New Optically Active tert-Butylarylthiophosphinic Acids and Their Selenium Analogues as the Potential Synthons of Supramolecular Organometallic Complexes: Syntheses and Crystallographic Structure Determination
Abstract
:1. Introduction
2. Results
2.1. Crystal and Molecular Structures of tert-Butyl-(4-methoxyphenyl)phosphinothioic Acid (1a) and tert-Butyl-(4-Trifluoromethylphenyl) Phosphinothioic Acid (1b)
2.2. Crystal and Molecular Structures of tert-Butyl-(4-methoxyphenyl)phosphinoselenoic Acid (Sp)-2a, (Rp)-2a, and (rac)-2a
3. Experimental
3.1. Synthesis
3.1.1. General Information
3.1.2. General Procedure and Characterization of the Racemic tert-Butylarylphosphinothioic Acids (1a–d)
3.1.3. General Procedure and Characterization of the Racemic tert-Butylarylphosphinoselenoic Acids (2a–d)
3.1.4. Synthetic Procedure and Characterization of Racemic tert-Butylarylphosphine Oxides (3a–d)

3.1.5. X-ray Crystallography
4. Conclusions
5. CCDC Accession Codes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Imamoto, T. Synthesis and application of high-performance P-chiral phosphine ligands. Proc. Jpn. Acad. Ser. B 2021, 97, 521–542. [Google Scholar] [CrossRef]
- Börner, A. (Ed.) Phosphorus Ligands in Asymmetric Catalysis: Synthesis and Application; Wiley-VCH: Weinheim, Germany, 2008; Volume 1. [Google Scholar]
- Grabulosa, A. P-Stereogenic Ligands in Enantioselective Catalysis; RSC Publishing: Cambridge, UK, 2011. [Google Scholar]
- Kolodiazhnyl, O.I. Recent advances in asymmetric synthesis of P-stereogenic phosphorus compounds. Top. Curr. Chem. 2014, 360, 161–236. [Google Scholar] [CrossRef]
- Dutartre, M.; Bayardon, J.; Jugé, S. Applications and stereoselective syntheses of P-chirogenic phosphor compounds. Chem. Soc. Rev. 2016, 45, 5771–5794. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Senanayake, C.H.; Tang, W. P-Chiral phosphorus ligands based on a 2,3-dihydrobenzo[d][1,3]oxaphosphole motif for asymmeric catalysis. Acc. Chem. Res. 2019, 52, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Carbré, A.; Riera, A.; Verdaguer, X. P-Stereogenic amino-phosphines as chiral ligands: From privileged intermediates to asymmetric catalysis. Acc. Chem. Res. 2020, 53, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Lemouzy, S.; Giordano, L.; Hérault, D.; Buono, G. Introducing chirality at phosphorus atoms: An update on the recent synthetic strategies for the preparation of optically pure P-stereogenic molecules. Eur. J. Org. Chem. 2020, 2020, 3351–3366. [Google Scholar] [CrossRef]
- Ye, F.; Xu, Z.; Xu, L.-W. The discovery of multifunctional chiral P ligands for the catalytic construction of quarternary carbon/ silicon and multiple stereogenic centers. Acc. Chem. Res. 2021, 54, 452–470. [Google Scholar] [CrossRef]
- Gavrilov, K.N.; Chuchelkin, I.V.; Trunina, V.M.; Firsin, I.D.; Bityak, Y.P.; Fedorov, D.A.; Zimarev, V.S.; Goulioukin, N.S. P,S-Bidentate Phosphoramidites with (Rα)-BINOL Core in Palladium-Catalyzed Asymmetric Allylic Substitution. Russ. J. Gen. Chem. 2022, 92, 2612–2619. [Google Scholar] [CrossRef]
- Wenzel, T.J. Discrimination of Chiral Compounds Using NMR Spectroscopy; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Wenzel, T.J.; Chisholm, C.D. Using NMR spectroscopic methods to determine enantiomeric purity and assign absolute stereochemistry. Prog. Nucl. Magn. Reson. Spectrosc. 2011, 59, 1–63. [Google Scholar] [CrossRef]
- De Vries, G. Science of Synthesis, Stereoselective Synthesis, 1st ed.; Georg Thieme Verlag: Stuttgart, Germany, 2011. [Google Scholar]
- Molander, G.A. Science of Synthesis, Stereoselective Synthesis, 2nd ed.; Georg Thieme Verlag: Stuttgart, Germany, 2011. [Google Scholar]
- Evans, P.A. Science of Synthesis, Stereoselective Synthesis, 3rd ed.; Georg Thieme Verlag: Stuttgart, Germany, 2011. [Google Scholar] [CrossRef]
- Pirkle, W.H. The nonequivalence of physical properties of enantiomers in optically active solvents. Differences in nuclear magnetic resonance spectra. J. Am. Chem. Soc. 1966, 88, 1837. [Google Scholar] [CrossRef]
- Wang, F.; Polavarapu, P.L.; Drabowicz, J.; Mikołajczyk, M.; Łyżwa, P. Absolute configurations, predominant conformations, and tautomeric structures of enantiomeric tert-butylphenylphosphinothioic acid. J. Org. Chem. 2001, 66, 9015–9019. [Google Scholar] [CrossRef]
- Harger, M.J.P. Proton magnetic resonance non-equivalence of the enantiomers of alkylphenylphosphinic amides. J. Chem. Soc. Perkin Trans. 1977, 2, 1882–1887. [Google Scholar] [CrossRef]
- Drabowicz, J.; Pokora-Sobczak, P.; Krasowska, D.; Czarnocki, Z. Optically Active t-butylphenylphosphinothioic acid: Synthesis, selected structural studies and applications as a chiral solvating agent. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 977–999. [Google Scholar] [CrossRef]
- Drabowicz, J.; Pokora-Sobczak, P.; Zając, A.; Wach-Panfiłow, P. A new procedure for the synthesis of optically active t-butylphenylphosphinothioic acid. Heteroatom Chem. 2014, 25, 674–677. [Google Scholar] [CrossRef]
- Drabowicz, J.; Pokora-Sobczak, P.; Zając, A.; Wach-Panfiłow, P. Sposób Wytwarzania Optycznie Czynnego Kwasu t-butylofenylotiofosfinowego. Polish Patent Pending P-405141, 27 August 2013. [Google Scholar]
- Jin, W.; Li, X.; Wan, B. A highly diastereo- and enantioselective copper(I)-catalyzed Henry reaction using a bis(sulfonamide)-diamine ligand. J. Org. Chem. 2011, 76, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.V.; Thomas, A.W. Modern synthetic methods for copper-mediated C(aryl)-O,C(aryl)-N, and C(aryl)-S bond formation. Angew. Chem. Int. Ed. 2003, 42, 5400–5449. [Google Scholar] [CrossRef]
- Deng, W.; Liu, L.; Zhang, C.; Liu, M.; Guo, Q.X. Copper-catalyzed cross-coupling of sulfonamides with aryl iodides and bromides facilitated by amino acid ligands. Tetrahedron Lett. 2005, 46, 7295–7298. [Google Scholar] [CrossRef]
- Tang, X.; Huang, L.; Qi, C.; Wu, X.; Wu, W.; Jiang, H. Copper-catalyzed sulfonamides formation from sodium sulfinates and amines. Chem. Commun. 2013, 49, 6102–6104. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, J.; Cheng, D. Mild and efficient indium metal catalyzed synthesis of sulfonamides and sulfonic esters. Synlett 2007, 16, 2501–2504. [Google Scholar] [CrossRef]
- Hassan, J.; Sevignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef]
- Wang, Z.; Bao, W.; Jiang, Y. L-proline promoted Ullmann-type reaction of vinyl bromides with imidazoles in ionic liquids. Chem. Commun. 2005, 22, 2849–2851. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, R.; Najera, C. The Sonogashira Reaction: A booming methodology in synthetic organic chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
- Firlik, S.; Skupiński, W.; Wielgosz, Z.; Stasiński, J. Application of the copper(II)-aminosilane catalysts in the oxidative polymerization of 2,6-dimethylphenol. Polimery 2015, 60, 372–376. [Google Scholar] [CrossRef]
- Flack, H.D.; Bernardinelli, G. Absolute structure and absolute configuration. Acta Cryst. 1999, A55, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, J.; Pokora-Sobczak, P.; Mielniczak, G.; Bujnicki, B.; Sieroń, L.; Drabowicz, J. The synthons containing sulfur and stereogenic phosphorus as heteroatoms: On the way to crystal structure determination of supramolecular complexes of organometallic and organic compounds. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 545–548. [Google Scholar] [CrossRef]
- Bujnicki, B.; Mielniczak, G.; Błaszczyk, J.; Pokora-Sobczak, P.; Sieroń, L.; Drabowicz, J. The progress on crystal structure determination of metalloorganic complexes and their organic synthons containing selenium and stereogenic phosphorus as heteroatoms. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 542–544. [Google Scholar] [CrossRef]
- Wang, F.; Polavarapu, P.L.; Drabowicz, J.; Kiełbasiński, P.; Potrzebowski, M.J.; Mikołajczyk, M.; Wieczorek, M.W.; Majzner, W.W.; Łażewska, I. Solution and crystal structures of chiral molecules can be significantly different: tert-butylphenylphosphinoselenoic acid. J. Phys. Chem. A 2004, 108, 2072–2079. [Google Scholar] [CrossRef]
- CrysAlis PRO; Rigaku Oxford Diffraction Ltd.: Yarnton, UK, 2015.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
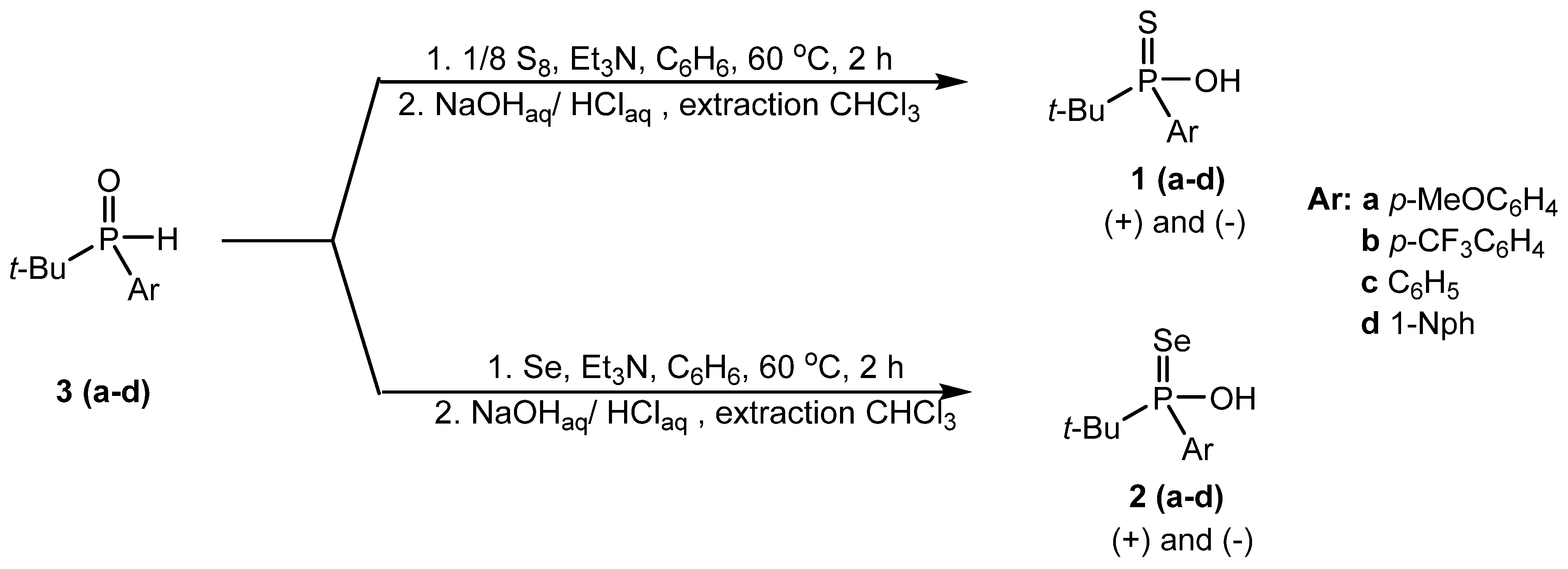









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błaszczyk, J.; Bujnicki, B.; Pokora-Sobczak, P.; Mielniczak, G.; Sieroń, L.; Kiełbasiński, P.; Drabowicz, J. New Optically Active tert-Butylarylthiophosphinic Acids and Their Selenium Analogues as the Potential Synthons of Supramolecular Organometallic Complexes: Syntheses and Crystallographic Structure Determination. Molecules 2023, 28, 4298. https://doi.org/10.3390/molecules28114298
Błaszczyk J, Bujnicki B, Pokora-Sobczak P, Mielniczak G, Sieroń L, Kiełbasiński P, Drabowicz J. New Optically Active tert-Butylarylthiophosphinic Acids and Their Selenium Analogues as the Potential Synthons of Supramolecular Organometallic Complexes: Syntheses and Crystallographic Structure Determination. Molecules. 2023; 28(11):4298. https://doi.org/10.3390/molecules28114298
Chicago/Turabian StyleBłaszczyk, Jarosław, Bogdan Bujnicki, Patrycja Pokora-Sobczak, Grażyna Mielniczak, Lesław Sieroń, Piotr Kiełbasiński, and Józef Drabowicz. 2023. "New Optically Active tert-Butylarylthiophosphinic Acids and Their Selenium Analogues as the Potential Synthons of Supramolecular Organometallic Complexes: Syntheses and Crystallographic Structure Determination" Molecules 28, no. 11: 4298. https://doi.org/10.3390/molecules28114298
APA StyleBłaszczyk, J., Bujnicki, B., Pokora-Sobczak, P., Mielniczak, G., Sieroń, L., Kiełbasiński, P., & Drabowicz, J. (2023). New Optically Active tert-Butylarylthiophosphinic Acids and Their Selenium Analogues as the Potential Synthons of Supramolecular Organometallic Complexes: Syntheses and Crystallographic Structure Determination. Molecules, 28(11), 4298. https://doi.org/10.3390/molecules28114298








