Purification of Natural Pigments Violacein and Deoxyviolacein Produced by Fermentation Using Yarrowia lipolytica
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pigment Recovery
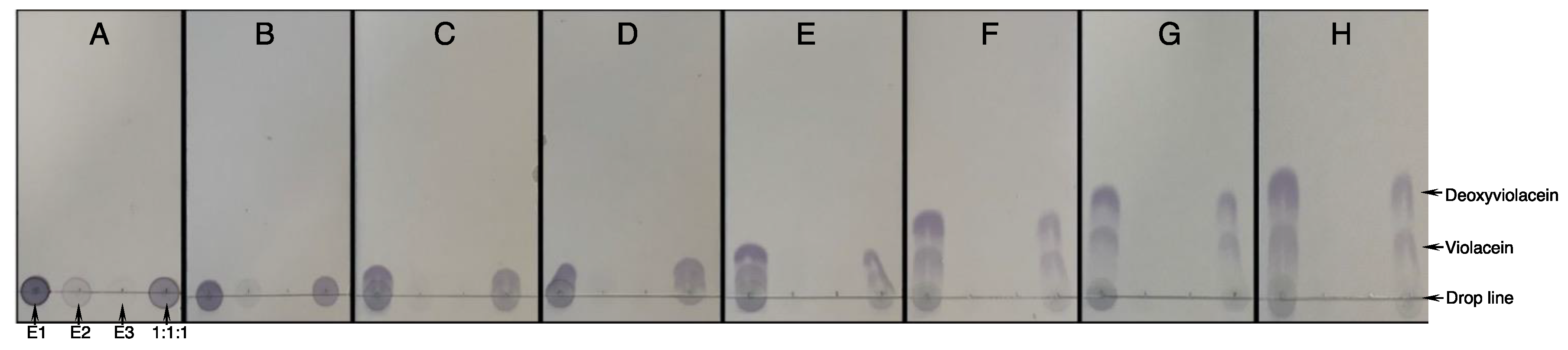
2.2. Optimization of Solvent Composition for Pigment Separation
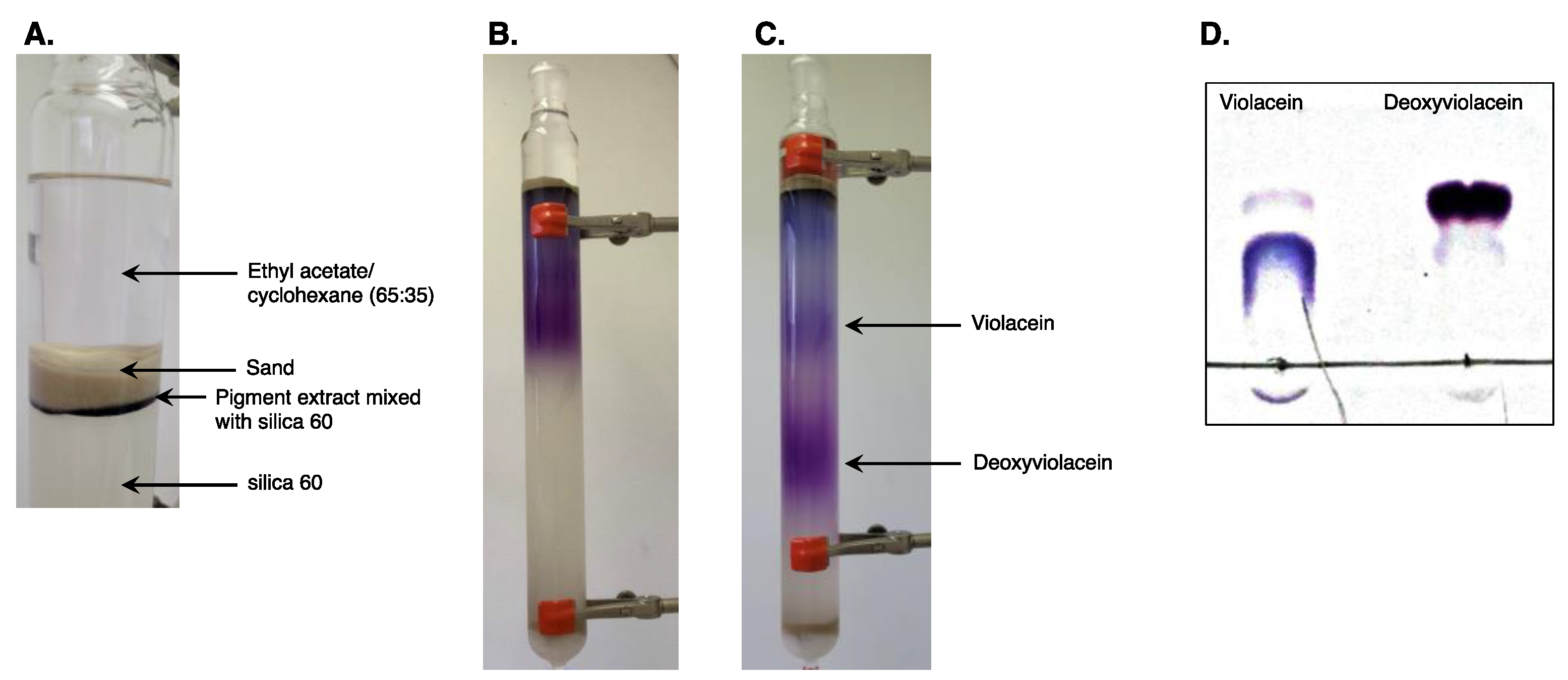
2.3. Separation of Violacein and Deoxyviolacein Using Column Chromatography
2.4. NMR Spectroscopy Analysis of Pigments
3. Materials and Methods
3.1. Yarrowia lipolytica Strain
3.2. Culture Medium
3.3. Pigment Production
3.4. Extraction of Pigments Using a Thermoregulated Double-Jacket Vessel
3.5. Pigment Quantification
3.6. Pigment Separation Using Thin-Layer Chromatography
3.7. Pigment Purification Using Column Chromatography
3.8. Pigment Analysis
3.8.1. Pigment Analysis Using High-Performance Thin-Layer Chromatography (HPTLC)
3.8.2. Pigment Analysis Using Nuclear Magnetic Resonance (NMR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Elshemy, N.S. Unconventional Natural Dyeing Using Microwave Heating with Cochineal as Natural Dyes. Res. J. Text. Appar. 2011, 15, 26–36. [Google Scholar] [CrossRef]
- Dufossé, L. Pigments, Microbial. In Encyclopedia of Microbiology, 3rd ed.; Elsevier/Academic Press: New York, NY, USA, 2009; pp. 457–471. [Google Scholar] [CrossRef]
- Sarwar, M.; Ali, S.; Hussain, M.T.; Atif, M.; Majeed, M.I. Cotton Fabric Dyeability Assessment of Floral Extracts Obtained from Binary Mixtures of Callistemon citrinus and Tagetes erecta L. J. Nat. Fibers 2019, 16, 484–493. [Google Scholar] [CrossRef]
- Carmen, Z.; Daniel, S. Textile Organic Dyes—Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents—A Critical Overview. In Organic Pollutants Ten Years after the Stockholm Convention—Environmental and Analytical Update; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, S.; Lyuck, S.; Kim, S.B.; Mitchell, R.J. High-Level Production of Violacein by the Newly Isolated Duganella violaceinigra str. NI28 and Its Impact on Staphylococcus aureus. Sci. Rep. 2015, 5, 15598. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Xue, Y.; Zhang, C.; Xing, X.H.; Lou, K.; Zhang, Z.; Li, Y.; Zhang, G.; Bi, J.; et al. Production of Violet Pigment by a Newly Isolated Psychrotrophic Bacterium from a Glacier in Xinjiang, China. Biochem. Eng. J. 2009, 43, 135–141. [Google Scholar] [CrossRef]
- Adams, J.S. Inequity In Social Exchange. In Advances in Experimental Social Psychology; Academic Press: Cambridge, MA, USA, 1965; Volume 2, pp. 267–299. [Google Scholar]
- Li, W.J.; Zhang, Y.Q.; Park, D.J.; Li, C.T.; Xu, L.H.; Kim, C.J.; Jiang, C.L. Duganella violaceinigra sp. nov., a Novel Mesophilic Bacterium Isolated from Forest Soil. Int. J. Syst. Evol. Microbiol. 2004, 54, 1811–1814. [Google Scholar] [CrossRef]
- Sasidharan, A.; Sasidharan, N.K.; Amma, D.B.N.S.; Vasu, R.K.; Nataraja, A.V.; Bhaskaran, K. Antifungal Activity of Violacein Purified from a Novel Strain of Chromobacterium sp. NIIST (MTCC 5522). J. Microbiol. 2015, 53, 694–701. [Google Scholar] [CrossRef]
- Wang, H.; Wang, F.; Zhu, X.; Yan, Y.; Yu, X.; Jiang, P.; Xing, X.H. Biosynthesis and Characterization of Violacein, Deoxyviolacein and Oxyviolacein in Heterologous Host, and Their Antimicrobial Activities. Biochem. Eng. J. 2012, 67, 148–155. [Google Scholar] [CrossRef]
- Platt, D.; Amara, S.; Mehta, T.; Vercuyssee, K.; Myles, E.L.; Johnson, T.; Tiriveedhi, V. Violacein Inhibits Matrix Metalloproteinase Mediated CXCR4 Expression: Potential Anti-Tumor Effect in Cancer Invasion and Metastasis. Biochem. Biophys. Res. Commun. 2014, 455, 107–112. [Google Scholar] [CrossRef]
- Durán, N.; Justo, G.Z.; Ferreira, C.V.; Melo, P.S.; Cordi, L.; Martins, D. Violacein: Properties and Biological Activities. Biotechnol. Appl. Biochem. 2007, 48, 127. [Google Scholar] [CrossRef]
- Andrighetti-Fröhner, C.R.; Antonio, R.V.; Creczynski-Pasa, T.B.; Barardi, C.R.M.; Simões, C.M.O. Cytotoxicity and Potential Antiviral Evaluation of Violacein Produced by Chromobacterium violaceum. Mem. Do Inst. Oswaldo Cruz 2003, 98, 843–848. [Google Scholar] [CrossRef]
- Antonisamy, P.; Ignacimuthu, S. Immunomodulatory, Analgesic and Antipyretic Effects of Violacein Isolated from Chromobacterium violaceum. Phytomedicine 2010, 17, 300–304. [Google Scholar] [CrossRef]
- Cazoto, L.L.; Martins, D.; Ribeiro, M.G.; Durán, N.; Nakazato, G. Antibacterial Activity of Violacein against Staphylococcus aureus Isolated from Bovine Mastitis. J. Antibiot. 2011, 64, 395–397. [Google Scholar] [CrossRef]
- Subramaniam, S.; Ravi, V.; Sivasubramanian, A. Synergistic Antimicrobial Profiling of Violacein with Commercial Antibiotics against Pathogenic Micro-Organisms. Pharm. Biol. 2014, 52, 86–90. [Google Scholar] [CrossRef]
- Sánchez, C.; Braña, A.F.; Méndez, C.; Salas, J.A. Reevaluation of the Violacein Biosynthetic Pathway and Its Relationship to Indolocarbazole Biosynthesis. ChemBioChem 2006, 7, 1231–1240. [Google Scholar] [CrossRef]
- Jiang, P.X.; Wang, H.S.; Zhang, C.; Lou, K.; Xing, X.H. Reconstruction of the Violacein Biosynthetic Pathway from Duganella sp. B2 in Different Heterologous Hosts. Appl. Microbiol. Biotechnol. 2010, 86, 1077–1088. [Google Scholar] [CrossRef]
- Gwon, D.A.; Seok, J.Y.; Jung, G.Y.; Lee, J.W. Biosensor-Assisted Adaptive Laboratory Evolution for Violacein Production. Int. J. Mol. Sci. 2021, 22, 6594. [Google Scholar] [CrossRef]
- Fang, M.Y.; Zhang, C.; Yang, S.; Cui, J.Y.; Jiang, P.X.; Lou, K.; Wachi, M.; Xing, X.H. High Crude Violacein Production from Glucose by Escherichia coli Engineered with Interactive Control of Tryptophan Pathway and Violacein Biosynthetic Pathway. Microb. Cell Factories 2015, 14, 8. [Google Scholar] [CrossRef]
- Rodrigues, A.L.; Trachtmann, N.; Becker, J.; Lohanatha, A.F.; Blotenberg, J.; Bolten, C.J.; Korneli, C.; de Souza Lima, A.O.; Porto, L.M.; Sprenger, G.A.; et al. Systems Metabolic Engineering of Escherichia coli for Production of the Antitumor Drugs Violacein and Deoxyviolacein. Metab. Eng. 2013, 20, 29–41. [Google Scholar] [CrossRef]
- Kholany, M.; Trébulle, P.; Martins, M.; Ventura, S.P.; Nicaud, J.-M.; Coutinho, J.A. Extraction and Purification of Violacein from Yarrowia lipolytica Cells Using Aqueous Solutions of Surfactants. J. Chem. Technol. Biotechnol. 2020, 95, 1126–1134. [Google Scholar] [CrossRef]
- Tong, Y.; Zhou, J.; Zhang, L.; Xu, P. A Golden-Gate Based Cloning Toolkit to Build Violacein Pathway Libraries in Yarrowia lipolytica. ACS Synth. Biol. 2021, 10, 115–124. [Google Scholar] [CrossRef]
- Al Sahyouni, W.; El Kantar, S.; Khelfa, A.; Park, Y.-K.; Nicaud, J.-M.; Louka, N.; Koubaa, M. Optimization of Cis-9-Heptadecenoic Acid Production from the Oleaginous Yeast Yarrowia lipolytica. Fermentation 2022, 8, 245. [Google Scholar] [CrossRef]
- El Kantar, S.; Koubaa, M. Valorization of Low-Cost Substrates for the Production of Odd Chain Fatty Acids by the Oleaginous Yeast Yarrowia lipolytica. Fermentation 2022, 8, 284. [Google Scholar] [CrossRef]
- Gu, Y.; Ma, J.; Zhu, Y.; Ding, X.; Xu, P. Engineering Yarrowia lipolytica as a Chassis for de Novo Synthesis of Five Aromatic-Derived Natural Products and Chemicals. ACS Synth. Biol. 2020, 9, 2096–2106. [Google Scholar] [CrossRef]
- Mitri, S.; Koubaa, M.; Maroun, R.G.; Rossignol, T.; Nicaud, J.-M.; Louka, N. Bioproduction of 2-Phenylethanol through Yeast Fermentation on Synthetic Media and on Agro-Industrial Waste and by-Products: A Review. Foods 2022, 11, 109. [Google Scholar] [CrossRef]
- Spagnuolo, M.; Hussain, M.S.; Gambill, L.; Blenner, M. Alternative Substrate Metabolism in Yarrowia lipolytica. Front. Microbiol. 2018, 9, 1077. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Amaro, R.; Nicaud, J.M. Metabolic Engineering for Expanding the Substrate Range of Yarrowia lipolytica. Trends Biotechnol. 2016, 34, 798–809. [Google Scholar] [CrossRef]
- Kanelli, M.; Mandic, M.; Kalakona, M.; Vasilakos, S.; Kekos, D.; Nikodinovic-Runic, J.; Topakas, E. Microbial Production of Violacein and Process Optimization for Dyeing Polyamide Fabrics with Acquired Antimicrobial Properties. Front. Microbiol. 2018, 9, 1495. [Google Scholar] [CrossRef]
- Alem, D.; Marizcurrena, J.J.; Saravia, V.; Davyt, D.; Martinez-Lopez, W.; Castro-Sowinski, S. Production and Antiproliferative Effect of Violacein, a Purple Pigment Produced by an Antarctic Bacterial Isolate. World J. Microbiol. Biotechnol. 2020, 36, 120. [Google Scholar] [CrossRef]
- Choi, S.Y.; Yoon, K.; Lee, J.I.; Mitchell, R.J. Violacein: Properties and Production of a Versatile Bacterial Pigment. BioMed. Res. Int. 2015, 2015, e465056. [Google Scholar] [CrossRef]
- Wille, G.; Steglich, W. A Short Synthesis of the Bacterial Pigments Violacein and Deoxyviolacein. Synthesis 2001, 2001, 0759–0762. [Google Scholar] [CrossRef]
- Masuelli, L.; Pantanella, F.; La Regina, G.; Benvenuto, M.; Fantini, M.; Mattera, R.; Di Stefano, E.; Mattei, M.; Silvestri, R.; Schippa, S.; et al. Violacein, an Indole-Derived Purple-Colored Natural Pigment Produced by Janthinobacterium lividum, Inhibits the Growth of Head and Neck Carcinoma Cell Lines Both In Vitro and In Vivo. Tumour Biol. 2016, 37, 3705–3717. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.A.; Venil, C.K.; Aruldass, C.A. Production of Violacein by Chromobacterium violaceum Grown in Liquid Pineapple Waste: Current Scenario. In Beneficial Microorganisms in Agriculture, Aquaculture and other Areas; Microbiology Monographs; Liong, M.-T., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 45–58. ISBN 978-3-319-23183-9. [Google Scholar]
- Bilsland, E.; Tavella, T.A.; Krogh, R.; Stokes, J.E.; Roberts, A.; Ajioka, J.; Spring, D.R.; Andricopulo, A.D.; Costa, F.T.M.; Oliver, S.G. Antiplasmodial and Trypanocidal Activity of Violacein and Deoxyviolacein Produced from Synthetic Operons. BMC Biotechnol. 2018, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.L.; Göcke, Y.; Bolten, C.; Brock, N.L.; Dickschat, J.S.; Wittmann, C. Microbial Production of the Drugs Violacein and Deoxyviolacein: Analytical Development and Strain Comparison. Biotechnol. Lett. 2012, 34, 717–720. [Google Scholar] [CrossRef]
- Füller, J.J.; Röpke, R.; Krausze, J.; Rennhack, K.E.; Daniel, N.P.; Blankenfeldt, W.; Schulz, S.; Jahn, D.; Moser, J. Biosynthesis of Violacein, Structure and Function of l-Tryptophan Oxidase VioA from Chromobacterium violaceum. J. Biol. Chem. 2016, 291, 20068–20084. [Google Scholar] [CrossRef]
- Dantas, C.; Tauler, R.; Ferreira, M.M.C. Exploring in Vivo Violacein Biosynthesis by Application of Multivariate Curve Resolution on Fused UV-VIS Absorption, Fluorescence, and Liquid Chromatography-Mass Spectrometry Data. Anal. Bioanal. Chem. 2013, 405, 1293–1302. [Google Scholar] [CrossRef]
- Dogancı, M.A.; Ay Sal, F.; Guler, H.I.; Katı, H.; Ceylan, E.; Belduz, A.O.; Bozdal, G.; Yaylı, N.; Canakcı, S. Investigation of Potential Inhibitor Properties of Violacein against HIV-1 RT and CoV-2 Spike RBD:ACE-2. World J. Microbiol. Biotechnol. 2022, 38, 161. [Google Scholar] [CrossRef]
- Stevens, W.C.; Hill, D.C. General Methods for Flash Chromatography Using Disposable Columns. Mol. Divers. 2009, 13, 247–252. [Google Scholar] [CrossRef]
- Still, W.C.; Kahn, M.; Mitra, A. Rapid Chromatographic Technique for Preparative Separations with Moderate Resolution. J. Org. Chem. 1978, 43, 2923–2925. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, P.; Lu, Y.; Ruan, Z.; Jiang, R.; Xing, X.-H.; Lou, K.; Wei, D. Optimization of Culture Conditions for Violacein Production by a New Strain of Duganella sp. B2. Biochem. Eng. J. 2009, 44, 119–124. [Google Scholar] [CrossRef]

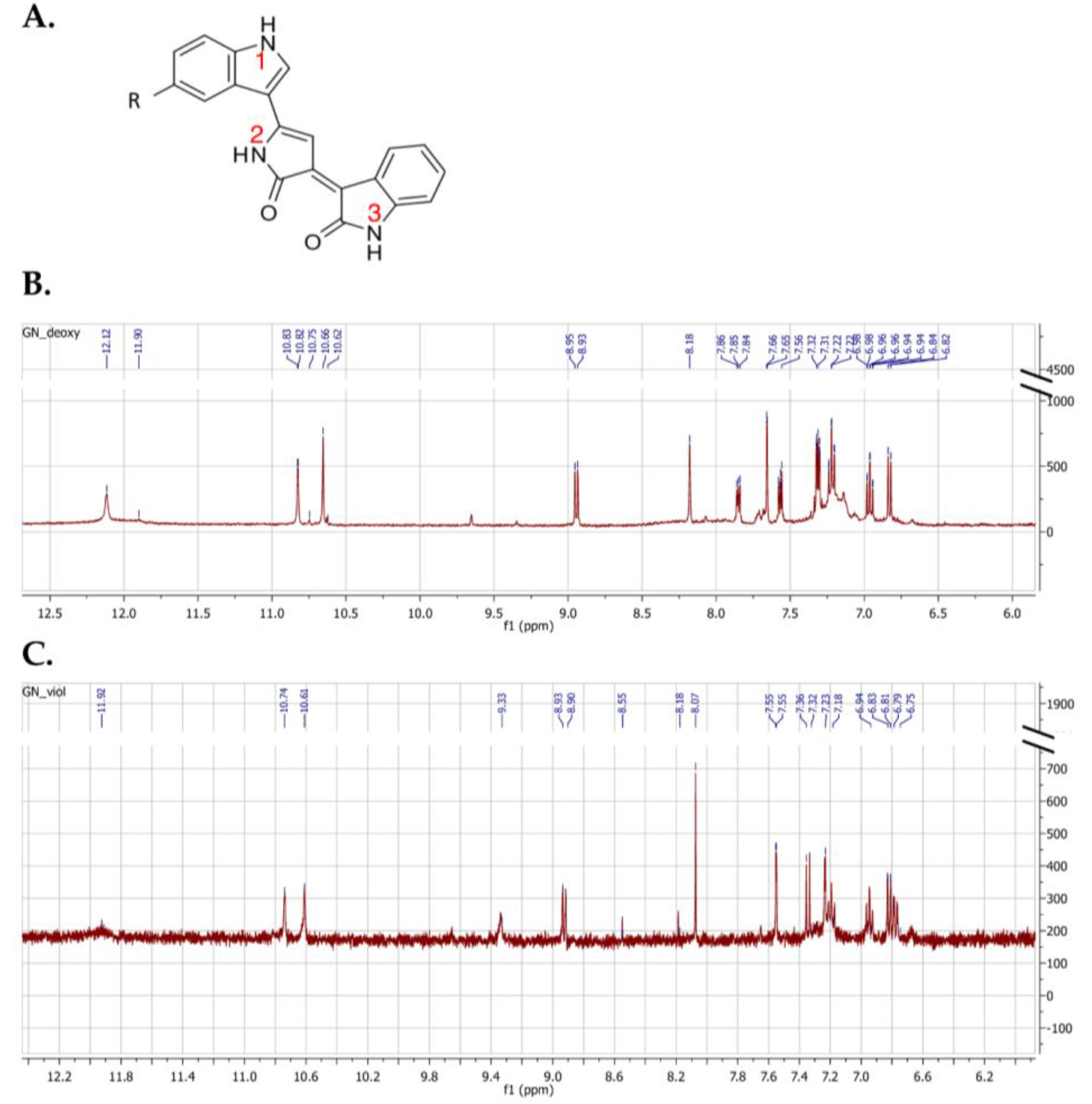
| Position of NH Functional Groups | Violacein | Deoxyviolacein | ||
|---|---|---|---|---|
| Wille and Steglich [33] | This Study | Wille and Steglich [33] | This Study | |
| 1 | 11.88 ppm | 11.92 ppm | 12.09 ppm | 12.12 ppm |
| 2 | 10.72 ppm | 10.74 ppm | 10.78 ppm | 10.83 ppm |
| 3 | 10.60 ppm | 10.61 ppm | 10.61 ppm | 10.66 ppm |
| A | Ethyl acetate/cyclohexane (10:90) | E | Ethyl acetate/cyclohexane (50:50) |
| B | Ethyl acetate/cyclohexane (20:80) | F | Ethyl acetate/cyclohexane (60:40) |
| C | Ethyl acetate/cyclohexane (30:70) | G | Ethyl acetate/cyclohexane (65:35) |
| D | Ethyl acetate/cyclohexane (40:60) | H | Ethyl acetate/cyclohexane (80:20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemer, G.; Louka, N.; Rabiller Blandin, P.; Maroun, R.G.; Vorobiev, E.; Rossignol, T.; Nicaud, J.-M.; Guénin, E.; Koubaa, M. Purification of Natural Pigments Violacein and Deoxyviolacein Produced by Fermentation Using Yarrowia lipolytica. Molecules 2023, 28, 4292. https://doi.org/10.3390/molecules28114292
Nemer G, Louka N, Rabiller Blandin P, Maroun RG, Vorobiev E, Rossignol T, Nicaud J-M, Guénin E, Koubaa M. Purification of Natural Pigments Violacein and Deoxyviolacein Produced by Fermentation Using Yarrowia lipolytica. Molecules. 2023; 28(11):4292. https://doi.org/10.3390/molecules28114292
Chicago/Turabian StyleNemer, Georgio, Nicolas Louka, Paul Rabiller Blandin, Richard G. Maroun, Eugène Vorobiev, Tristan Rossignol, Jean-Marc Nicaud, Erwann Guénin, and Mohamed Koubaa. 2023. "Purification of Natural Pigments Violacein and Deoxyviolacein Produced by Fermentation Using Yarrowia lipolytica" Molecules 28, no. 11: 4292. https://doi.org/10.3390/molecules28114292
APA StyleNemer, G., Louka, N., Rabiller Blandin, P., Maroun, R. G., Vorobiev, E., Rossignol, T., Nicaud, J.-M., Guénin, E., & Koubaa, M. (2023). Purification of Natural Pigments Violacein and Deoxyviolacein Produced by Fermentation Using Yarrowia lipolytica. Molecules, 28(11), 4292. https://doi.org/10.3390/molecules28114292









