Photosensitive Dye as an Ideal Peroxymonosulfate Activator for Efficient Self-Degradation: A Novel Idea of Using Waste to Treat Waste
Abstract
:1. Introduction
2. Results and Discussion
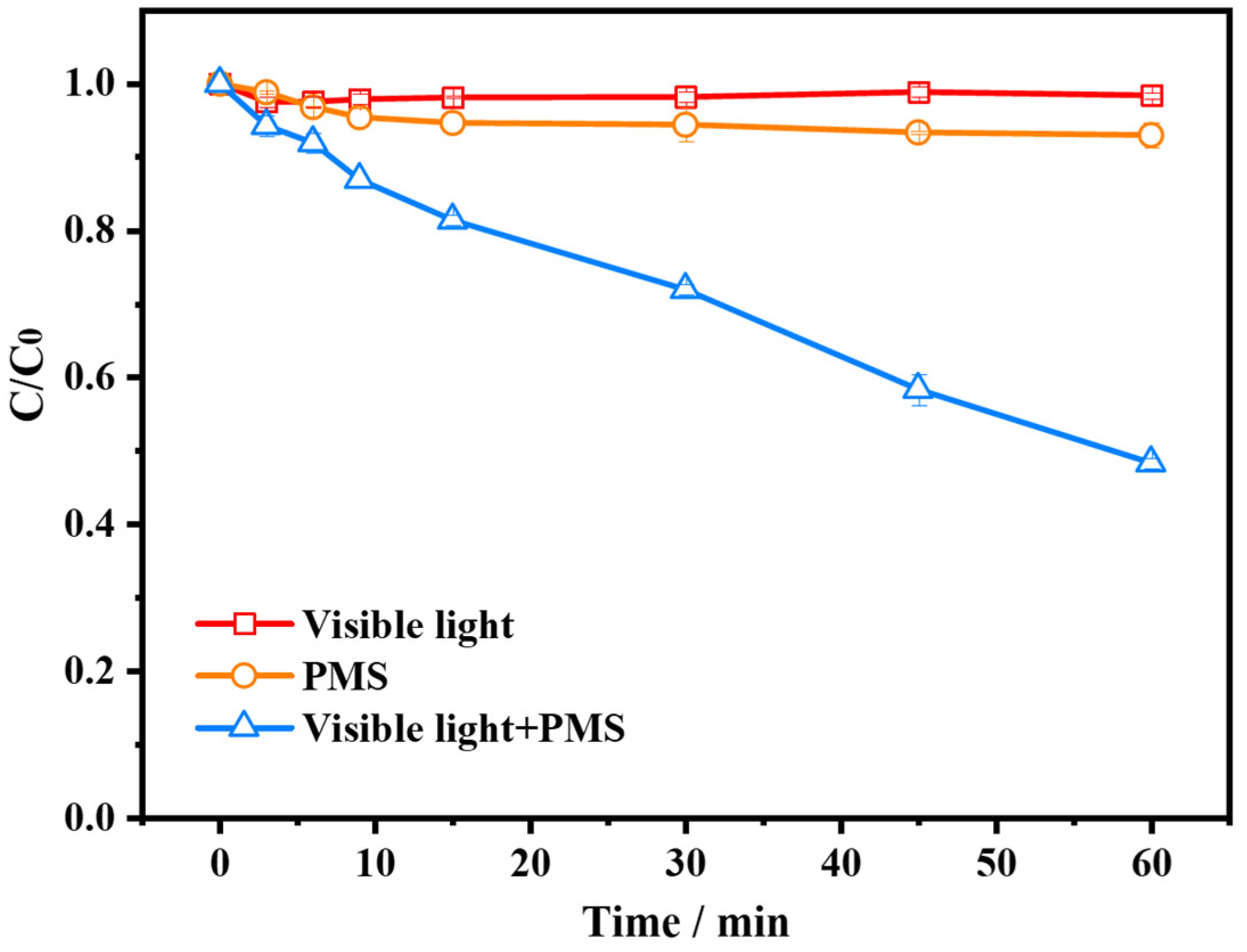
2.1. Performance of AG/PMS Self-Catalytic System
2.2. Effects of Different Parameters on AG/PMS Self-Catalytic System
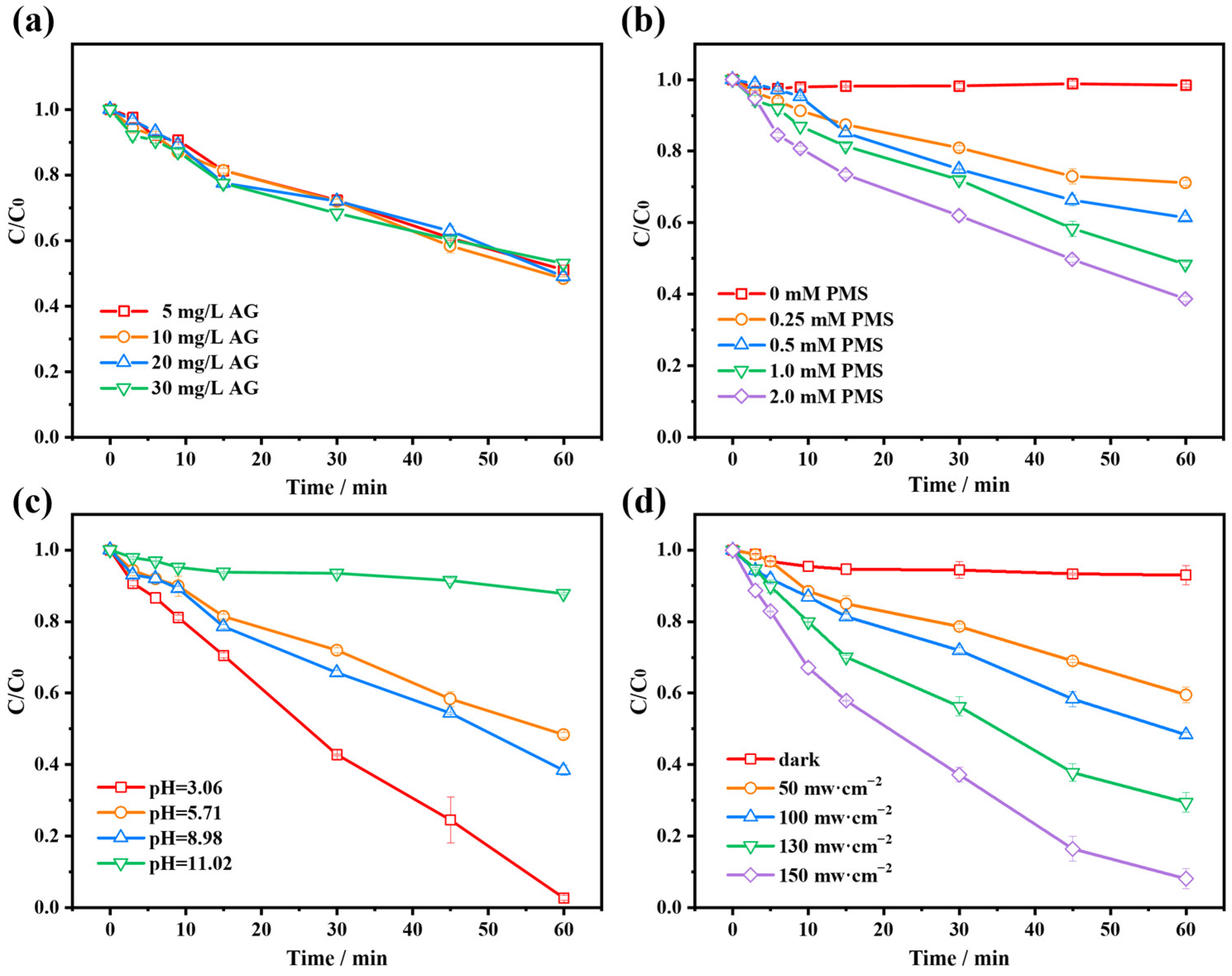
2.2.1. Initial AG Concentration
2.2.2. PMS Dosage
2.2.3. Initial Solution pH
2.2.4. Light Intensity
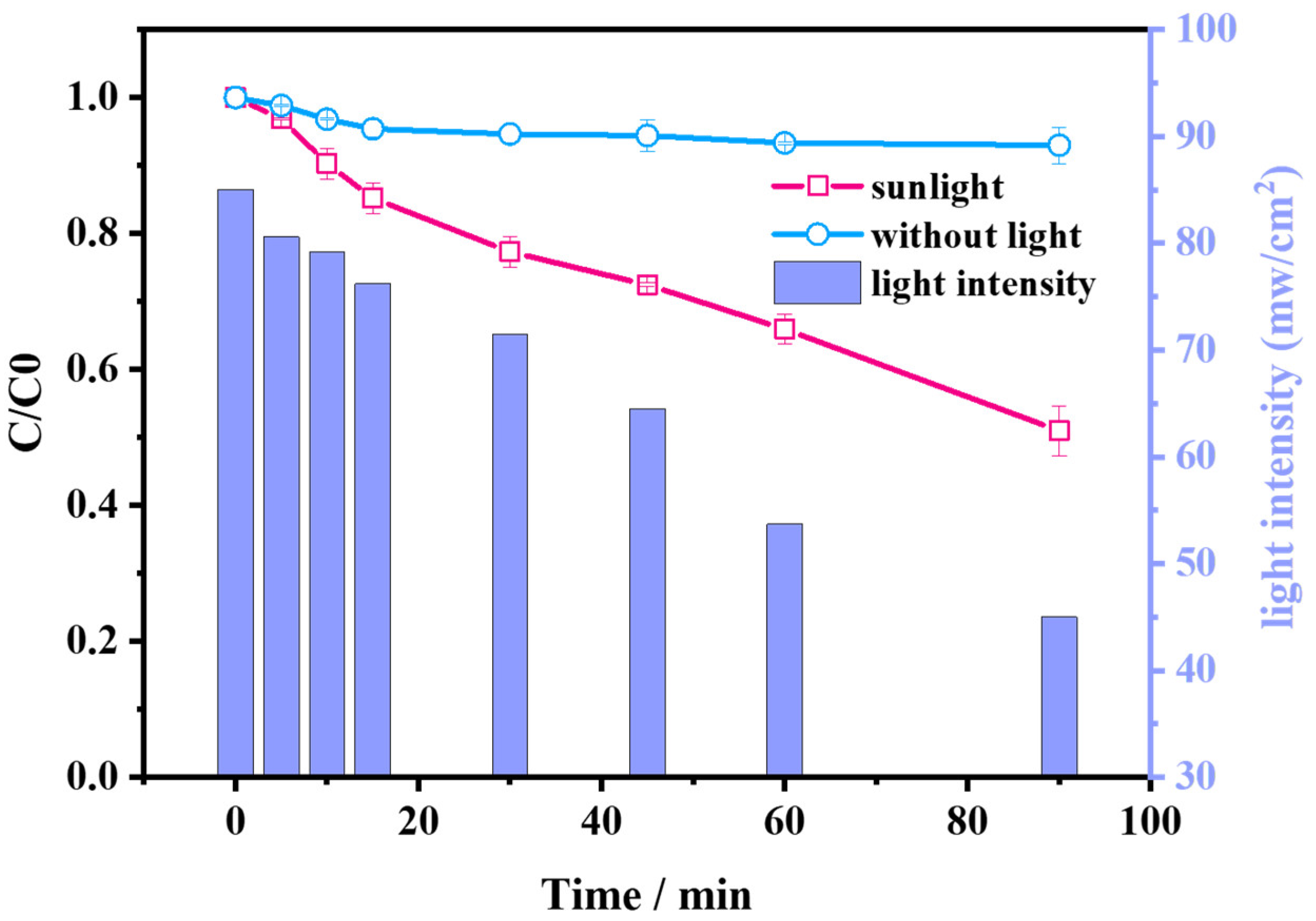
2.3. Application of AG/PMS Self-Catalytic System under Natural Sunlight Irradiation
2.4. Mechanism of AG Self-Activating PMS
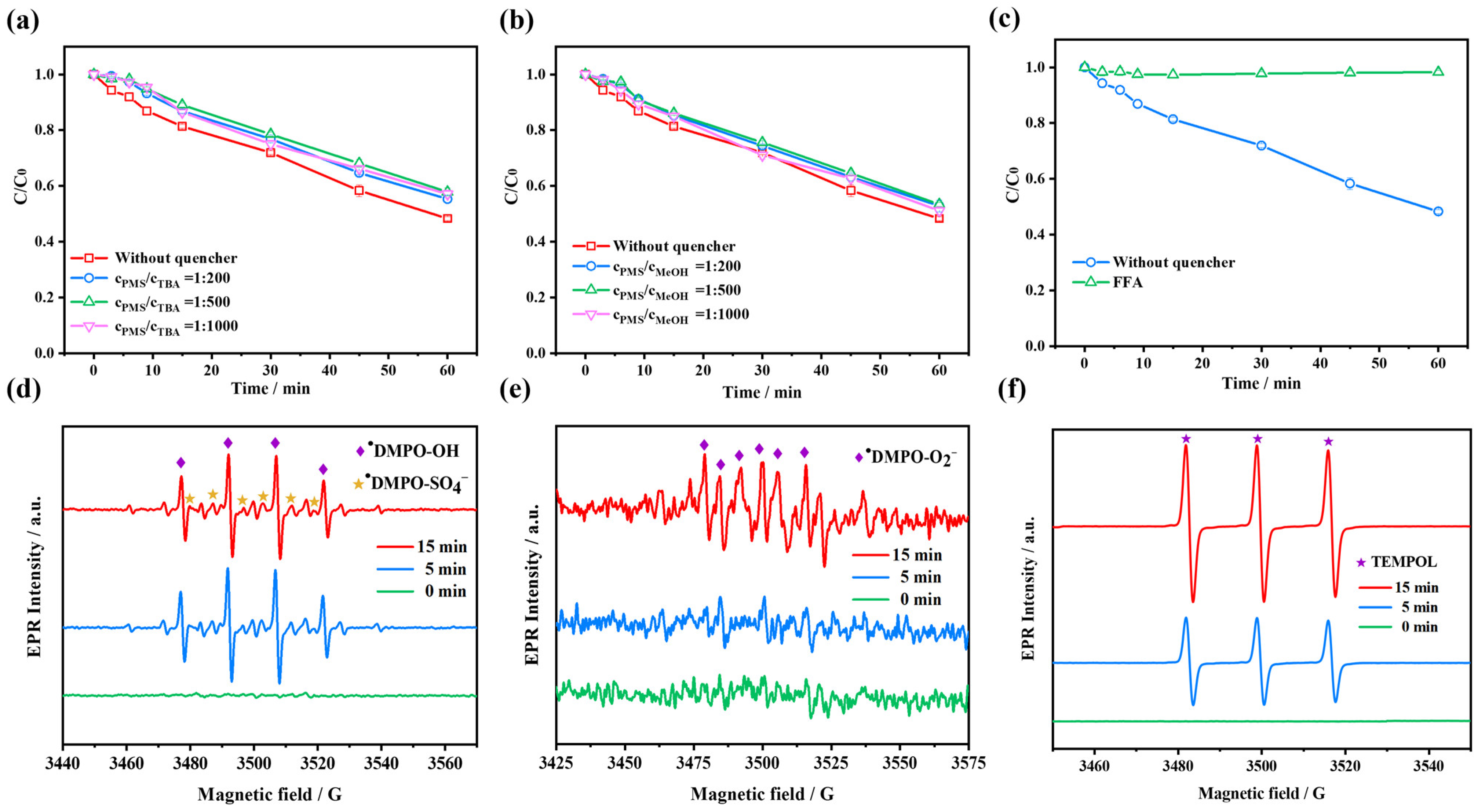
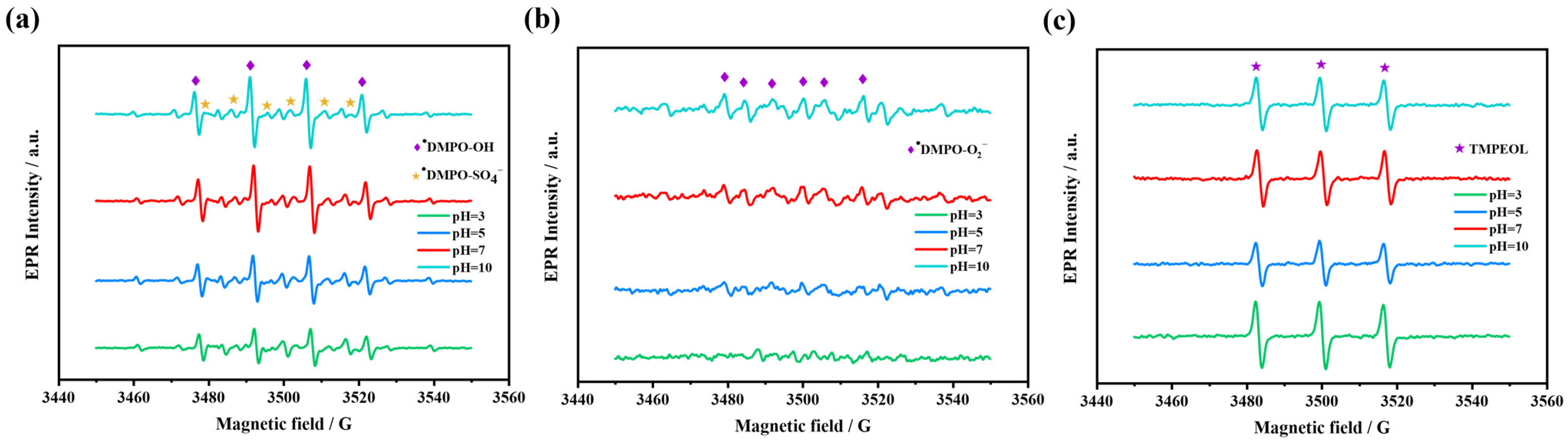
2.4.1. Identification of Reactive Oxidizing Species
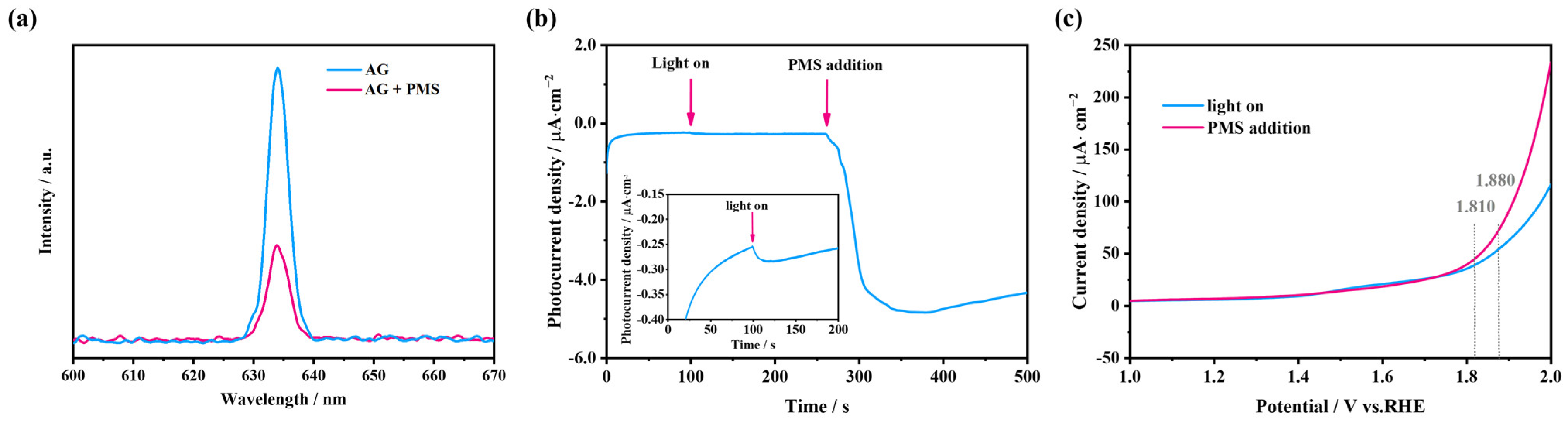
2.4.2. Characterization of Electron Transfer Processes
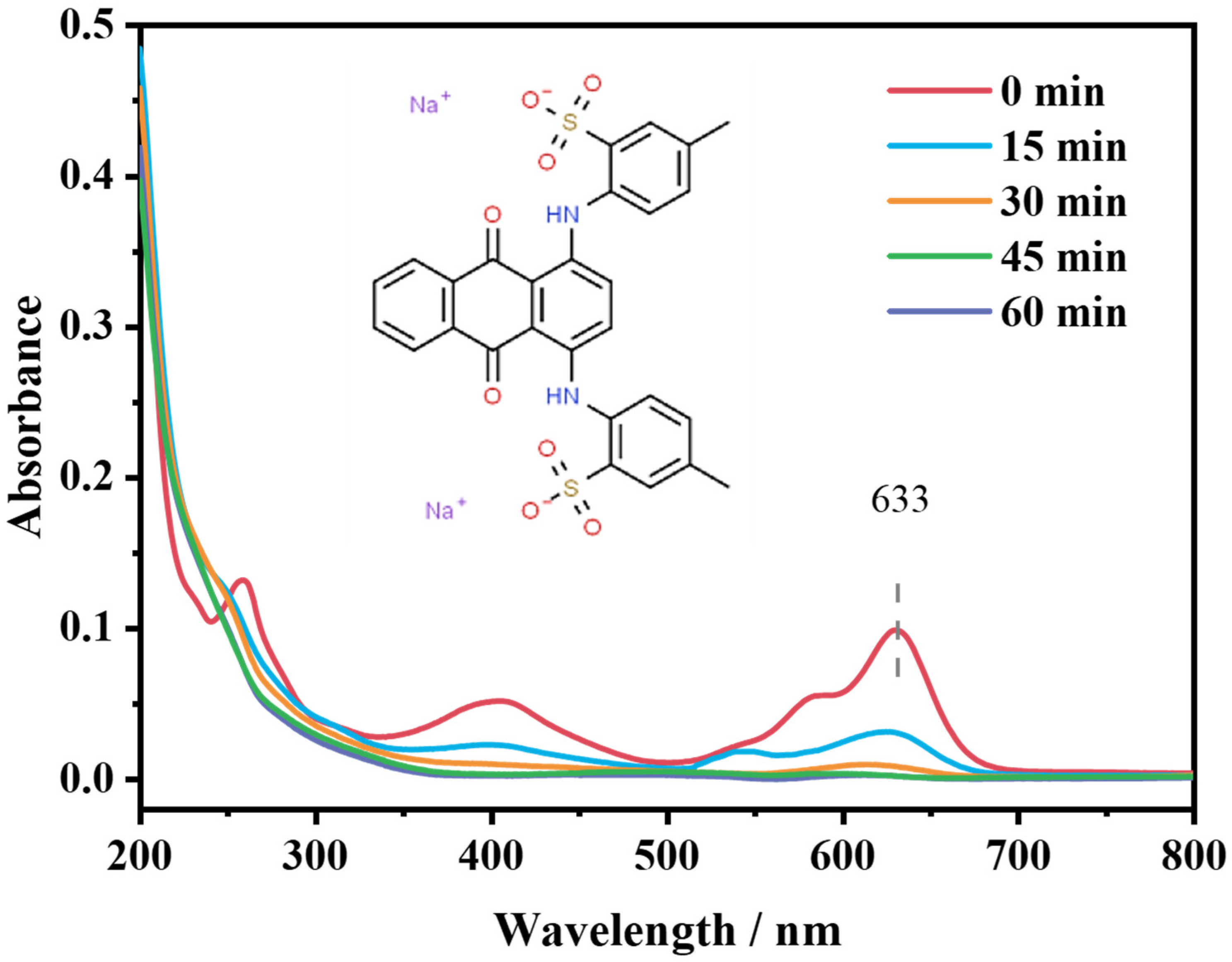
2.4.3. Degradation Route of AG
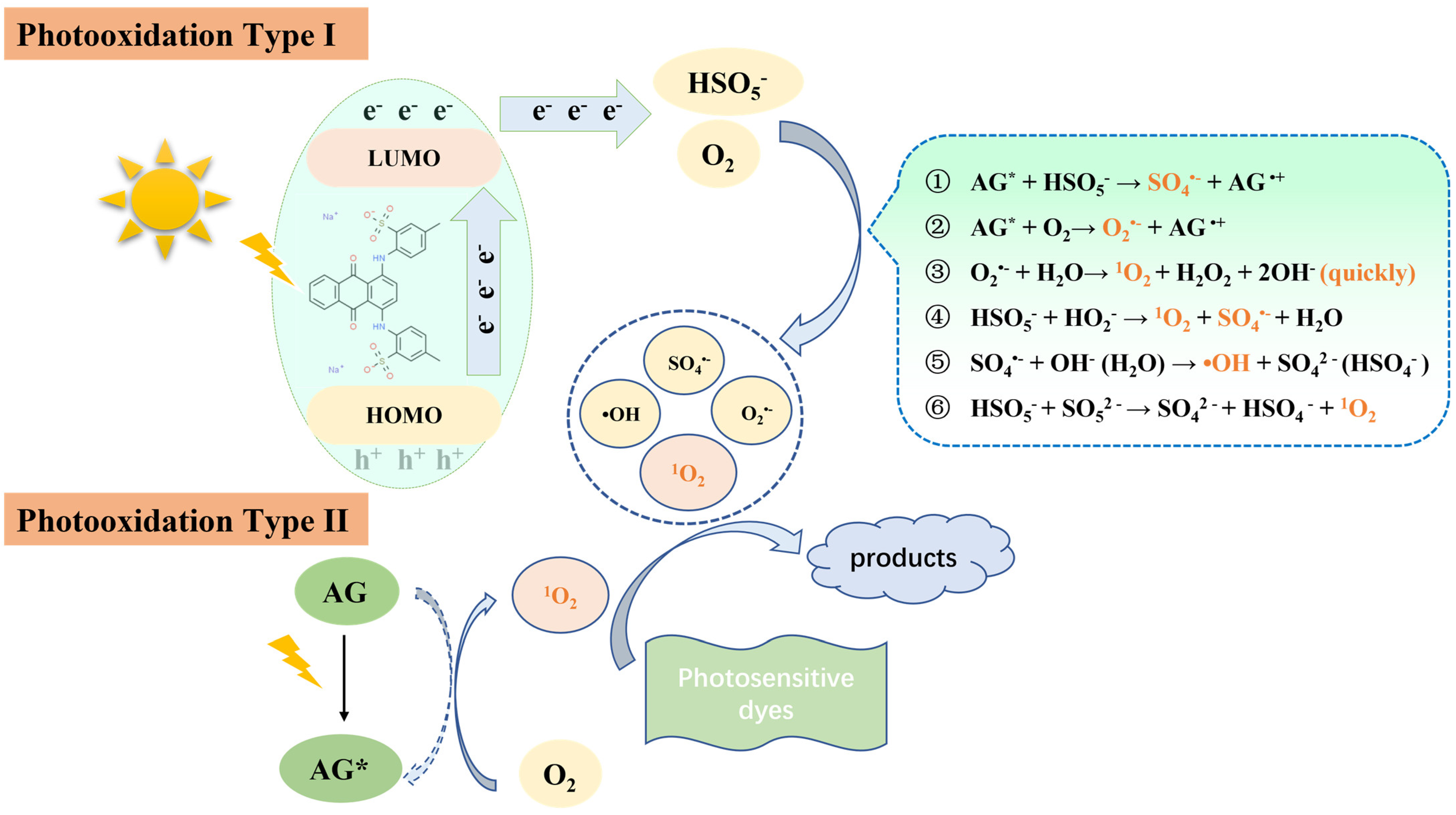
2.4.4. Mechanism of AG Self-Activating PMS
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Procedures
3.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, Z.; Fu, J.; Wang, M.; Wang, X.; Zhang, J.; Xu, Q. Adsorption of cationic dye (methylene blue) from aqueous solution using poly (cyclotriphosphazene-co-4,4′-sulfonyldiphenol) nanospheres. Appl. Surf. Sci. 2014, 289, 495–501. [Google Scholar] [CrossRef]
- Sharma, P.; Kaur, H.; Sharma, M.; Sahore, V. A review on applicability of naturally available adsorbents for the removal of hazardous dyes from aqueous waste. Environ. Monit. Assess. 2011, 183, 151–195. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.-H.; Liu, W.-Z.; Tang, Z.-E.; Cui, D. Recent advancements in azo dye decolorization in bio-electrochemical systems (BESs): Insights into decolorization mechanism and practical application. Water Res. 2021, 203, 117512. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Liu, Y.; Wang, L.; Dong, W.; Chen, H.; Zeng, W.; Xia, X.; Zeng, G. Activation of persulfate by photoexcited dye for antibiotic degradation: Radical and nonradical reactions. Chem. Eng. J. 2019, 375, 122070. [Google Scholar] [CrossRef]
- Chu, W. Dye removal from textile dye wastewater using recycled alum sludge. Water Res. 2001, 35, 3147–3152. [Google Scholar] [CrossRef]
- Deng, D.; Guo, J.; Zeng, G.; Sun, G. Decolorization of anthraquinone, triphenylmethane and azo dyes by a new isolated Bacillus cereus strain DC11. Int. Biodeterior. Biodegrad. 2008, 62, 263–269. [Google Scholar] [CrossRef]
- Li, H.; Gong, D. On the mineralization process of the anthraquinone dyes and the related intermediate products analysis via photocatalysis. J. Saf. Environ. 2016, 16, 254–257. [Google Scholar]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Xie, P.; Guo, Y.; Chen, Y.; Wang, Z.; Shang, R.; Wang, S.; Ding, J.; Wan, Y.; Jiang, W.; Ma, J. Application of a novel advanced oxidation process using sulfite and zero-valent iron in treatment of organic pollutants. Chem. Eng. J. 2017, 314, 240–248. [Google Scholar] [CrossRef]
- Khatri, J.; Nidheesh, P.; Singh, T.A.; Kumar, M.S. Advanced oxidation processes based on zero-valent aluminium for treating textile wastewater. Chem. Eng. J. 2018, 348, 67–73. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shao, Y.; Gao, N.; Chu, W.; Chen, J.; Lu, X.; Zhu, Y.; An, N. Activation of peroxymonosulfate by Al2O3-based CoFe2O4 for the degradation of sulfachloropyridazine sodium: Kinetics and mechanism. Sep. Purif. Technol. 2017, 189, 176–185. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I. Comparison of sulfate and hydroxyl radical based advanced oxidation of phenol. Chem. Eng. J. 2013, 224, 10–16. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Z.; Wang, S.; Liu, J.; Zhang, Y.; Wang, B.; Gong, Z.; Wang, M.; Dong, H.; Shi, J. Enhanced removal of methylparaben mediated by cobalt/carbon nanotubes (Co/CNTs) activated peroxymonosulfate in chloride-containing water: Reaction kinetics, mechanisms and pathways. Chem. Eng. J. 2021, 409, 128176. [Google Scholar] [CrossRef]
- Qi, C.; Wen, Y.; Zhao, Y.; Dai, Y.; Li, Y.; Xu, C.; Yang, S.; He, H. Enhanced degradation of organic contaminants by Fe (III)/peroxymonosulfate process with L-cysteine. Chin. Chem. Lett. 2022, 33, 2125–2128. [Google Scholar] [CrossRef]
- Ahmad, M.; Teel, A.L.; Watts, R.J. Mechanism of persulfate activation by phenols. Environ. Sci. Technol. 2013, 47, 5864–5871. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, M.; Dionysiou, D.D. What is the role of light in persulfate-based advanced oxidation for water treatment? Water Res. 2021, 189, 116627. [Google Scholar] [CrossRef]
- Lee, S.S.; Bai, H.; Liu, Z.; Sun, D.D. Novel-structured electrospun TiO2/CuO composite nanofibers for high efficient photocatalytic cogeneration of clean water and energy from dye wastewater. Water Res. 2013, 47, 4059–4073. [Google Scholar] [CrossRef]
- Fang, G.; Gao, J.; Dionysiou, D.D.; Liu, C.; Zhou, D. Activation of persulfate by quinones: Free radical reactions and implication for the degradation of PCBs. Environ. Sci. Technol. 2013, 47, 4605–4611. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, J.; Gao, Y.; Ma, J.; Pang, S.-Y.; Li, J.; Lu, X.-T.; Yuan, L.-P. Activation of peroxymonosulfate by benzoquinone: A novel nonradical oxidation process. Environ. Sci. Technol. 2015, 49, 12941–12950. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.J.; Ahmad, M.; Hohner, A.K.; Teel, A.L. Persulfate activation by glucose for in situ chemical oxidation. Water Res. 2018, 133, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhan, G.; Huang, X.; Wang, N.; Ai, Z.; Zhang, L. Persulfate activation induced by ascorbic acid for efficient organic pollutants oxidation. Chem. Eng. J. 2020, 382, 122355. [Google Scholar] [CrossRef]
- Bai, X.; Yang, L.; Hagfeldt, A.; Johansson, E.M.; Jin, P. D35-TiO2 nano-crystalline film as a high performance visible-light photocatalyst towards the degradation of bis-phenol A. Chem. Eng. J. 2019, 355, 999–1010. [Google Scholar] [CrossRef]
- Yang, L.; Bai, X.; Shi, J.; Du, X.; Xu, L.; Jin, P. Quasi-full-visible-light absorption by D35-TiO2/g-C3N4 for synergistic persulfate activation towards efficient photodegradation of micropollutants. Appl. Catal. B Environ. 2019, 256, 117759. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Ji, Q.; Cheng, X.; Wu, Y.; Xiang, W.; He, H.; Xu, Z.; Xu, C.; Qi, C.; Li, S.; Zhang, L. Visible light absorption by perylene diimide for synergistic persulfate activation towards efficient photodegradation of bisphenol A. Appl. Catal. B Environ. 2021, 282, 119579. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Wang, E. Electroreduction of alizarin green. Chin. J. Appl. Chem. 1985, 1, 19–23. [Google Scholar]
- Li, H.; Shan, C.; Pan, B. Fe (III)-doped g-C3N4 mediated peroxymonosulfate activation for selective degradation of phenolic compounds via high-valent iron-oxo species. Environ. Sci. Technol. 2018, 52, 2197–2205. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Ma, J.; Lin, C.; Li, X.; Zhang, H. Activation of peroxymonosulfate by base: Implications for the degradation of organic pollutants. Chemosphere 2016, 151, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.; Kumawat, K.; Biswas, S.; Krupanidhi, S.B. Solvothermal synthesis of Cu2SnS3 quantum dots and their application in near-infrared photodetectors. Inorg. Chem. 2017, 56, 2198–2203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, D.; Lv, X.; Wang, Y.; Chang, H.; Li, J. Energy-efficient photodegradation of azo dyes with TiO2 nanoparticles based on photoisomerization and alternate UV− visible light. Environ. Sci. Technol. 2010, 44, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Wang, C.; Yao, Y.; Qi, J.; Li, J. Insight into the Relationship of Reactive Oxygen Species and Anions in Persulfate-Based Advanced Oxidation Processes for Saline Organic Wastewater Treatment. Environ. Sci. Water Res. Technol. 2022, 8, 465–483. [Google Scholar] [CrossRef]
- Liu, G.; You, S.; Tan, Y.; Ren, N. In situ photochemical activation of sulfate for enhanced degradation of organic pollutants in water. Environ. Sci. Technol. 2017, 51, 2339–2346. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, X.; Zhang, H.; Yang, B.; Xiao, K.; Guo, T.; Zhang, J.; Shao, H.; Wang, Y.; Yu, G. MOF-derived nitrogen doped carbon modified g-C3N4 heterostructure composite with enhanced photocatalytic activity for bisphenol A degradation with peroxymonosulfate under visible light irradiation. Appl. Catal. B Environ. 2018, 233, 35–45. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Nie, M.; Deng, Y.; Nie, S.; Yan, C.; Ding, M.; Dong, W.; Dai, Y.; Zhang, Y. Simultaneous removal of bisphenol A and phosphate from water by peroxymonosulfate combined with calcium hydroxide. Chem. Eng. J. 2019, 369, 35–45. [Google Scholar] [CrossRef]
- Guan, C.; Jiang, J.; Pang, S.; Zhou, Y.; Gao, Y.; Li, J.; Wang, Z. Formation and control of bromate in sulfate radical-based oxidation processes for the treatment of waters containing bromide: A critical review. Water Res. 2020, 176, 115725. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Liu, B.; Wu, Q.; Luo, H.; Zhao, Q.; Si, Q.; Sseguya, F.; Ren, N. Edge-nitrogenated biochar for efficient peroxydisulfate activation: An electron transfer mechanism. Water Res. 2019, 160, 405–414. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Yin, R.; Du, J.; Wu, Q.; Luo, H.; Liu, B.; Sseguya, F.; Ren, N. Biochar-induced Fe (III) reduction for persulfate activation in sulfamethoxazole degradation: Insight into the electron transfer, radical oxidation and degradation pathways. Chem. Eng. J. 2019, 362, 561–569. [Google Scholar] [CrossRef]
- Chauvin, J.; Judée, F.; Yousfi, M.; Vicendo, P.; Merbahi, N. Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Sci. Rep. 2017, 7, 4562. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Zou, J.; Yan, S.; Song, W. Photosensitized transformation of peroxymonosulfate in dissolved organic matter solutions under simulated solar irradiation. Environ. Sci. Technol. 2022, 56, 1963–1972. [Google Scholar] [CrossRef]
- Bai, X.; Shi, J.; Xu, L.; Jin, X.; Shi, X.; Jin, P. Fe-g-C3N4/reduced graphene oxide lightless application for efficient peroxymonosulfate activation and pollutant mineralization: Comprehensive exploration of reactive sites. Sci. Total Environ. 2023, 855, 158799. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Quan, W.-Z.; Cao, Y.; Niu, Q.; Lu, Y.; Xiao, X.; Cheng, L. Boosting the singlet oxygen production from H2O2 activation with highly dispersed Co–N-graphene for pollutant removal. RSC Adv. 2022, 12, 17864–17872. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, C.; Luo, Z.; Zhu, M.; Li, B.; Zhou, L.; Zhang, G. Anchoring Co3O4 nanoparticles on conjugated polyimide ultrathin nanosheets: Construction of a Z-scheme nano-heterostructure for enhanced photocatalytic performance. RSC Adv. 2023, 13, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Fischer, M.K.; Bäuerle, P. Metal-free organic dyes for dye-sensitized solar cells: From structure: Property relationships to design rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Lu, Z.; Zeng, L.; Song, W.; Qin, Z.; Zeng, D.; Xie, C. In situ synthesis of C-TiO2/g-C3N4 heterojunction nanocomposite as highly visible light active photocatalyst originated from effective interfacial charge transfer. Appl. Catal. B Environ. 2017, 202, 489–499. [Google Scholar] [CrossRef]
- Srisantitham, S.; Sukwattanasinitt, M.; Unarunotai, S. Effect of pH on fluorescence quenching of organic dyes by graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2018, 550, 123–131. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, Y.; Shi, J.; Xu, L.; Wang, Y.; Jin, P. A new application pattern for sludge-derived biochar adsorbent: Ideal persulfate activator for the high-efficiency mineralization of pollutants. J. Hazard. Mater. 2021, 419, 126343. [Google Scholar] [CrossRef]
- Yin, X.; Li, X.; Gu, W.; Wang, F.; Zou, Y.; Sun, S.; Fu, Z.; Lu, Y. Enhanced photocatalytic activities of g-C3N4 via hybridization with a Bi–Fe–Nb-containing ferroelectric pyrochlore. ACS Appl. Mater. Interfaces 2017, 9, 19908–19916. [Google Scholar] [CrossRef] [PubMed]
- Dadigala, R.; Bandi, R.; Gangapuram, B.R.; Guttena, V. Construction of in situ self-assembled FeWO4/gC3N4 nanosheet heterostructured Z-scheme photocatalysts for enhanced photocatalytic degradation of rhodamine B and tetracycline. Nanoscale Adv. 2019, 1, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Cao, W.; Xiong, Z.; Xia, D.; Zeng, Q.; Xu, A. Activity and mechanism of photocatalytic degradation of Alizarin Green by cerium ions. Chin. J. Environ. Eng. 2014, 8, 448–452. [Google Scholar]
- Xiong, Z.; Xu, A.; Li, H.; Ruan, X.; Xia, D.; Zeng, Q. Highly efficient photodegradation of alizarin green in TiO2 suspensions using a microwave powered electrodeless discharged lamp. Ind. Eng. Chem. Res. 2013, 52, 362–369. [Google Scholar]
- Peng, J.; Wu, E.; Lou, X.; Deng, Q.; Hou, X.; Lv, C.; Hu, Q. Anthraquinone removal by a metal-organic framework/polyvinyl alcohol cryogel-immobilized laccase: Effect and mechanism exploration. Chem. Eng. J. 2021, 418, 129473. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Sharma, V.K.; Xiao, R.; Dionysiou, D.D. Revisit the alkaline activation of peroxydisulfate and peroxymonosulfate. Curr. Opin. Chem. Eng. 2022, 37, 100854. [Google Scholar] [CrossRef]
- Burgett, R.A.; Bao, X.; Villamena, F.A. Superoxide radical anion adduct of 5,5-dimethyl-1-pyrroline N-oxide (DMPO). 3.Effect of mildly acidic pH on the thermodynamics and kinetics of adduct formation. J. Phys. Chem. A 2008, 112, 2447–2455. [Google Scholar] [CrossRef]
- Takayanagi, T.; Kimiya, H.; Ohyama, T. Formation of artifactual DMPO-OH spin adduct in acid solutions containing nitrite ions. Free Radic. Res. 2017, 51, 739–748. [Google Scholar] [CrossRef]
- Fadda, A.; Barberis, A.; Sanna, D. Influence of pH, buffers and role of quinolinic acid, a novel iron chelating agent, in the determination of hydroxyl radical scavenging activity of plant extracts by Electron Paramagnetic Resonance (EPR). Food Chem. 2018, 240, 174–182. [Google Scholar] [CrossRef]
- Da, M.; Baptista, S.; Cadet, J.; Mascio, P.D.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; et al. Type I and II photosensitized oxidation reactions: Guidelines and mechanistic pathways HHS public access author manuscript. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar]
- Buglak, A.A.; Kapitonova, M.A.; Vechtomova, Y.L.; Telegina, T.A. Insights into Molecular Structure of Pterins Suitable for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 15222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Song, S.; Gu, P.; Ma, R.; Wei, D.; Zhao, G.; Wen, T.; Jehan, R.; Hu, B.; Wang, X. Visible-light-driven activation of persulfate over cyano and hydroxyl group co-modified mesoporous gC3N4 for boosting bisphenol A degradation. J. Mater. Chem. A 2019, 7, 5552–5560. [Google Scholar] [CrossRef]
- Lim, J.; Yang, Y.; Hoffmann, M.R. Activation of peroxymonosulfate by oxygen vacancies-enriched cobalt-doped black TiO2 nanotubes for the removal of organic pollutants. Environ. Sci. Technol. 2019, 53, 6972–6980. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhao, Y. Photooxidation of phytochemicals in food and control: A review. Ann. N. Y. Acad. Sci. 2017, 1398, 72–82. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Pan, Y.; Xiong, Z.; Yao, G.; Xie, R.; Lai, B. Peroxymonosulfate activation on FeCo2S4 modified g-C3N4 (FeCo2S4-CN): Mechanism of singlet oxygen evolution for nonradical efficient degradation of sulfamethoxazole. Chem. Eng. J. 2020, 384, 123361. [Google Scholar] [CrossRef]
- Peng, X.; Wu, J.; Zhao, Z.; Wang, X.; Dai, H.; Xu, L.; Xu, G.; Jian, Y.; Hu, F. Activation of peroxymonosulfate by single-atom Fe-g-C3N4 catalysts for high efficiency degradation of tetracycline via nonradical pathways: Role of high-valent iron-oxo species and Fe–Nx sites. Chem. Eng. J. 2022, 427, 130803. [Google Scholar] [CrossRef]
- Luo, R.; Li, M.; Wang, C.; Zhang, M.; Khan, M.A.N.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Li, J. Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition. Water Res. 2019, 148, 416–424. [Google Scholar] [CrossRef]
- Mohanty, P.; Matysik, J. Effect of proline on the production of singlet oxygen. Amino Acids 2001, 21, 195–200. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, Z.; Bai, X.; Shi, J.; Hu, M.; Chai, J.; Li, K.; Jin, P. Photosensitive Dye as an Ideal Peroxymonosulfate Activator for Efficient Self-Degradation: A Novel Idea of Using Waste to Treat Waste. Molecules 2023, 28, 4237. https://doi.org/10.3390/molecules28104237
Zhang Z, Li Z, Bai X, Shi J, Hu M, Chai J, Li K, Jin P. Photosensitive Dye as an Ideal Peroxymonosulfate Activator for Efficient Self-Degradation: A Novel Idea of Using Waste to Treat Waste. Molecules. 2023; 28(10):4237. https://doi.org/10.3390/molecules28104237
Chicago/Turabian StyleZhang, Zhiyao, Zhaolin Li, Xue Bai, Juan Shi, Min Hu, Jin Chai, Keqian Li, and Pengkang Jin. 2023. "Photosensitive Dye as an Ideal Peroxymonosulfate Activator for Efficient Self-Degradation: A Novel Idea of Using Waste to Treat Waste" Molecules 28, no. 10: 4237. https://doi.org/10.3390/molecules28104237
APA StyleZhang, Z., Li, Z., Bai, X., Shi, J., Hu, M., Chai, J., Li, K., & Jin, P. (2023). Photosensitive Dye as an Ideal Peroxymonosulfate Activator for Efficient Self-Degradation: A Novel Idea of Using Waste to Treat Waste. Molecules, 28(10), 4237. https://doi.org/10.3390/molecules28104237






