Design and Synthesis of Brefeldin A-Isothiocyanate Derivatives with Selectivity and Their Potential for Cervical Cancer Therapy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Antiproliferative Activity
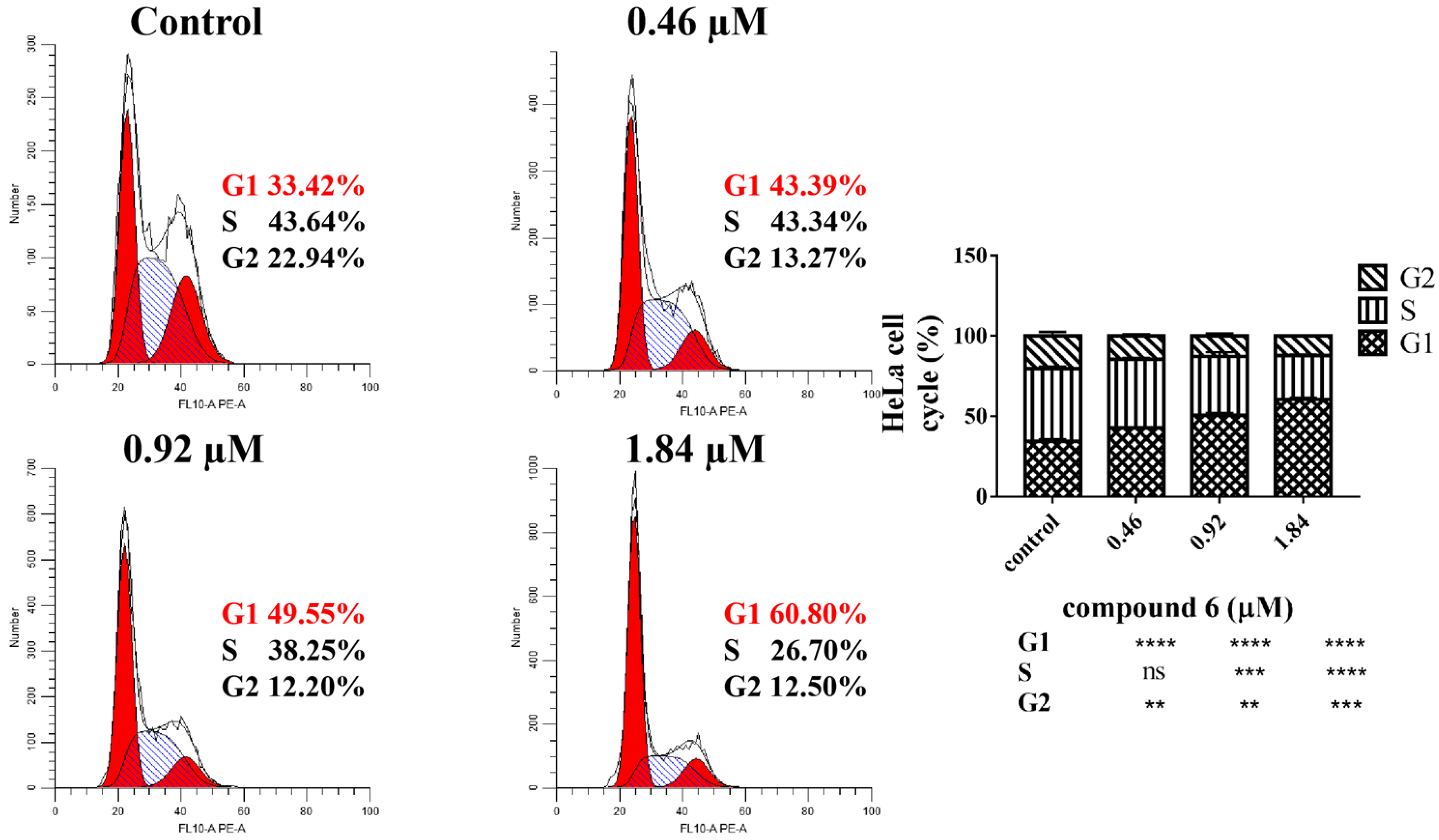
2.2.2. 6 Induced HeLa Cell Cycle Arrest in G1 Phase
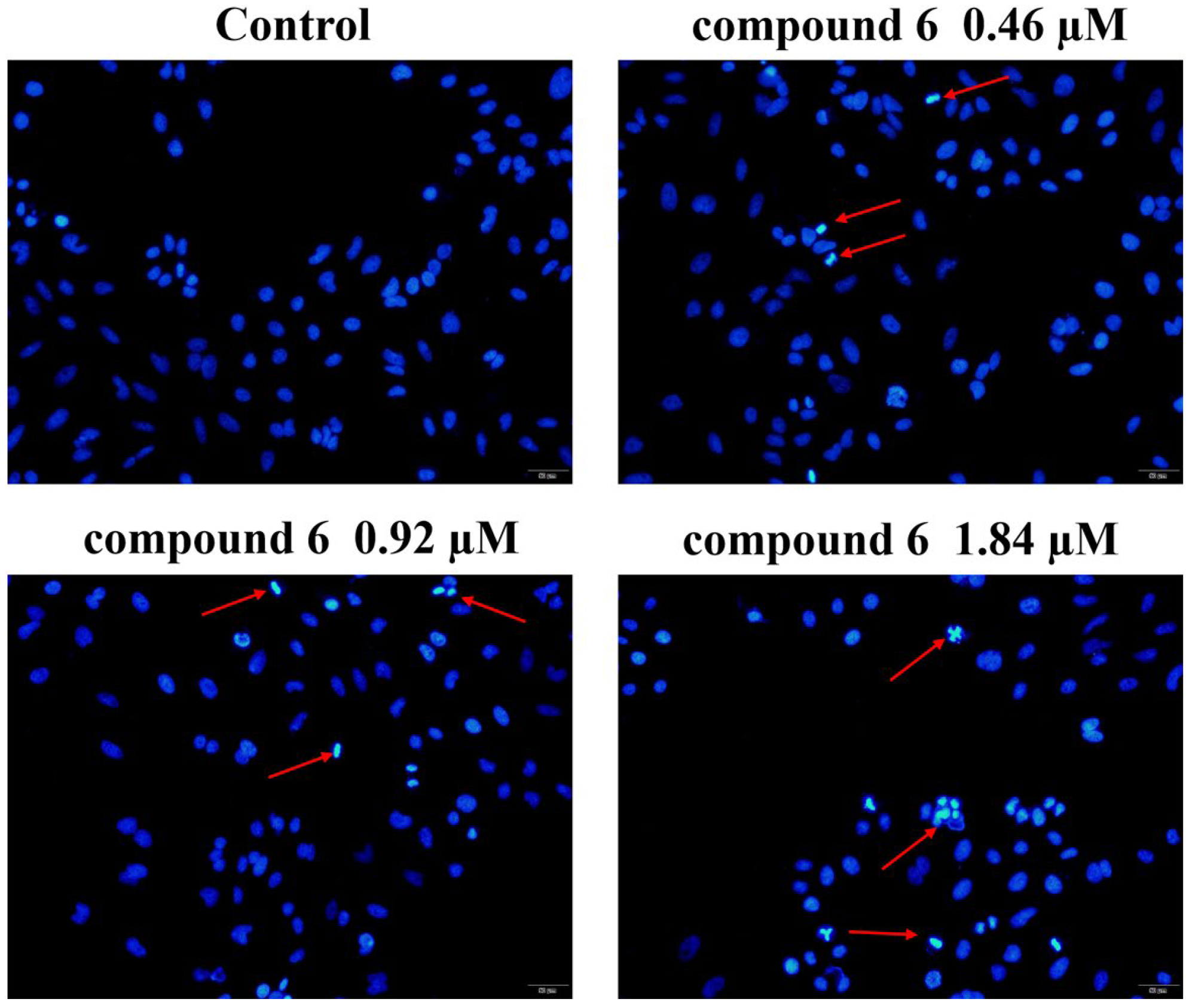
2.2.3. 6 Induced HeLa Cell Nucleus Fragmentation
2.2.4. 6 Induced Apoptosis in HeLa Cells
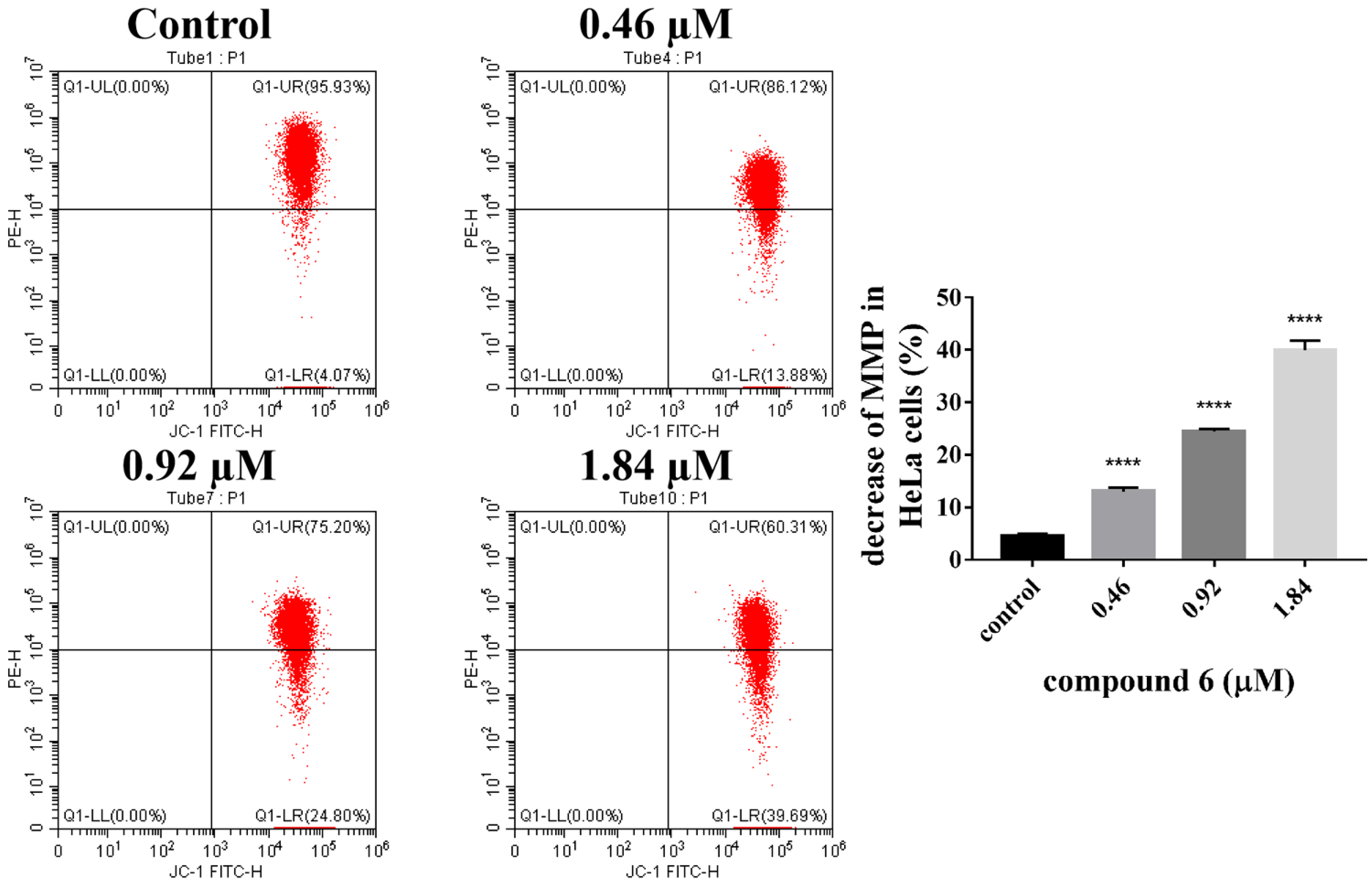
2.2.5. 6 Decreased Mitochondrial Membrane Potential in HeLa Cells
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of Intermediates 4a–k
2-Hydroxyethyl 4-Isothiocyanatobenzoate (3a)
4-Hydroxybutyl 4-Isothiocyanatobenzoate (3b)
4-Hydroxybut-2-yn-1-yl 4-Isothiocyanatobenzoate (3c)
2-(2-Hydroxyethoxy)ethyl 4-Isothiocyanatobenzoate (3d)
6-Hydroxyhexyl 4-Isothiocyanatobenzoate (3e)
4-Hydroxyphenyl 4-Isothiocyanatobenzoate (3f)
3.1.2. Synthesis of Compounds 5–7
(1R,2E,6S,10E,11aS,13S,14aR)-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecine-1,13-diyl bis(4-isothiocyanatobenzoate) (5)
(1R,2E,6S,10E,11aS,13S,14aR)-13-Hydroxy-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecin-1-yl 4-isothiocyanatobenzoate (6)
(1R,2E,6S,10E,11aS,13S,14aR)-1-Hydroxy-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecin-13-yl 4-isothiocyanatobenzoate (7)
3.1.3. Synthesis of Compounds 8a–k and 9a–k
Bis(2-((4-isothiocyanatobenzoyl)oxy)ethyl) O,O’-((1R,2E,6S,10E,11aS,13S,14aR)-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecine-1,13-diyl) disuccinate (8a)
(1R,2E,6S,10E,11aS,13S,14aR)-13-Hydroxy-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecin-1-yl (2-((4-isothiocyanatobenzoyl)oxy)ethyl) succinate (9a)
Bis(4-((4-isothiocyanatobenzoyl)oxy)butyl) O,O’-((1R,2E,6S,10E,11aS,13S,14aR)-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecine-1,13-diyl) disuccinate (8b)
(1R,2E,6S,10E,11aS,13S,14aR)-13-Hydroxy-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecin-1-yl (4-((4-isothiocyanatobenzoyl)oxy)butyl) succinate (9b)
Bis(4-((4-isothiocyanatobenzoyl)oxy)but-2-yn-1-yl) O,O’-((1R,2E,6S,10E,11aS,13S,14aR)-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecine-1,13-diyl) disuccinate (8c)
(1R,2E,6S,10E,11aS,13S,14aR)-13-Hydroxy-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecin-1-yl (4-((4-isothiocyanatobenzoyl)oxy)but-2-yn-1-yl) succinate (9c)
Bis(2-(2-((4-isothiocyanatobenzoyl)oxy)ethoxy)ethyl) O,O’-((1R,2E,6S,10E,11aS,13S,14aR)-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecine-1,13-diyl) disuccinate (8d)
(1R,2E,6S,10E,11aS,13S,14aR)-13-Hydroxy-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecin-1-yl (2-(2-((4-isothiocyanatobenzoyl)oxy)ethoxy)ethyl) succinate (9d)
Bis(6-((4-isothiocyanatobenzoyl)oxy)hexyl) O,O’-((1R,2E,6S,10E,11aS,13S,14aR)-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecine-1,13-diyl) disuccinate (8e)
(1R,2E,6S,10E,11aS,13S,14aR)-13-Hydroxy-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecin-1-yl (6-((4-isothiocyanatobenzoyl)oxy)hexyl) succinate (9e)
Bis(4-((4-isothiocyanatobenzoyl)oxy)phenyl) O,O’-((1R,2E,6S,10E,11aS,13S,14aR)-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecine-1,13-diyl) disuccinate (8f)
(1R,2E,6S,10E,11aS,13S,14aR)-13-Hydroxy-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecin-1-yl (4-((4-isothiocyanatobenzoyl)oxy)phenyl) succinate (9f)
Bis(2-((4-isothiocyanatobenzoyl)oxy)ethyl) O,O’-((1R,2E,6S,10E,11aS,13S,14aR)-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecine-1,13-diyl) dimaleate (8g)
(1R,2E,6S,10E,11aS,13S,14aR)-13-Hydroxy-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecin-1-yl (2-((4-isothiocyanatobenzoyl)oxy)ethyl) maleate(9g)
Bis(4-((4-isothiocyanatobenzoyl)oxy)butyl) O,O’-((1R,2E,6S,10E,11aS,13S,14aR)-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecine-1,13-diyl) dimaleate (8h)
(1R,2E,6S,10E,11aS,13S,14aR)-13-Hydroxy-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecin-1-yl (4-((4-isothiocyanatobenzoyl)oxy)butyl) maleate (9h)
Bis(4-((4-isothiocyanatobenzoyl)oxy)but-2-yn-1-yl) O,O’-((1R,2E,6S,10E,11aS,13S,14aR)-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecine-1,13-diyl) dimaleate (8i)
(1R,2E,6S,10E,11aS,13S,14aR)-13-Hydroxy-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecin-1-yl (4-((4-isothiocyanatobenzoyl)oxy)but-2-yn-1-yl) maleate (9i)
Bis(2-(2-((4-isothiocyanatobenzoyl)oxy)ethoxy)ethyl) O,O’-((1R,2E,6S,10E,11aS,13S,14aR)-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecine-1,13-diyl) dimaleate (8j)
(1R,2E,6S,10E,11aS,13S,14aR)-13-Hydroxy-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecin-1-yl (2-(2-((4-isothiocyanatobenzoyl)oxy)ethoxy)ethyl) maleate (9j)
Bis(6-((4-isothiocyanatobenzoyl)oxy)hexyl) O,O’-((1R,2E,6S,10E,11aS,13S,14aR)-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecine-1,13-diyl) dimaleate (8k)
(1R,2E,6S,10E,11aS,13S,14aR)-13-Hydroxy-6-methyl-4-oxo-1,6,7,8,9,11a,12,13,14,14a-decahydro-4H-cyclopenta[f][1]oxacyclotridecin-1-yl (6-((4-isothiocyanatobenzoyl)oxy)hexyl) maleate (9k)
3.2. Cell Viability Analysis
3.3. Cell Cycle Analysis
3.4. Hoechst 33258 Staining Assay
3.5. Cell Apoptosis Analysis
3.6. Mitochondrial Membrane Potential Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Meinardi, M.T.; Gietema, J.A.; van Veldhuisen, D.J.; van der Graaf, W.T.; de Vries, E.G.; Sleijfer, D.T. Long-term chemotherapy-related cardiovascular morbidity. Cancer Treat. Rev. 2000, 26, 429–447. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Shangguan, D.; Wu, Y.; Chen, Y.; Liu, N.; Tang, J.; Yao, D.; Shi, Y. Curcumin protects against doxorubicin induced oxidative stress by regulating the Keap1-Nrf2-ARE and autophagy signaling pathways. Psychopharmacology 2023, 240, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Tayem, Y.; Green, C.J.; Motterlini, R.; Foresti, R. Isothiocyanate-cysteine conjugates protect renal tissue against cisplatin-induced apoptosis via induction of heme oxygenase-1. Pharmacol. Res. 2014, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jonan, S.; Hamouda, N.; Fujiwara, A.; Iwata, K.; Fujita, T.; Kato, S.; Amagase, K. Alleviative effects of glutamate against chemotherapeutic agent-induced intestinal mucositis. J. Physiol. Pharmacol. 2022, 73, 539–546. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Anadu, N.O.; Davisson, V.J.; Cushman, M. Synthesis and anticancer activity of brefeldin A ester derivatives. J. Med. Chem. 2006, 49, 3897–3905. [Google Scholar] [CrossRef]
- Singleton, V.L.; Bohonos, N.; Ullstrup, A.J. Decumbin, A new compound from a species of Penicillium. Nature 1958, 181, 1072–1073. [Google Scholar] [CrossRef]
- Sam, C.T. Effect of brefeldin A and actinomycin D on culture growth and brefeldin A yield in Curvularia lunata. Folia Microbiol. 1978, 23, 133–136. [Google Scholar] [CrossRef]
- Abraham, W.R.; Arfmann, H.A. Penicillium camemberti a new source of brefeldin A. Planta Med. 1992, 58, 484. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wu, Y.F.; Xue, F.; Wu, Z.X.; Xue, Y.P.; Zheng, Y.G.; Shen, Y.C. Isolation of brefeldin A from Eupenicillium brefeldianum broth using macroporous resin adsorption chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 895–896, 146–153. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, C.; Al Chnani, A.A.; Zhou, Q.; Tong, Q.; Wang, W.; Zang, Y.; Gong, J.; Wu, Z.; Liu, J.; et al. Dibrefeldins A and B, a pair of epimers representing the first brefeldin A dimers with cytotoxic activities from Penicillium janthinellum. Bioorg. Chem. 2019, 86, 176–182. [Google Scholar] [CrossRef]
- Lippincott-Schwartz, J.; Yuan, L.C.; Bonifacino, J.S.; Klausner, R.D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: Evidence for membrane cycling from Golgi to ER. Cell 1989, 56, 801–813. [Google Scholar] [CrossRef]
- Donaldson, J.G.; Finazzi, D.; Klausner, R.D. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature 1992, 360, 350–352. [Google Scholar] [CrossRef]
- Mossessova, E.; Corpina, R.A.; Goldberg, J. Crystal structure of ARF1*Sec7 complexed with brefeldin A and its implications for the guanine nucleotide exchange mechanism. Mol. Cell 2003, 12, 1403–1411. [Google Scholar] [CrossRef]
- Alexander, B.; Fishman, A.I.; Green, D.; Choudhury, M.S.; Konno, S. Attenuation of androgenic regulation by brefeldin A in androgen-responsive prostate cancer cells. Urol. Oncol. 2013, 31, 104–109. [Google Scholar] [CrossRef]
- Wlodkowic, D.; Skommer, J.; Pelkonen, J. Brefeldin A triggers apoptosis associated with mitochondrial breach and enhances HA14-1- and anti-Fas-mediated cell killing in follicular lymphoma cells. Leuk. Res. 2007, 31, 1687–1700. [Google Scholar] [CrossRef]
- Wang, J.; Fang, Y.; Yan, L.; Yuan, N.; Zhang, S.; Xu, L.; Nie, M.; Zhang, X.; Wang, J. Erythroleukemia cells acquire an alternative mitophagy capability. Sci. Rep. 2016, 6, 24641. [Google Scholar] [CrossRef]
- Phillips, L.R.; Wolfe, T.L.; Malspeis, L.; Supko, J.G. Analysis of brefeldin A and the prodrug breflate in plasma by gas chromatography with mass selective detection. J. Pharm. Biomed. Anal. 1998, 16, 1301–1309. [Google Scholar] [CrossRef]
- Kikuchi, S.; Shinpo, K.; Tsuji, S.; Yabe, I.; Niino, M.; Tashiro, K. Brefeldin A-induced neurotoxicity in cultured spinal cord neurons. J. Neurosci. Res. 2003, 71, 591–599. [Google Scholar] [CrossRef]
- Tanii, I.; Yoshinaga, K.; Toshimori, K. The effects of brefeldin A on acrosome formation and protein transport to the acrosome in organ cultures of rat seminiferous tubules. J. Electron. Microsc. 1998, 47, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Ignashkova, T.I.; Gendarme, M.; Peschk, K.; Eggenweiler, H.M.; Lindemann, R.K.; Reiling, J.H. Cell survival and protein secretion associated with Golgi integrity in response to Golgi stress-inducing agents. Traffic 2017, 18, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Paek, S.M. Recent synthesis and discovery of brefeldin A analogs. Mar. Drugs 2018, 16, 133. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Tian, K.; Pan, H.; Liu, Y.; Xu, F.; Li, Z.; Uchita, T.; Gao, M.; Hua, H.; Li, D. Novel hybrids of brefeldin A and nitrogen mustards with improved antiproliferative selectivity: Design, synthesis and antitumor biological evaluation. Eur. J. Med. Chem. 2018, 150, 53–63. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Gao, Y.; Liu, J.Y.; Xu, Y.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. Design and characterization of a natural Arf-GEFs inhibitor prodrug CHNQD-01255 with potent anti-hepatocellular carcinoma efficacy in vivo. J. Med. Chem. 2022, 65, 11970–11984. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Wu, S.; Wu, Y.W.; Gao, Y.; Chong, D.; Sun, C.; Wei, M.Y.; Gu, Y.C.; Shao, C.L.; Gu, Y. New brefeldin A-cinnamic acid ester derivatives as potential antitumor agents: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2022, 240, 114598. [Google Scholar] [CrossRef]
- Soundararajan, P.; Kim, J.S. Anti-carcinogenic glucosinolates in cruciferous vegetables and their antagonistic effects on prevention of cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef]
- Ramirez, D.; Abellan-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional ingredients from Brassicaceae species: Overview and perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef]
- Nyein, C.M.; Zhong, X.; Lu, J.; Luo, H.; Wang, J.; Rapposelli, S.; Li, M.; Ou-Yang, Y.; Pi, R.; He, X. Synthesis and anti-glioblastoma effects of artemisinin-isothiocyanate derivatives. RSC Adv. 2018, 8, 40974–40983. [Google Scholar] [CrossRef]
- Yano, S.; Wu, S.; Sakao, K.; Hou, D.X. Involvement of ERK1/2-mediated ELK1/CHOP/DR5 pathway in 6-(methylsulfinyl)hexyl isothiocyanate-induced apoptosis of colorectal cancer cells. Biosci. Biotechnol. Biochem. 2019, 83, 960–969. [Google Scholar] [CrossRef]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef]
- Singh, D.; Arora, R.; Bhatia, A.; Singh, H.; Singh, B.; Arora, S. Molecular targets in cancer prevention by 4-(methylthio)butyl isothiocyanate—A comprehensive review. Life Sci. 2020, 241, 117061. [Google Scholar] [CrossRef]
- Dinh, T.N.; Parat, M.O.; Ong, Y.S.; Khaw, K.Y. Anticancer activities of dietary benzyl isothiocyanate: A comprehensive review. Pharmacol. Res. 2021, 169, 105666. [Google Scholar] [CrossRef]
- Ioannides, C.; Konsue, N. A principal mechanism for the cancer chemopreventive activity of phenethyl isothiocyanate is modulation of carcinogen metabolism. Drug Metab. Rev. 2015, 47, 356–373. [Google Scholar] [CrossRef]
- Minarini, A.; Milelli, A.; Tumiatti, V.; Ferruzzi, L.; Marton, M.R.; Turrini, E.; Hrelia, P.; Fimognari, C. Design, synthesis and biological evaluation of new naphtalene diimides bearing isothiocyanate functionality. Eur. J. Med. Chem. 2012, 48, 124–131. [Google Scholar] [CrossRef]
- Citi, V.; Martelli, A.; Testai, L.; Marino, A.; Breschi, M.C.; Calderone, V. Hydrogen sulfide releasing capacity of natural isothiocyanates: Is it a reliable explanation for the multiple biological effects of Brassicaceae? Planta Med. 2014, 80, 610–613. [Google Scholar] [CrossRef]
- Hu, X.; Xiao, Y.; Sun, J.; Ji, B.; Luo, S.; Wu, B.; Zheng, C.; Wang, P.; Xu, F.; Cheng, K. New possible silver lining for pancreatic cancer therapy: Hydrogen sulfide and its donors. Acta Pharm. Sin. B 2021, 11, 1148–1157. [Google Scholar] [CrossRef]
- Wallace, J.L.; Nagy, P.; Feener, T.D.; Allain, T.; Ditrói, T.; Vaughan, D.J.; Muscara, M.N.; de Nucci, G.; Buret, A.G. A proof-of-concept, Phase 2 clinical trial of the gastrointestinal safety of a hydrogen sulfide-releasing anti-inflammatory drug. Br. J. Pharmacol. 2020, 177, 769–777. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. Gasotransmitter hydrogen sulfide signaling in neuronal health and disease. Biochem. Pharmacol. 2018, 149, 101–109. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Whiteman, M.; Wood, M.E.; Torregrossa, R.; Baxter, G.F. Pharmacological postconditioning against myocardial infarction with a slow-releasing hydrogen sulfide donor, GYY4137. Pharmacol. Res. 2016, 111, 442–451. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Lucarini, E.; Micheli, L.; Mosca, I.; Ambrosino, P.; Soldovieri, M.V.; Martelli, A.; Testai, L.; Taglialatela, M.; Calderone, V.; et al. Effects of natural and synthetic isothiocyanate-based H2S-releasers against chemotherapy-induced neuropathic pain: Role of Kv7 potassium channels. Neuropharmacology 2017, 121, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Yanaka, A. Role of sulforaphane in protection of gastrointestinal tract against H. pylori and NSAID-induced oxidative stress. Curr. Pharm. Des. 2017, 23, 4066–4075. [Google Scholar] [CrossRef]
- Kim, M.W.; Kang, J.H.; Jung, H.J.; Park, S.Y.; Phan, T.H.L.; Namgung, H.; Seo, S.Y.; Yoon, Y.S.; Oh, S.H. Allyl isothiocyanate protects acetaminophen-induced liver injury via NRF2 activation by decreasing spontaneous degradation in hepatocyte. Nutrients 2020, 12, 3585. [Google Scholar] [CrossRef] [PubMed]
- Sestito, S.; Daniele, S.; Pietrobono, D.; Citi, V.; Bellusci, L.; Chiellini, G.; Calderone, V.; Martini, C.; Rapposelli, S. Memantine prodrug as a new agent for Alzheimer’s disease. Sci. Rep. 2019, 9, 4612. [Google Scholar] [CrossRef] [PubMed]
- Sestito, S.; Pruccoli, L.; Runfola, M.; Citi, V.; Martelli, A.; Saccomanni, G.; Calderone, V.; Tarozzi, A.; Rapposelli, S. Design and synthesis of H2S-donor hybrids: A new treatment for Alzheimer’s disease? Eur. J. Med. Chem. 2019, 184, 111745. [Google Scholar] [CrossRef]
- Pan, H.Q.; Hu, J.C.; Wang, S.J. A Plant Endophytic Fungus Eupenicillium brefeldianum F4a and Its Application. Chinese Patent No. ZL201410072690.1, 2 September 2015. [Google Scholar]


| Compound | a IC50 (μM) | b SIHeLa | |||||
|---|---|---|---|---|---|---|---|
| HepG2 | MDA-MB-231 | A375 | A549 | HeLa | L-02 | ||
| BFA | 0.12 ± 0.01 | 0.054 ± 0.001 | 0.039 ± 0.001 | 0.056 ± 0.002 | 0.044 ± 0.004 | 0.094 ± 0.004 | 2.14 |
| paclitaxel | 0.55 ± 0.03 | 0.33 ± 0.03 | 0.059 ± 0.001 | 0.014 ± 0.002 | 0.034 ± 0.002 | 0.13 ± 0.00 | 3.82 |
| 2 | >100 | 82.06 ± 2.70 | 42.99 ± 3.34 | 79.25 ± 2.52 | 45.73 ± 1.74 | 67.82 ± 2.00 | 1.48 |
| 5 | >40 | >40 | >40 | 20.9 ± 1.42 | >40 | >80 | c NC |
| 6 | 20.76 ± 1.22 | 6.83 ± 0.61 | 3.08 ± 0.43 | 7.18 ± 0.51 | 1.84 ± 0.08 | >80 | >43.48 |
| 7 | >40 | 17.03 ± 1.21 | 5.78 ± 0.10 | 20.35 ± 1.31 | 3.02 ± 0.23 | 8.60 ± 0.71 | 2.85 |
| 8a | >40 | >40 | >40 | >40 | 11.98 ± 1.47 | >80 | >6.68 |
| 8b | >40 | >40 | >40 | >40 | 16.41 ± 1.35 | >80 | >4.88 |
| 8c | >40 | >40 | >40 | >40 | 39.03 ± 1.43 | >80 | >2.05 |
| 8d | >40 | >40 | 22.77 ± 1.58 | >40 | 7.67 ± 0.33 | >80 | >10.43 |
| 8e | >40 | >40 | >40 | >40 | 26.78 ± 0.70 | >80 | >2.99 |
| 8f | >40 | >40 | >40 | >40 | >40 | >80 | c NC |
| 8g | >40 | >40 | >40 | >40 | 9.74 ± 0.37 | >80 | >8.21 |
| 8h | >40 | >40 | >40 | >40 | 8.48 ± 0.72 | >80 | >9.43 |
| 8i | >40 | >40 | >40 | >40 | >40 | >80 | c NC |
| 8j | >40 | >40 | >40 | 23.79 ± 0.02 | 30.59 ± 2.12 | 21.27 ± 0.16 | 0.70 |
| 8k | >40 | >40 | >40 | >40 | >40 | >80 | c NC |
| 9a | 5.93 ± 0.03 | 8.04 ± 0.01 | 2.07 ± 0.09 | 8.29 ± 0.10 | 2.69 ± 0.30 | 15.48 ± 1.22 | 5.75 |
| 9b | 6.54 ± 0.18 | 2.23 ± 0.04 | 2.79 ± 0.25 | 2.84 ± 0.02 | 2.28 ± 0.04 | 10.56 ± 0.60 | 4.63 |
| 9c | 8.84 ± 0.28 | 9.77 ± 0.22 | 10.41 ± 0.16 | 13.19 ± 0.03 | 3.63 ± 0.15 | 17.37 ± 0.44 | 4.79 |
| 9d | 2.34 ± 0.31 | 2.05 ± 0.27 | 2.93 ± 0.12 | 2.85 ± 0.10 | 0.99 ± 0.02 | 9.18 ± 0.13 | 9.27 |
| 9e | 5.77 ± 0.07 | 1.56 ± 0.05 | 3.87 ± 0.35 | 2.59 ± 0.13 | 0.81 ± 0.03 | 7.87 ± 0.33 | 9.72 |
| 9f | 27.73 ± 0.47 | 32.08 ± 1.03 | 21.31 ± 0.33 | 16.18 ± 0.06 | 13.12 ± 0.45 | 25.1 ± 0.82 | 1.91 |
| 9g | 12.8 ± 0.15 | 7.51 ± 0.07 | 11.88 ± 0.54 | 10.42 ± 0.59 | 3.63 ± 0.23 | 20.88 ± 0.49 | 5.75 |
| 9h | 13.27 ± 0.10 | 5.71 ± 0.20 | 11.2 ± 0.29 | 8.54 ± 0.26 | 9.68 ± 0.24 | 27.81 ± 1.39 | 2.87 |
| 9i | 11.87 ± 0.32 | 10.00 ± 0.26 | 9.10 ± 0.46 | 10.3 ± 0.35 | 6.59 ± 0.75 | 28.14 ± 0.14 | 4.27 |
| 9j | 3.69 ± 0.14 | 2.27 ± 0.03 | 1.5 ± 0.05 | 4.61 ± 0.12 | 1.71 ± 0.02 | 10.76 ± 0.57 | 6.29 |
| 9k | 3.28 ± 0.27 | 2.61 ± 0.08 | 7.27 ± 0.57 | 8.34 ± 0.24 | 2.39 ± 0.10 | 23.95 ± 1.23 | 10.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Chen, X.; Qu, Y.; Ma, Q.; Pan, H.; Li, H.; Hua, H.; Li, D. Design and Synthesis of Brefeldin A-Isothiocyanate Derivatives with Selectivity and Their Potential for Cervical Cancer Therapy. Molecules 2023, 28, 4284. https://doi.org/10.3390/molecules28114284
Wang M, Chen X, Qu Y, Ma Q, Pan H, Li H, Hua H, Li D. Design and Synthesis of Brefeldin A-Isothiocyanate Derivatives with Selectivity and Their Potential for Cervical Cancer Therapy. Molecules. 2023; 28(11):4284. https://doi.org/10.3390/molecules28114284
Chicago/Turabian StyleWang, Mingying, Xiaoyuan Chen, Ying Qu, Qingyinglu Ma, Huaqi Pan, Haonan Li, Huiming Hua, and Dahong Li. 2023. "Design and Synthesis of Brefeldin A-Isothiocyanate Derivatives with Selectivity and Their Potential for Cervical Cancer Therapy" Molecules 28, no. 11: 4284. https://doi.org/10.3390/molecules28114284
APA StyleWang, M., Chen, X., Qu, Y., Ma, Q., Pan, H., Li, H., Hua, H., & Li, D. (2023). Design and Synthesis of Brefeldin A-Isothiocyanate Derivatives with Selectivity and Their Potential for Cervical Cancer Therapy. Molecules, 28(11), 4284. https://doi.org/10.3390/molecules28114284






