Synthesis, In Vitro Evaluation and Molecular Docking Studies of Novel Thiophenyl Thiazolyl-Pyridine Hybrids as Potential Anticancer Agents
Abstract
:1. Introduction
2. Results and Discussion
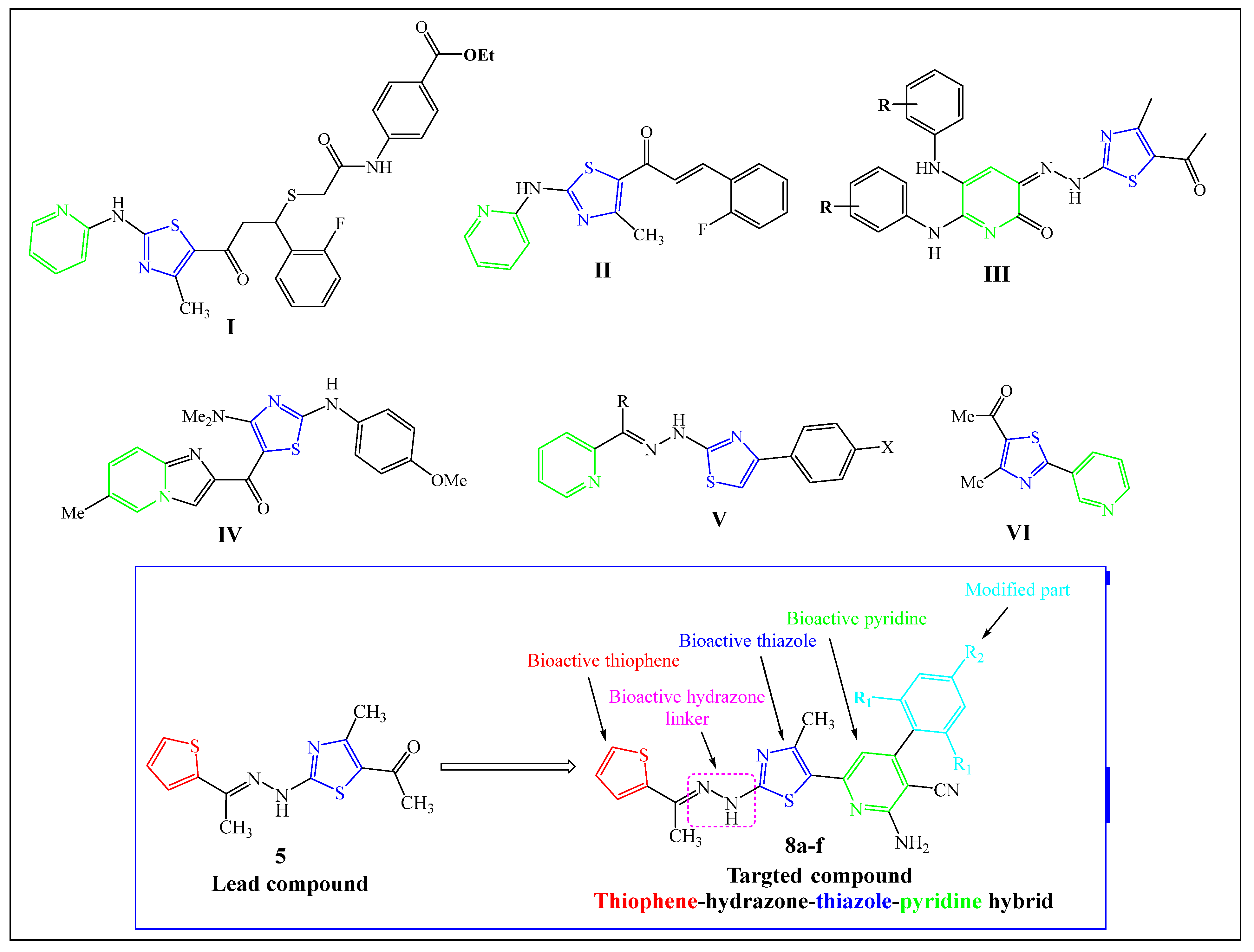
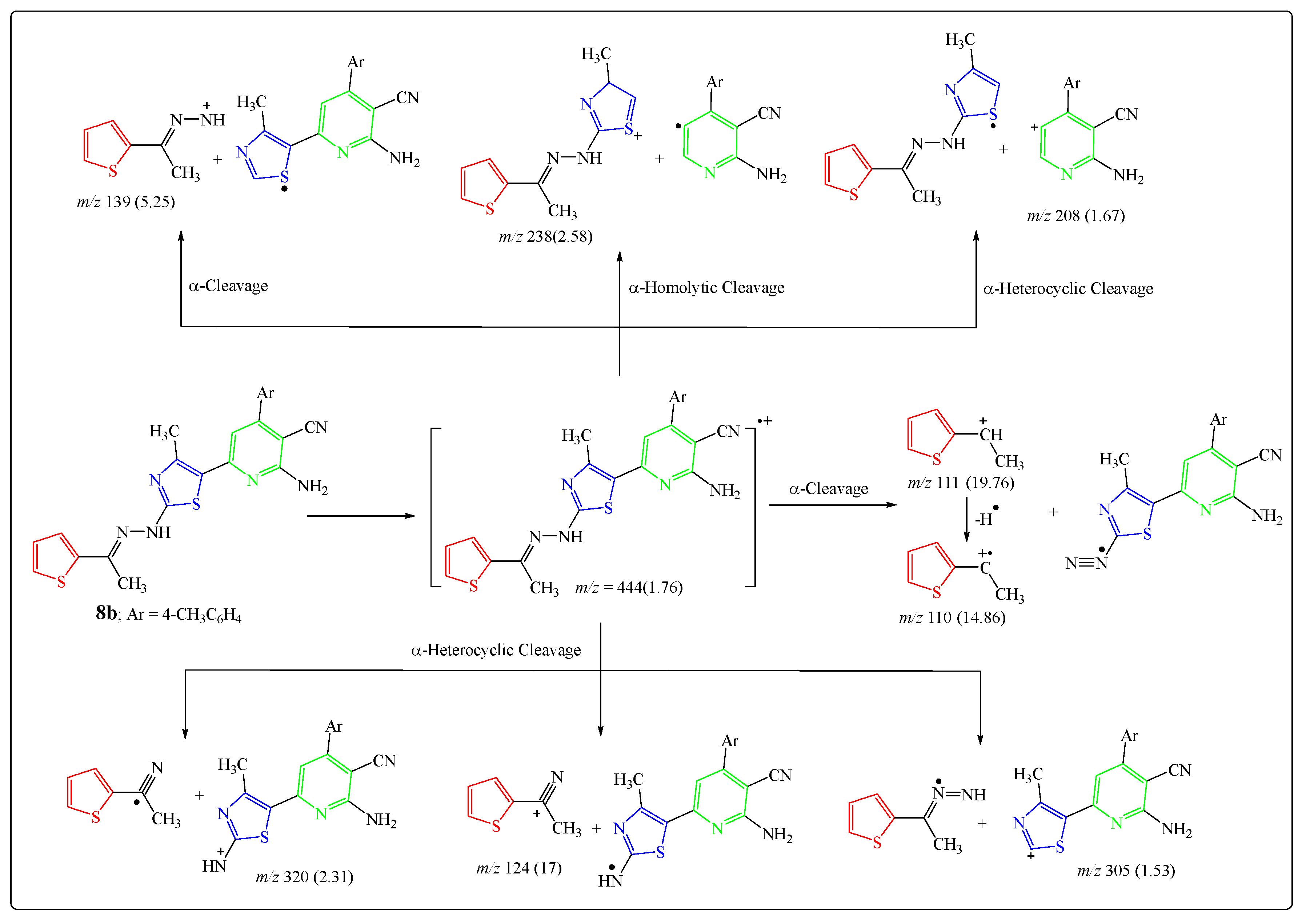
2.1. Chemistry
2.2. Cytotoxic Activity
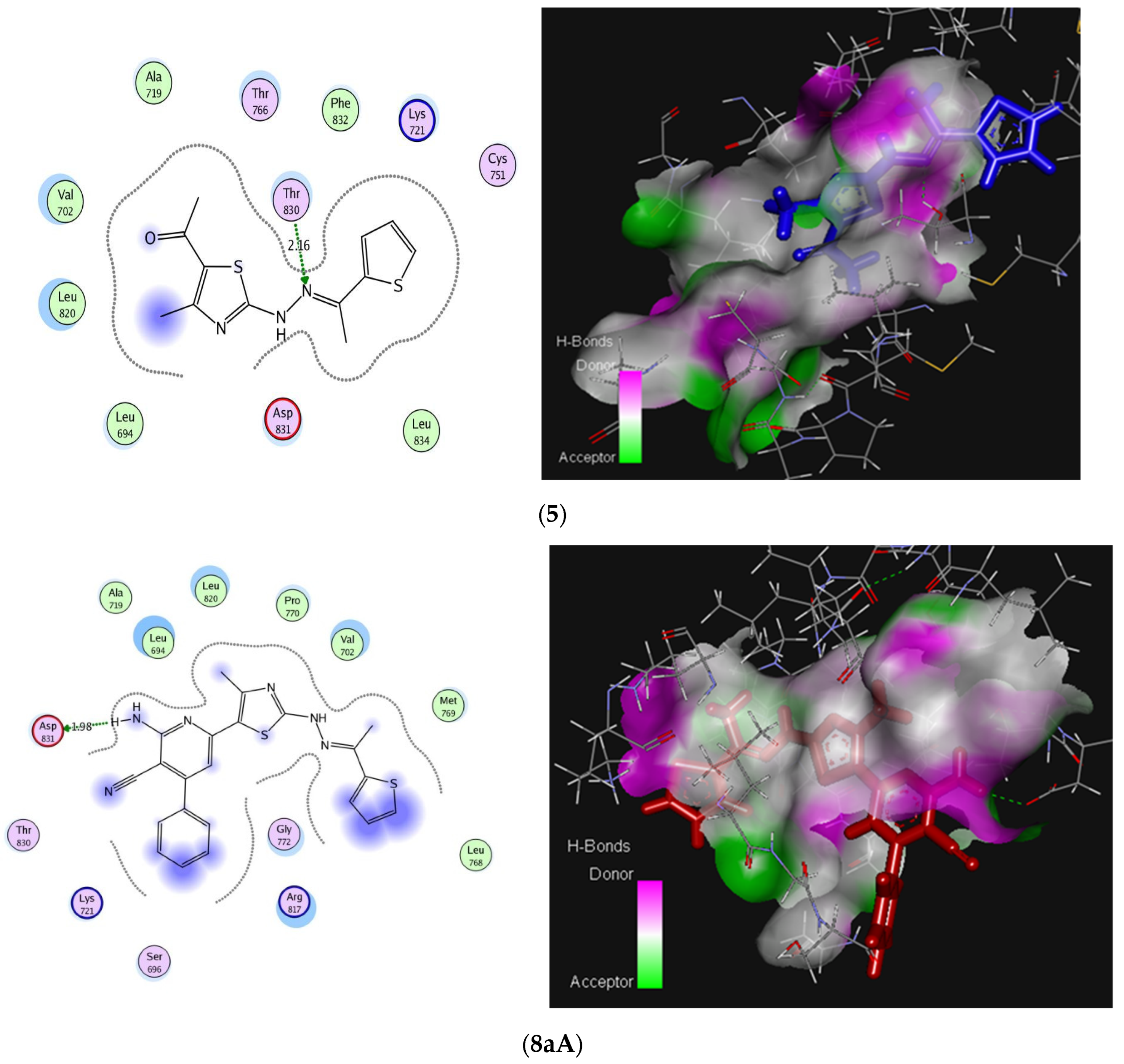
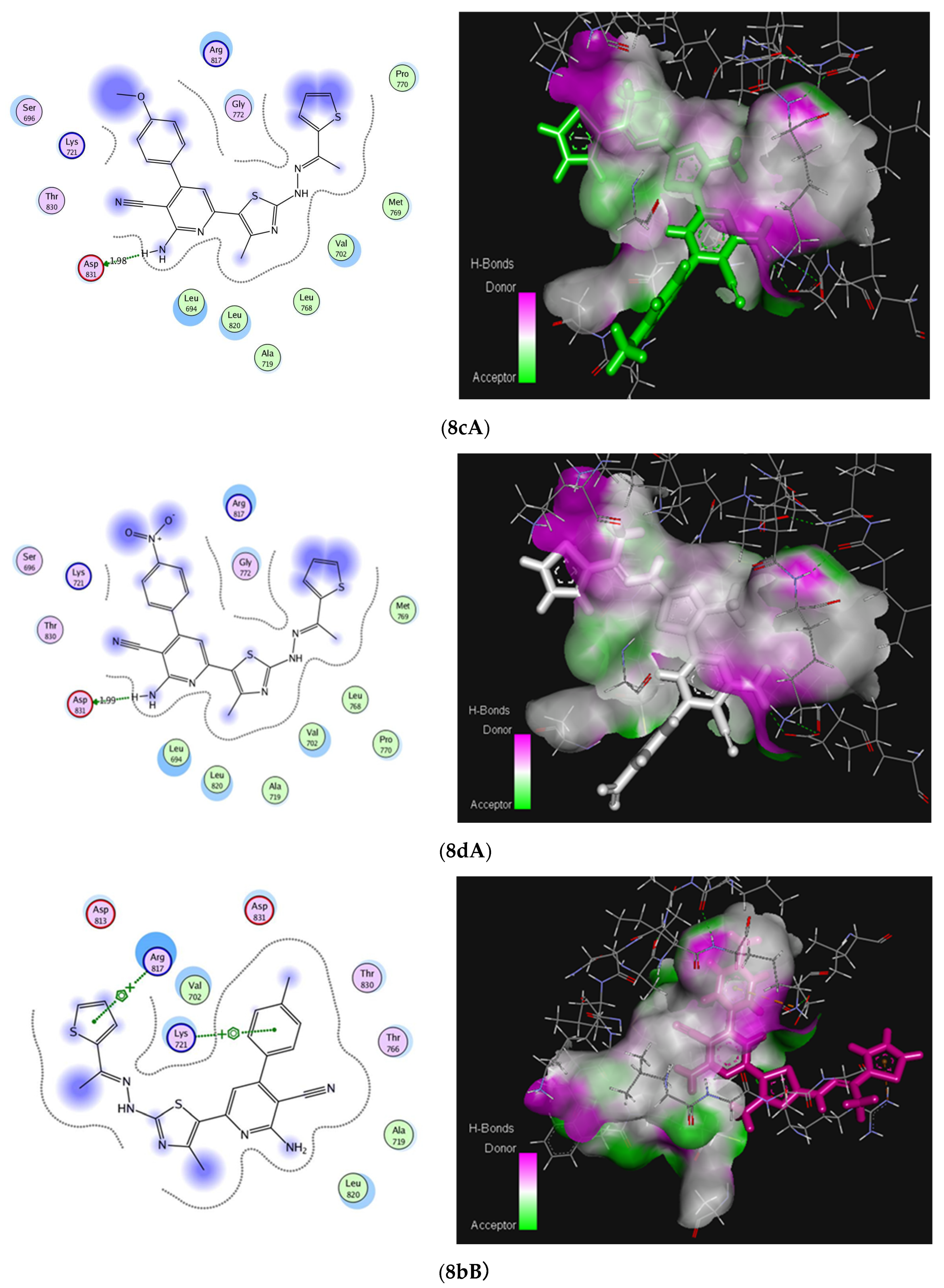
2.3. Molecular Docking Studies
3. Experimental Section
3.1. Reagents Used
3.2. Chemistry
3.3. Maintenance of Cell Line
3.4. Cytotoxicity Assay
3.5. Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sanduja, M.; Gupta, J.; Virmani, T. Recent advancements in Uracil and 5-Fluorouracil hybrids as potential anticancer agents, A review. J. Appl. Pharm. Sci. 2020, 10, 129–146. [Google Scholar] [CrossRef]
- Teli, G.; Chawla, P.A. Hybridization of imidazole with various heterocycles in targeting cancer: A decade’s work. ChemistrySelect 2021, 6, 4803–4836. [Google Scholar] [CrossRef]
- Rajendran, P.; Maheshwari, U.; Muthukrishnan, A.; Muthuswamy, R.; Anand, K.; Ravindran, B.; Dhanaraj, P.; Balamuralikrishnan, B.; Chang, W.S.; Chung, W.J. Myricetin: Versatile plant based flavonoid for cancer treatment by inducing cell cycle arrest and ROS–reliant mitochondria-facilitated apoptosis in A549 lung cancer cells and in silico prediction. Mol. Cell. Biochem. 2021, 476, 57–68. [Google Scholar] [CrossRef]
- Ponte, L.G.S.; Pavan, I.C.B.; Mancini, M.C.S.; da Silva, L.G.S.; Morelli, A.P.; Severino, M.B.; Bezerra, R.M.N.; Simabuco, F.M. The hallmarks of flavonoids in cancer. Molecules 2021, 26, 2029. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Véras Of Aguiar, A.C.; Olímpio of Moura, R.; Mendonça Junior, J.F.B.; de Oliveira Rocha, H.A.; Câmara, R.B.G.; Schiavon, M.D.S.C. Evaluation of the antiproliferative activity of 2-amino thiophene derivatives against human cancer cells lines. Biomed. Pharmacother. 2016, 84, 403–414. [Google Scholar] [CrossRef]
- Dos Santos, F.A.; Pereira, M.C.; de Oliveira, T.B.; Mendonça Junior, F.J.B.; de Lima, M.D.C.A.; Pitta, M.G.D.R.; Pitta, I.D.R.; de Melo Rêgo, M.J.B.; da Rocha Pitta, M.G. Anticancer properties of thiophene derivatives in breast cancer MCF-7 cells. Anti-Cancer Drugs 2018, 29, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Verma, P.K. Synthesis of thiophene derivatives and their anti-microbial, antioxidant, anticorrosion and anticancer activity. BMC Chem. 2019, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Riyadh, S.M.; Huwaimel, B.; Zayed, M.E.M.; Abdellattif, M.H. Synthesis, molecular docking study and cytotoxic activity on MCF cells of some new thiazole clubbed thiophene scaffolds. Molecules 2022, 27, 4639. [Google Scholar] [CrossRef]
- De Vasconcelos, A.; Campos, V.F.; Nedel, F.; Seixas, F.K.; Dellagostin, O.A.; Smith, K.R.; de Pereira, C.M.P.; Stefanello, F.M.; Collares, T.; Barschak, A.G. Cytotoxic and apoptotic effects of chalcone derivatives of 2-acetyl thiophene on human colon adenocarcinoma cells. Cell Biochem. Funct. 2013, 31, 289–297. [Google Scholar] [CrossRef]
- Gulipalli, K.C.; Bodige, S.; Ravula, P.; Endoori, S.; Vanaja, G.R.; Babu, G.S.; Chandra, J.N.N.; Seelam, N. Design, synthesis, in silico and in vitro evaluation of thiophene derivatives: A potent tyrosine phosphatase 1B inhibitor and anticancer activity. Bioorg. Med. Chem. Lett. 2017, 27, 3558–3564. [Google Scholar] [CrossRef] [PubMed]
- Bondock, S.; Albormani, O.; Fouda, A.M.; Abu Safieh, K.A. Progress in the chemistry of 5-acetylthiazoles. Synth. Commun. 2016, 46, 1081–1111. [Google Scholar] [CrossRef]
- Sharma, P.C.; Bansal, K.K.; Sharma, A.; Sharma, D.; Deep, A. Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur. J. Med. Chem. 2020, 188, 112016–112062. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Ruiz, C.H.; Koenig, M.; Cameron, M.D. Characterization of dasatinib and its structural analogs as CYP3A4 mechanism-based inactivators and the proposed bioactivation pathway. Drug Metab. Dispos. 2009, 37, 1242–1250. [Google Scholar] [CrossRef]
- Rashid, M.A.; Gustafson, K.R.; Cardellina, J.H.; Boyd, M.R. Patellamide F, a new cytotoxic cyclic peptide from the colonial ascidian Lissoclinum patella. J. Nat. Prod. 1995, 58, 594–597. [Google Scholar] [CrossRef]
- Franchetti, P.; Cappellacci, L.; Grifantini, M.; Barzi, A.; Nocentini, G.; Yang, H.; O’Connor, A.; Jayaram, H.N.; Carrell, C.; Goldstein, B.M. Furanfurin and Thiophenfurin: Two Novel Tiazofurin Analogs. Synthesis, Structure, Antitumor Activity, and Interactions with Inosine Monophosphate Dehydrogenase. J. Med. Chem. 1995, 38, 3829–3837. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, S.; Zhou, X.; Xie, L.; Chen, A. 5-FU and ixabepilone modify the microRNA expression profiles in MDA-MB-453 triple-negative breast cancer cells. Oncol. Lett. 2014, 7, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Hu-Lieskovan, S.; Mok, S.; Homet Moreno, B.; Tsoi, J.; Robert, L.; Goedert, L.; Pinheiro, E.M.; Koya, R.C.; Graeber, T.; Comin-Anduix, B.; et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF V600E melanoma. Sci. Transl. Med. 2015, 7, 279ra41. [Google Scholar] [CrossRef]
- Altmann, K.H. Epothilone B and its analogs-a new family of anticancer agents. Mini Rev. Med. Chem. 2003, 3, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.A.; Gomha, S.M.; Abbas, I.M.; Kazem, M.S.H.; Alterary, S.S.; Mabkhot, Y.N. An Efficient synthesis of novel pyrazole-based heterocycles as potential antitumor agents. Appl. Sci. 2017, 7, 785. [Google Scholar] [CrossRef]
- Alqahtani, A.M.; Bayazeed, A.A. Synthesis and antiproliferative activity studies of new functionalized pyridine linked thiazole derivatives. Arab. J. Chem. 2021, 14, 102914–102927. [Google Scholar] [CrossRef]
- Singh, H.; Singh, J.V.; Gupta, M.K.; Saxena, A.K.; Sharma, S.; Nepali, K.; Bedi, P.M.S. Triazole tethered isatin-coumarin based molecular hybrids as novel antitubulin agents: Design, synthesis, biological investigation and docking studies. BMCL 2017, 27, 3974–3979. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.E.; Ghadari, R.; Mohammadian, R.; Shaabani, A.; Khavasi, H.R. Rhodanine-Furan Bis-Heterocyclic Frameworks Synthesis via Green One-Pot Sequential Six-Component Reactions: A Synthetic and Computational Study. ChemistrySelect 2019, 4, 11893–11898. [Google Scholar] [CrossRef]
- Kumar, D.; Khan, S.I.; Ponnan, P.; Rawat, D.S. Triazine–pyrimidine based molecular hybrids: Synthesis, docking studies and evaluation of antimalarial activity. New J. Chem. 2014, 38, 5087–5095. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdel-aziz, H.M. Synthesis and antitumor activity of 1,3,4-thiadiazole derivatives bearing coumarine ring. Heterocycles 2015, 91, 583–592. [Google Scholar] [CrossRef]
- Kekeçmuhammed, H.; Tapera, M.; Tüzün, B.; Akkoç, S.; Zorlu, Y.; Sarıpınar, E. Synthesis, Molecular Docking and Antiproliferative Activity Studies of a Thiazole-Based Compound Linked to Hydrazone Moiety. ChemistrySelect 2022, 7, e202201502. [Google Scholar] [CrossRef]
- Bhavya, K.; Mantipally, M.; Roy, S.; Arora, L.; Badavath, V.N.; Gangireddy, M.; Dasgupta, S.; Gundla, R.; Pal, D. Novel imidazo [1, 2-a] pyridine derivatives induce apoptosis and cell cycle arrest in non-small cell lung cancer by activating NADPH oxidase mediated oxidative stress. Life Sci. 2022, 294, 120334–120346. [Google Scholar] [CrossRef]
- Ivasechko, I.; Yushyn, I.; Roszczenko, P.; Senkiv, J.; Finiuk, N.; Lesyk, D.; Holota, S.; Czarnomysy, R.; Klyuchivska, O.; Khyluk, D.; et al. Development of novel pyridine-thiazole hybrid molecules as potential anticancer agents. Molecules 2022, 27, 6219. [Google Scholar] [CrossRef]
- Xie, W.; Wu, Y.; Zhang, J.; Mei, Q.; Zhang, Y.; Zhu, N.; Liu, R.; Zhang, H. Design, synthesis and biological evaluations of novel pyridone-thiazole hybrid molecules as antitumor agents. Eur. J. Med. Chem. 2018, 145, 35–40. [Google Scholar] [CrossRef]
- Vasu, K.K.; Digwal, C.S.; Pandya, A.N.; Pandya, D.H.; Sharma, J.A.; Patel, S.; Agarwal, M. Imidazo[1,2-a]pyridines linked with thiazoles/thiophene motif through keto spacer as potential cytotoxic agents and NF-ĸB inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 5463–5466. [Google Scholar] [CrossRef]
- Dos Santos Silva, T.D.; Bomfim, L.M.; da Cruz Rodrigues, A.C.B.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.B.P.; Bezerra, D.P.; de Oliveira Cardoso, M.V.; Leite, A.C.L.; et al. Anti-liver cancer activity in vitro and in vivo induced by 2-pyridyl 2,3-thiazole derivatives. Toxicol. Appl. Pharm. 2017, 329, 212–223. [Google Scholar] [CrossRef]
- Amin, H.K.; El-Araby, A.M.; Eid, S.; Nasr, T.; Bondock, S.; Leheta, O.; Dawoud, M.E. A thiazole analogue exhibits an antiproliferative effect in different human carcinoma cell lines and its mechanism based on molecular modeling. Adv. Biol. Chem. 2017, 7, 76–87. [Google Scholar] [CrossRef]
- Ayati, A.; Moghimi, S.; Salarinejad, S.; Safavi, M.; Pouramiri, B.; Foroumadi, A. A review on progression of epidermal growth factor receptor (EGFR) inhibitors as an efficient approach in cancer targeted therapy. Bioorg. Chem. 2020, 99, 103811–103826. [Google Scholar] [CrossRef]
- Palabindela, R.; Guda, R.; Ramesh, G.; Myadaraveni, P.; Banothu, D.; Ravi, G.; Korra, R.; Mekala, H.; Kasula, M. Novel tryptanthrin hybrids bearing aminothiazoles as potential EGFR inhibitors: Design, synthesis, biological screening, molecular docking studies, and ADME/T predictions. J. Heterocycl. Chem. 2022, 59, 1533–1550. [Google Scholar] [CrossRef]
- Abbas, I.M.; Gomha, S.M.; Elaasser, M.M.; Bauomi, M.A. Synthesis and biological evaluation of new pyridines containing imidazole moiety as antimicrobial and anticancer agents. Turk. J. Chem. 2015, 39, 334–346. [Google Scholar] [CrossRef]
- Gomha, S.M.; Muhammad, Z.A.; Abdel-aziz, M.R.; Abdel-aziz, H.M.; Gaber, H.M.; Elaasser, M.M. One-pot synthesis of new thiadiazolyl-pyridines as anticancer and antioxidant agents. J. Heterocycl. Chem. 2018, 55, 530–536. [Google Scholar] [CrossRef]
- Gomha, S.M.; Kheder, N.A.; Abdelaziz, M.R.; Mabkhot, Y.N.; Alhajoj, A.M. A facile synthesis and anticancer activity of some novel thiazoles carrying 1,3,4-thiadiazole moiety. Chem. Cent. J. 2017, 11, 25. [Google Scholar] [CrossRef]
- Gomha, S.M.; Edrees, M.M.; Muhammad, Z.A.; El-Reedy, A.A.M. 5-(Thiophen-2-yl)-1,3,4- thiadiazole derivatives: Synthesis, molecular docking and in-vitro cytotoxicity evaluation as potential anticancer agents. Drug Des. Devel. Ther. 2018, 12, 1511–1523. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.A.; Abdelrazek, F.M.; Gomha, S.M.; Hebishy, A.S.; Abdelfattah, M.S.; Hassan, S.M.; Sayed, A.R.; Elaasser, M.M. Synthesis and Biological Evaluation of Thiazolyl-ethylidene hydrazino-thiazole Derivatives: A Novel Heterocyclic System. Appl. Sci. 2021, 11, 8908. [Google Scholar] [CrossRef]
- Edrees, M.M.; Abu-Melha, S.; Saad, A.M.; Kheder, N.A.; Gomha, S.M.; Muhammad, Z.A. Eco-friendly synthesis, characterization and biological evaluation of some novel pyrazolines containing thiazole moiety as potential anticancer and antimicrobial agents. Molecules 2018, 23, 2970. [Google Scholar] [CrossRef]
- Abu-Melha, S.; Edrees, M.M.; Salem, H.H.; Kheder, N.A.; Gomha, S.M.; Abdelaziz, M.R. Synthesis and biological evaluation of some novel thiazole-based heterocycles as potential anticancer and antimicrobial agents. Molecules 2019, 24, 539. [Google Scholar] [CrossRef]
- Gomha, S.M.; Muhammad, Z.A.; Abdel-aziz, H.M.; Matar, I.K.; El-Sayed, A.A. Green synthesis, molecular docking and anticancer activity of novel 1,4-dihydropyridine-3,5-Dicarbohydrazones under grind-stone chemistry. Green Chem. Lett. Rev. 2020, 13, 6–17. [Google Scholar] [CrossRef]
- Makam, P.; Kankanala, R.; Prakash, A.; Kannan, T. 2-(2-Hydrazinyl) thiazole derivatives: Design, synthesis and in vitro antimycobacterial studies. Eur. J. Med. Chem. 2013, 69, 564–576. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; El-Hashash, M.A.; Elkaeed, E.B. Eco-friendly sequential one-pot synthesis, molecular docking, and anticancer evaluation of arylidene-hydrazinyl-thiazole derivatives as CDK2 inhibitors. Bioorg. Chem. 2021, 108, 104615–104629. [Google Scholar] [CrossRef]
- Makam, P.; Thakur, P.K.; Kannan, T. In vitro and in silico antimalarial activity of 2-(2-hydrazinyl)thiazole derivatives. Eur. J. Pharmaceut. Sci. 2014, 52, 138–145. [Google Scholar] [CrossRef]
- Mahmoud, H.K.; Abdelhady, H.A.; Elaasser, M.M.; Hassain, D.Z.H.; Gomha, S.M. Microwave-Assisted One-Pot Three Component Synthesis of Some Thiazolyl(Hydrazonoethyl)Thiazoles as Potential Anti-Breast Cancer Agents. Polycycl. Aromat. Comp. 2022, 42, 7232–7246. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Shirazi, A.N.; El-Meligy, M.G.; El-Ziaty, A.K.; Rowley, D.; Sun, J.; Nagib, Z.A.; Parang, K. Synthesis of 4-aryl-6-indolylpyridine-3-carbonitriles and evaluation of their antiproliferative activity. Tetrahedron Lett. 2014, 55, 1154–1158. [Google Scholar] [CrossRef]
- Rodríguez, M.R.; Balsa, L.M.; Piro, O.E.; Etcheverría, G.A.; García-Tojal, J.; Pis-Diez, R.; León, I.E.; Parajón-Costa, B.P.; González-Baró, A.C. Synthesis, Crystal Structure, Spectroscopic Characterization, DFT Calculations and Cytotoxicity Assays of a New Cu(II) Complex with an Acylhydrazone Ligand Derived from Thiophene. Inorganics 2021, 9, 9–23. [Google Scholar] [CrossRef]
- Yurttaş, L.; Temel, H.E.; Aksoy, M.O.; Bülbül, E.F.; Çiftçi, G.A. New chromanone derivatives containing thiazoles: Synthesis and antitumor activity evaluation on A549 lung cancer cell line. Drug Dev. Res. 2022, 83, 470–484. [Google Scholar] [CrossRef]
- Suma, V.R.; Sreenivasulu, R.; Rao, M.V.B.; Subramanyam, M.; Ahsan, M.J.; Alluri, R.; Rao, K.R.M. Design, synthesis, and biological evaluation of chalcone-linked thiazole-imidazopyridine derivatives as anticancer agents. Med. Chem. Res. 2020, 29, 1643–1654. [Google Scholar] [CrossRef]
- Esmail, E.A.; Fathy, H.M.; Sedik, M.Z.; Mohamed, A.F. Assessment The effect of prebiotics, probiotics and synbiotics on Hyperlipidemia. Egypt. J. Chem. 2022, 65, 421–432. [Google Scholar] [CrossRef]
- Cory, A.H.; Owen, T.C.; Barltrop, J.A.; Cory, J.G. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991, 3, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [PubMed]
- Chen, X.; Lv, P.; Liu, J.; Xu, K. Apoptosis of human hepatocellular carcinoma cell (HepG2) induced by cardiotoxin III through S-phase arrest. Exp. Toxicol. Pathol. 2009, 61, 307–315. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Liu, T.; He, W.; Chen, Y.; Chen, X.; Li, X.; Zhou, W.; Yi, J.; Ren, Z. Antitumor effect of matrine in human hepatoma G2 cells by inducing apoptosis and autophagy. World J. Gastroenterol. 2010, 16, 4281–4290. [Google Scholar] [CrossRef]




| Compound | IC50 (µM) |
|---|---|
| 5 | 0.452 |
| 8a | 0.428 |
| 8b | 0.412 |
| 8c | 0.549 |
| 8d | 0.433 |
| 8e | 0.302 |
| 8f | 0.788 |
| Doxorubicin | 0.460 |
| Compound(s) | Compound 5 | Group A (8a,c,d) | Group B (8b,e,f) | |
|---|---|---|---|---|
| Parameter | ||||
| E (Kcal/mol) a | 41.2253 | 82.7210 | 75.8467 | |
| S (Kcal/mol) a | −20.1495 | −24.2234 | −24.4538 | |
| Residues involved in interaction | Thr830 (H-bond) | Asp831 (H-bond) | Lys721, Arg817 (Arene-cation) | |
| No. of hydrogen bonding | 1 | 1 | - | |
| Bond length [Å] | 2.16 | 1.989 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashmawy, F.O.; Gomha, S.M.; Abdallah, M.A.; Zaki, M.E.A.; Al-Hussain, S.A.; El-desouky, M.A. Synthesis, In Vitro Evaluation and Molecular Docking Studies of Novel Thiophenyl Thiazolyl-Pyridine Hybrids as Potential Anticancer Agents. Molecules 2023, 28, 4270. https://doi.org/10.3390/molecules28114270
Ashmawy FO, Gomha SM, Abdallah MA, Zaki MEA, Al-Hussain SA, El-desouky MA. Synthesis, In Vitro Evaluation and Molecular Docking Studies of Novel Thiophenyl Thiazolyl-Pyridine Hybrids as Potential Anticancer Agents. Molecules. 2023; 28(11):4270. https://doi.org/10.3390/molecules28114270
Chicago/Turabian StyleAshmawy, Fayza O., Sobhi M. Gomha, Magda A. Abdallah, Magdi E. A. Zaki, Sami A. Al-Hussain, and Mohamed A. El-desouky. 2023. "Synthesis, In Vitro Evaluation and Molecular Docking Studies of Novel Thiophenyl Thiazolyl-Pyridine Hybrids as Potential Anticancer Agents" Molecules 28, no. 11: 4270. https://doi.org/10.3390/molecules28114270






