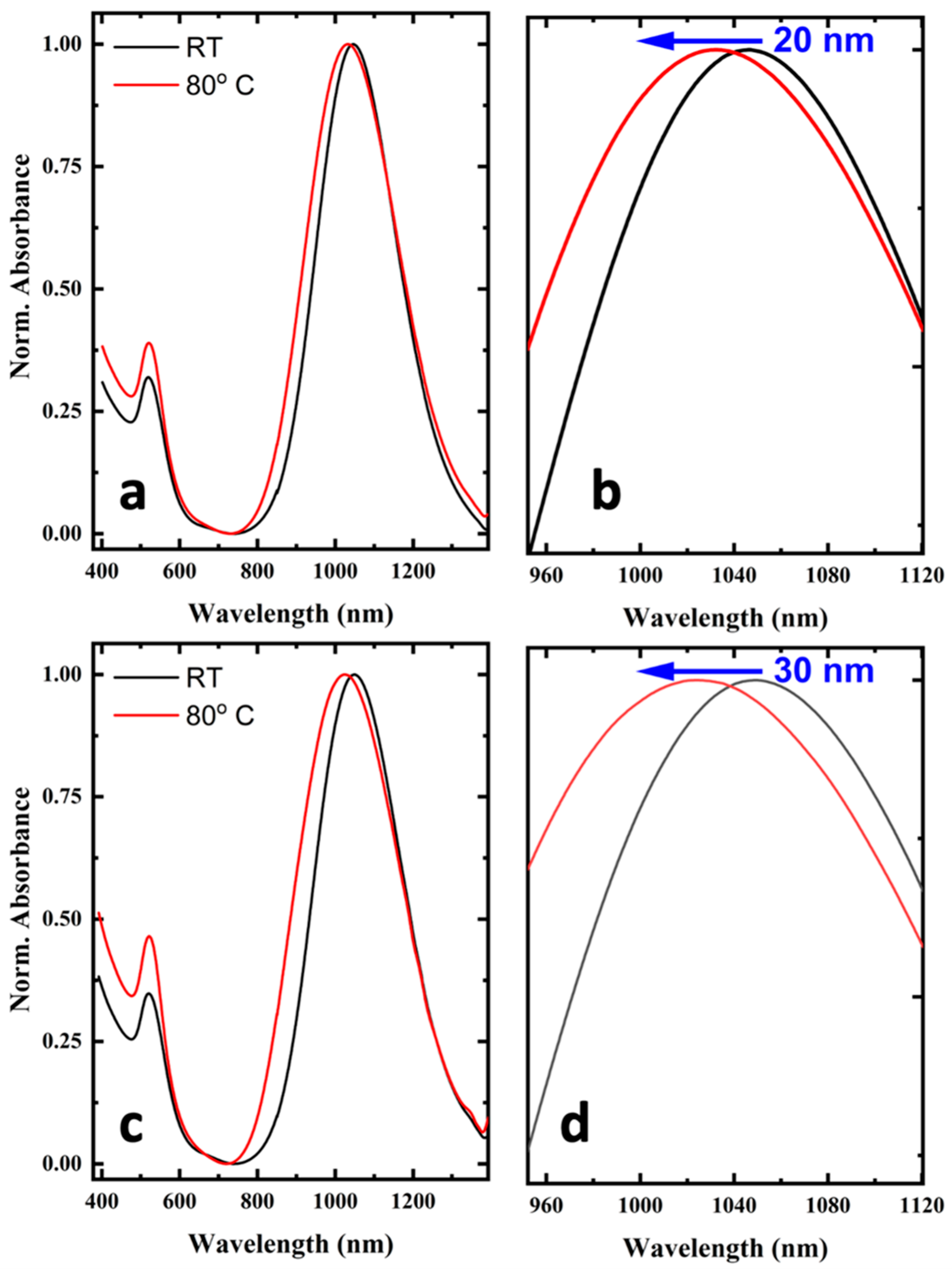
2.2. UV–Vis–NIR Absorption Measurements of AuNRs@SCO
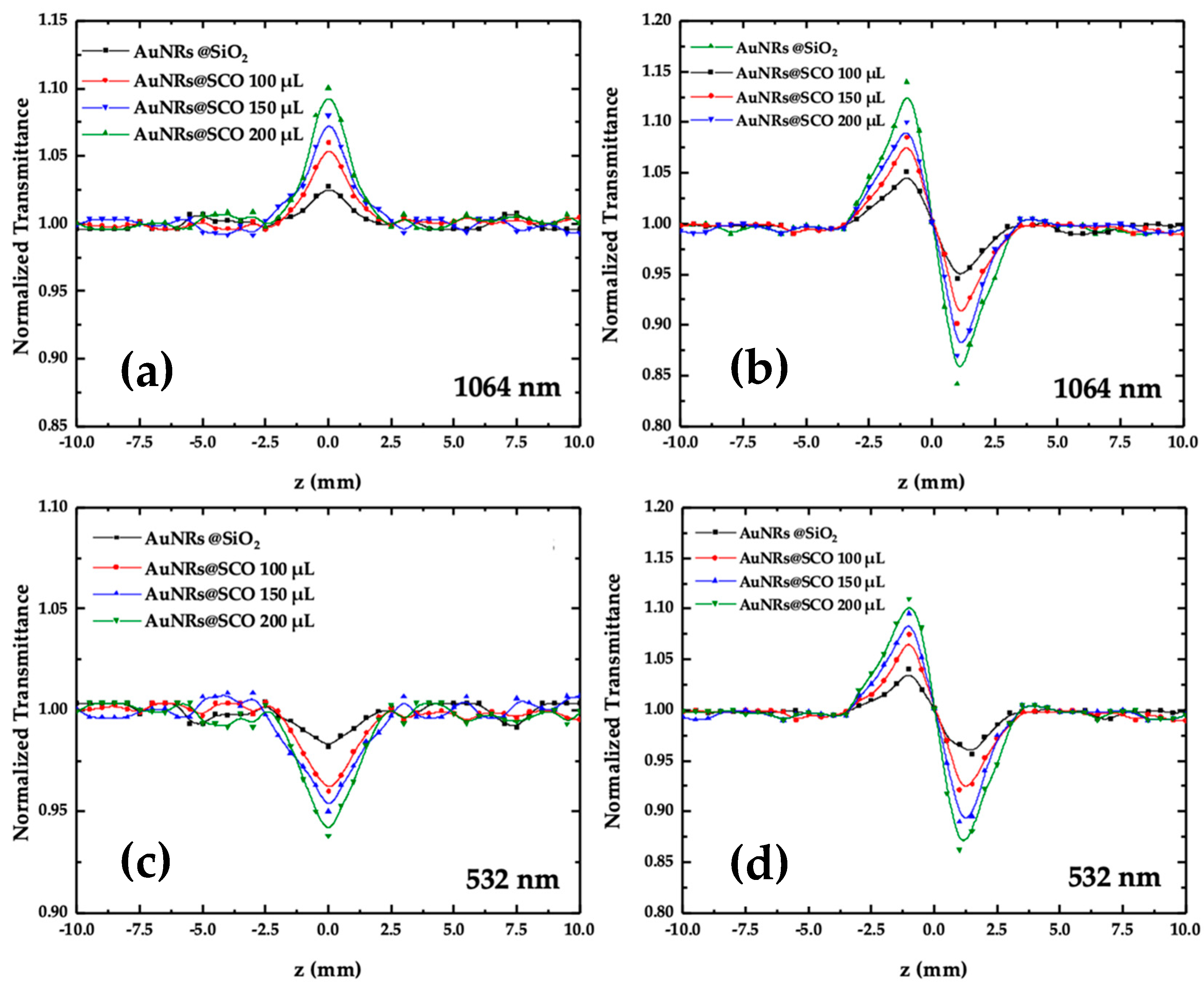
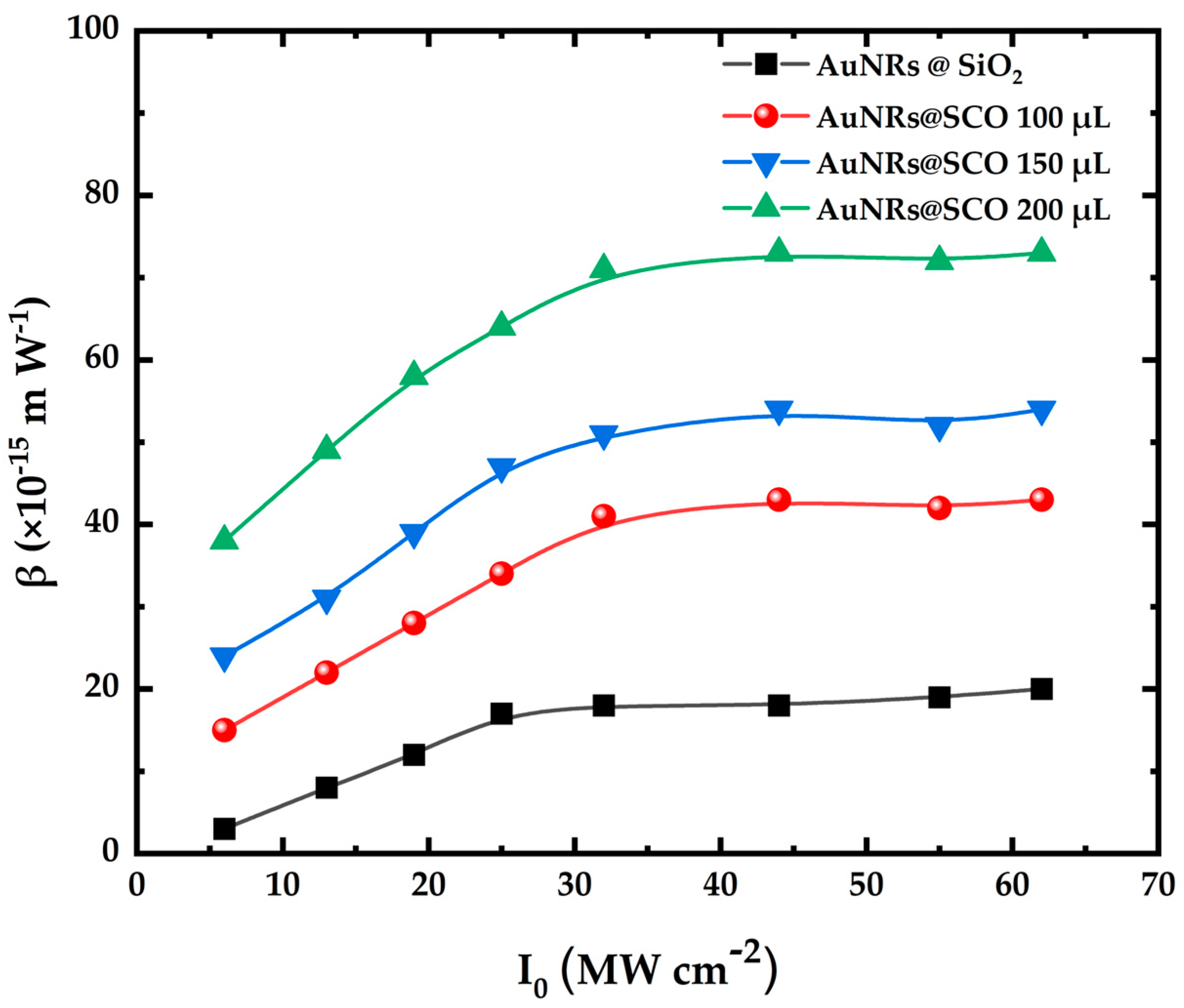
2.3. Nonlinear Optical Properties of AuNRs@SCO
2.4. Investigation of SCO Phenomenon in AuNRs@SCO and Dependence of NLO Response on Spin Transition
3. Materials and Methods
3.1. Material–Instrumentation–Physical Measurements
3.2. Synthesis of Gold Nanorods (AuNRs) and Silica-Covered AuNRs@SiO2
3.3. Synthesis of Spin-Crossover Nanoparticles [Fe(Htrz)2(trz)](BF4).1.4SiO2.0.7H2O.0.4Acetone (SCO)
3.4. Synthesis of AuNRs@SiO2@SCO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hayami, S.; Holmes, S.M.; Halcrow, M.A. Spin-state switches in molecular materials chemistry. J. Mater. Chem. C 2015, 3, 7775–7778. [Google Scholar] [CrossRef]
- Gutlich, P.; Gaspar, A.B.; Garcia, Y. Spin state switching in iron coordination compounds. Beilstein J. Org. Chem. 2013, 9, 342–391. [Google Scholar] [CrossRef]
- Bousseksou, A.; Molnar, G.; Salmon, L.; Nicolazzi, W. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef]
- Murray, K.S.; Oshio, H.; Real, J.A. Spin-Crossover Complexes. Eur. J. Inorg. Chem. 2013, 2013, 577–580. [Google Scholar] [CrossRef]
- Lefter, C.; Davesne, V.; Salmon, L.; Molnár, G.; Demont, P.; Rotaru, A.; Bousseksou, A. Charge Transport and Electrical Properties of Spin Crossover Materials: Towards Nanoelectronic and Spintronic Devices. Magnetochemistry 2016, 2, 18. [Google Scholar] [CrossRef]
- Aragones, A.C.; Aravena, D.; Valverde-Munoz, F.J.; Real, J.A.; Sanz, F.; Diez-Perez, I.; Ruiz, E. Metal-Controlled Magnetoresistance at Room Temperature in Single-Molecule Devices. J. Am. Chem. Soc. 2017, 139, 5768–5778. [Google Scholar] [CrossRef]
- Prins, F.; Monrabal-Capilla, M.; Osorio, E.A.; Coronado, E.; van der Zant, H.S.J. Room-Temperature Electrical Addressing of a Bistable Spin-Crossover Molecular System. Adv. Mater. 2011, 23, 1545–1549. [Google Scholar] [CrossRef]
- Lefter, C.; Tan, R.; Tricard, S.; Dugay, J.; Molnar, G.; Salmon, L.; Carrey, J.; Rotaru, A.; Bousseksou, A. On the stability of spin crossover materials: From bulk samples to electronic devices. Polyhedron 2015, 102, 434–440. [Google Scholar] [CrossRef]
- Zhang, X.; Palamarciuc, T.; Letard, J.F.; Rosa, P.; Lozada, E.V.; Torres, F.; Rosa, L.G.; Doudin, B.; Dowben, P.A. The spin state of a molecular adsorbate driven by the ferroelectric substrate polarization. Chem. Commun. 2014, 50, 2255–2257. [Google Scholar] [CrossRef]
- Kumar, K.S.; Ruben, M. Emerging trends in spin crossover (SCO) based functional materials and devices. Coord. Chem. Rev. 2017, 346, 176–205. [Google Scholar] [CrossRef]
- Bellec, A.; Lagoute, J.; Repain, V. Molecular electronics: Scanning tunneling microscopy and single-molecule devices. C. R. Chim. 2018, 21, 1287–1299. [Google Scholar] [CrossRef]
- Martinho, P.N.; Rajnak, C.; Ruben, M. Spin-Crossover Materials: Properties and Applications, 1st ed.; Halcrow, M.A., Ed.; John Wiley & Sons: Chichester, UK, 2013; p. 375. [Google Scholar]
- Tissot, A.; Rechignat, L.; Bousseksou, A.; Boillot, M.L. Micro- and nanocrystals of the iron(III) spin-transition material [FeIII(3-MeO-SalEen)2]PF6. J. Mater. Chem. 2012, 22, 3411–3419. [Google Scholar] [CrossRef]
- Salmon, L.; Catala, L. Spin-crossover nanoparticles and nanocomposite materials. C. R. Chim. 2018, 21, 1230–1269. [Google Scholar] [CrossRef]
- Coronado, E.; Galan-Mascaros, J.R.; Monrabal-Capilla, M.; Garcia-Martinez, J.; Pardo-Ibanez, P. Bistable spin-crossover nanoparticles showing magnetic thermal hysteresis near room temperature. Adv. Mater. 2007, 19, 1359–1361. [Google Scholar] [CrossRef]
- Forestier, T.; Kaiba, A.; Pechev, S.; Denux, D.; Guionneau, P.; Etrillard, C.; Daro, N.; Freysz, E.; Letard, J.F. Nanoparticles of [Fe(NH2-trz)3]Br2·3H2O (NH2-trz=2-Amino-1,2,4-triazole) Prepared by the Reverse Micelle Technique: Influence of Particle and Coherent Domain Sizes on Spin-Crossover Properties. Chem.-Eur. J. 2009, 15, 6122–6130. [Google Scholar] [CrossRef]
- Gural’skiy, I.A.; Quintero, C.M.; Molnar, G.; Fritsky, I.O.; Salmon, L.; Bousseksou, A. Synthesis of Spin-Crossover Nano- and Micro-objects in Homogeneous Media. Chem.-Eur. J. 2012, 18, 9946–9954. [Google Scholar] [CrossRef] [PubMed]
- Bartual-Murgui, C.; Natividad, E.; Roubeau, O. Critical assessment of the nature and properties of Fe(II) triazole-based spin-crossover nanoparticles. J. Mater. Chem. C 2015, 3, 7916–7924. [Google Scholar] [CrossRef]
- Moulet, L.; Daro, N.; Etrillard, C.; Létard, J.-F.; Grosjean, A.; Guionneau, P. Rational Control of Spin-Crossover Particle Sizes: From Nano- to Micro-Rods of [Fe(Htrz)2(trz)](BF4). Magnetochemistry 2016, 2, 10. [Google Scholar] [CrossRef]
- Gimenez-Marques, M.; de Larrea, M.L.G.S.; Coronado, E. Unravelling the chemical design of spin-crossover nanoparticles based on iron(II)-triazole coordination polymers: Towards a control of the spin transition. J. Mater. Chem. C 2015, 3, 7946–7953. [Google Scholar] [CrossRef]
- Galan-Mascaros, J.R.; Coronado, E.; Forment-Aliaga, A.; Monrabal-Capilla, M.; Pinilla-Cienfuegos, E.; Ceolin, M. Tuning Size and Thermal Hysteresis in Bistable Spin Crossover Nanoparticles. Inorg. Chem. 2010, 49, 5706–5714. [Google Scholar] [CrossRef]
- Titos-Padilla, S.; Herrera, J.M.; Chen, X.W.; Delgado, J.J.; Colacio, E. Bifunctional Hybrid SiO2 Nanoparticles Showing Synergy between Core Spin Crossover and Shell Luminescence Properties. Angew. Chem. Int. Ed. 2011, 50, 3290–3293. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.M.; Titos-Padilla, S.; Pope, S.J.A.; Berlanga, I.; Zamora, F.; Delgado, J.J.; Kamenev, K.V.; Wang, X.; Prescimone, A.; Brechin, E.K.; et al. Studies on bifunctional Fe(II)-triazole spin crossover nanoparticles: Time-dependent luminescence, surface grafting and the effect of a silica shell and hydrostatic pressure on the magnetic properties. J. Mater. Chem. C 2015, 3, 7819–7829. [Google Scholar] [CrossRef]
- Rat, S.; Piedrahita-Bello, M.; Salmon, L.; Molnar, G.; Demont, P.; Bousseksou, A. Coupling Mechanical and Electrical Properties in Spin Crossover Polymer Composites. Adv. Mater. 2018, 30, 1705275. [Google Scholar] [CrossRef] [PubMed]
- Piedrahita-Bello, M.; Ridier, K.; Mikolasek, M.; Molnar, G.; Nicolazzi, W.; Salmon, L.; Bousseksou, A. Drastic lattice softening in mixed triazole ligand iron(ii) spin crossover nanoparticles. Chem. Commun. 2019, 55, 4769–4772. [Google Scholar] [CrossRef] [PubMed]
- Torres-Cavanillas, R.; Lima-Moya, L.; Tichelaar, F.D.; Zandbergen, H.W.; Gimenez-Marques, M.; Coronado, E. Downsizing of robust Fe-triazole@SiO2 spin-crossover nanoparticles with ultrathin shells. Dalton Trans. 2019, 48, 15465–15469. [Google Scholar] [CrossRef]
- Gkolfi, P.; Tsivaka, D.; Tsougos, I.; Vassiou, K.; Malina, O.; Polaskova, M.; Polyzou, C.; Chasapis, C.; Tangoulis, V. A facile approach to prepare silica hybrid, spin-crossover water-soluble nanoparticles as potential candidates for thermally responsive MRI agents. Dalton Trans. 2021, 50, 13227–13231. [Google Scholar] [CrossRef]
- Polyzou, C.D.; Gkolfi, P.; Chasapis, C.T.; Bekiari, V.; Zianna, A.; Psomas, G.; Ondrej, M.; Tangoulis, V. Stimuli-responsive spin crossover nanoparticles for drug delivery and DNA-binding studies. Dalton Trans. 2022, 51, 12427–12431. [Google Scholar] [CrossRef]
- Lalioti, N.; Giannopoulou, E.; Charitos, A.; Parthenios, J.; Malina, O.; Polaskova, M.; Kalarakis, A.; Tangoulis, V. Observation of two-step spin transition in iron(ii) 4-amino-1,2,4-triazole based spin crossover nanoparticles. Dalton Trans. 2023, 52, 2937–2941. [Google Scholar] [CrossRef]
- Suleimanov, I.; Kraieva, O.; Costa, J.S.; Fritsky, I.O.; Molnar, G.; Salmon, L.; Bousseksou, A. Electronic communication between fluorescent pyrene excimers and spin crossover complexes in nanocomposite particles. J. Mater. Chem. C 2015, 3, 5026–5032. [Google Scholar] [CrossRef]
- Suleimanov, I.; Kraieva, O.; Molnar, G.; Salmon, L.; Bousseksou, A. Enhanced luminescence stability with a Tb-spin crossover nanocomposite for spin state monitoring. Chem. Commun. 2015, 51, 15098–15101. [Google Scholar] [CrossRef]
- Suleimanov, I.; Costa, J.S.; Molnar, G.; Salmon, L.; Bousseksou, A. The photo-thermal plasmonic effect in spin crossover@silica-gold nanocomposites. Chem. Commun. 2014, 50, 13015–13018. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Gu, L.; Sun, X.L.; Ren, D.H.; Gu, Z.G.; Li, Z.J. SCO@SiO2@Au core-shell nanomaterials: Enhanced photo-thermal plasmonic effect and spin-crossover properties. RSC Adv. 2014, 4, 61313–61319. [Google Scholar] [CrossRef]
- Palluel, M.; Tran, N.M.; Daro, N.; Buffiere, S.; Mornet, S.; Freysz, E.; Chastanet, G. The Interplay between Surface Plasmon Resonance and Switching Properties in Gold@Spin Crossover Nanocomposites. Adv. Funct. Mater. 2020, 30, 2000447. [Google Scholar] [CrossRef]
- Torres-Cavanillas, R.; Sanchis-Gual, R.; Dugay, J.; Coronado-Puchau, M.; Gimenez-Marques, M.; Coronado, E. Design of Bistable Gold@Spin-Crossover Core-Shell Nanoparticles Showing Large Electrical Responses for the Spin Switching. Adv. Mater. 2019, 31, 190039. [Google Scholar] [CrossRef]
- Sanchis-Gual, R.; Torres-Cavanillas, R.; Coronado-Puchau, M.; Gimenez-Marques, M.; Coronado, E. Plasmon-assisted spin transition in gold nanostar@spin crossover heterostructures. J Mater. Chem. C 2021, 9, 10811–10818. [Google Scholar] [CrossRef]
- Abdul-Kader, K.; Lopes, M.; Bartual-Murgui, C.; Kraieva, O.; Hernandez, E.M.; Salmon, L.; Nicolazzi, W.; Carcenac, F.; Thibault, C.; Molnar, G.; et al. Synergistic switching of plasmonic resonances and molecular spin states. Nanoscale 2013, 5, 5288–5293. [Google Scholar] [CrossRef]
- Lacroix, P.G.; Malfant, I.; Real, J.A.; Rodriguez, V. From Magnetic to Nonlinear Optical Switches in Spin-Crossover Complexes. Eur. J. Inorg. Chem. 2013, 5–6, 615–627. [Google Scholar] [CrossRef]
- Sheik-Bahae, M.; Said, A.A.; Van Stryland, E.W. High-sensitivity, single-beam n(2) measurements. Opt. Lett. 1989, 14, 955–957. [Google Scholar] [CrossRef]
- Ye, X.C.; Zheng, C.; Chen, J.; Gao, Y.Z.; Murray, C.B. Using Binary Surfactant Mixtures to Simultaneously Improve the Dimensional Tunability and Monodispersity in the Seeded Growth of Gold Nanorods. Nano Lett. 2013, 13, 765–771. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Pellas, V.; Blanchard, J.; Guibert, C.; Krafft, J.M.; Miche, A.; Salmain, M.; Boujday, S. Gold Nanorod Coating with Silica Shells Having Controlled Thickness and Oriented Porosity: Tailoring the Shells for Biosensing. ACS Appl. Nano Mater. 2021, 4, 9842–9854. [Google Scholar] [CrossRef]
- Rowe, L.R.; Chapman, B.S.; Tracy, J.B. Understanding and Controlling the Morphology of Silica Shells on Gold Nanorods. Chem. Mater. 2018, 30, 6249–6258. [Google Scholar] [CrossRef]
- Chapman, B.S.; Wu, W.C.; Li, Q.C.; Holten-Andersen, N.; Tracy, J.B. Heteroaggregation Approach for Depositing Magnetite Nanoparticles onto Silica-Overcoated Gold Nanorods. Chem. Mater. 2017, 29, 10362–10368. [Google Scholar] [CrossRef]
- Zhai, Y.; Han, L.; Wang, P.; Li, G.; Ren, W.; Liu, L.; Wang, E.; Dong, S. Superparamagnetic Plasmonic Nanohybrids: Shape-Controlled Synthesis, TEM-Induced Structure Evolution, and Efficient Sunlight-Driven Inactivation of Bacteria. ACS Nano 2011, 5, 8562–8570. [Google Scholar] [CrossRef]
- Truby, R.L.; Emelianov, S.Y.; Homan, K.A. Ligand-Mediated Self-Assembly of Hybrid Plasmonic and Superparamagnetic Nanostructures. Langmuir 2013, 29, 2465–2470. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA, 1990. [Google Scholar]
- Inouye, H.; Tanaka, K.; Tanahashi, I.; Hirao, K. Ultrafast dynamics of nonequilibrium electrons in a gold nanoparticle system. Phys. Rev. B 1998, 57, 11334–11340. [Google Scholar] [CrossRef]
- Watanabe, K.; Menzel, D.; Nilius, N.; Freund, H.-J. Photochemistry on metal nanoparticles. Chem. Rev. 2006, 106, 4301–4320. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Christodoulides, D.; Khoo, I.; Salamo, G.; Stegeman, G.; Van Stryland, E. Nonlinear refraction and absorption: Mechanisms and magnitudes. Adv. Opt. Photonics 2010, 2, 60–200. [Google Scholar] [CrossRef]
- Rumi, M.; Perry, J. Two-photon absorption: An overview of measurements and principles. Adv. Opt. Photonics 2010, 2, 451–518. [Google Scholar] [CrossRef]
- Zygouri, E.; Bekiari, V.; Malis, G.; Karamanos, N.K.; Koutsakis, C.; Psomas, G.; Tangoulis, V. pH-Sensitive Gold Nanorods for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Delivery and DNA-Binding Studies. Molecules 2023, 28, 3780. [Google Scholar] [CrossRef] [PubMed]


| Excitation at 532 nm | Excitation at 1064 nm | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | β (×10−15 m/W) | γ′ (×10−18 m2/W) | n2 (×10−12 esu) | χ(3) (×10−16 esu) | β (×10−11 m/W) | γ′ (×10−18 m2/W) | n2 (×10−12 esu) | χ(3) (×10−13 esu) |
| AuNRs | 20 ± 4 | −37 ± 3 | −125 ± 10 | 48 ± 4 | −68 ± 8 | −80 ± 7 | −272 ± 24 | 57 ± 4 |
| AuNRs@SCO 100 μL | 43 ± 4 | −51 ± 6 | −173 ± 14 | 68 ± 5 | −86 ± 4 | −125 ± 8 | −425 ± 27 | 105 ± 6 |
| AuNRs@SCO 150 μL | 54 ± 5 | −66 ± 4 | −224 ± 20 | 90 ± 5 | −118 ± 15 | −152 ± 12 | −516 ± 41 | 120 ± 11 |
| AuNRs@SCO 200 μL | 73 ± 8 | −85 ± 4 | −289 ± 20 | 115 ± 11 | −130 ± 15 | −190 ± 20 | −646 ± 68 | 161 ± 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zygouri, E.; Stathis, A.; Couris, S.; Tangoulis, V. Nanocomposites Based on Spin-Crossover Nanoparticles and Silica-Coated Gold Nanorods: A Nonlinear Optical Study. Molecules 2023, 28, 4200. https://doi.org/10.3390/molecules28104200
Zygouri E, Stathis A, Couris S, Tangoulis V. Nanocomposites Based on Spin-Crossover Nanoparticles and Silica-Coated Gold Nanorods: A Nonlinear Optical Study. Molecules. 2023; 28(10):4200. https://doi.org/10.3390/molecules28104200
Chicago/Turabian StyleZygouri, Eleni, Aristeidis Stathis, Stelios Couris, and Vassilis Tangoulis. 2023. "Nanocomposites Based on Spin-Crossover Nanoparticles and Silica-Coated Gold Nanorods: A Nonlinear Optical Study" Molecules 28, no. 10: 4200. https://doi.org/10.3390/molecules28104200
APA StyleZygouri, E., Stathis, A., Couris, S., & Tangoulis, V. (2023). Nanocomposites Based on Spin-Crossover Nanoparticles and Silica-Coated Gold Nanorods: A Nonlinear Optical Study. Molecules, 28(10), 4200. https://doi.org/10.3390/molecules28104200








