The Combined Effect of Lactic Acid Bacteria and Galactomyces geotrichum Fermentation on the Aroma Composition of Sour Whey
Abstract
1. Introduction
2. Results and Discussion
2.1. Cultivation Experiments
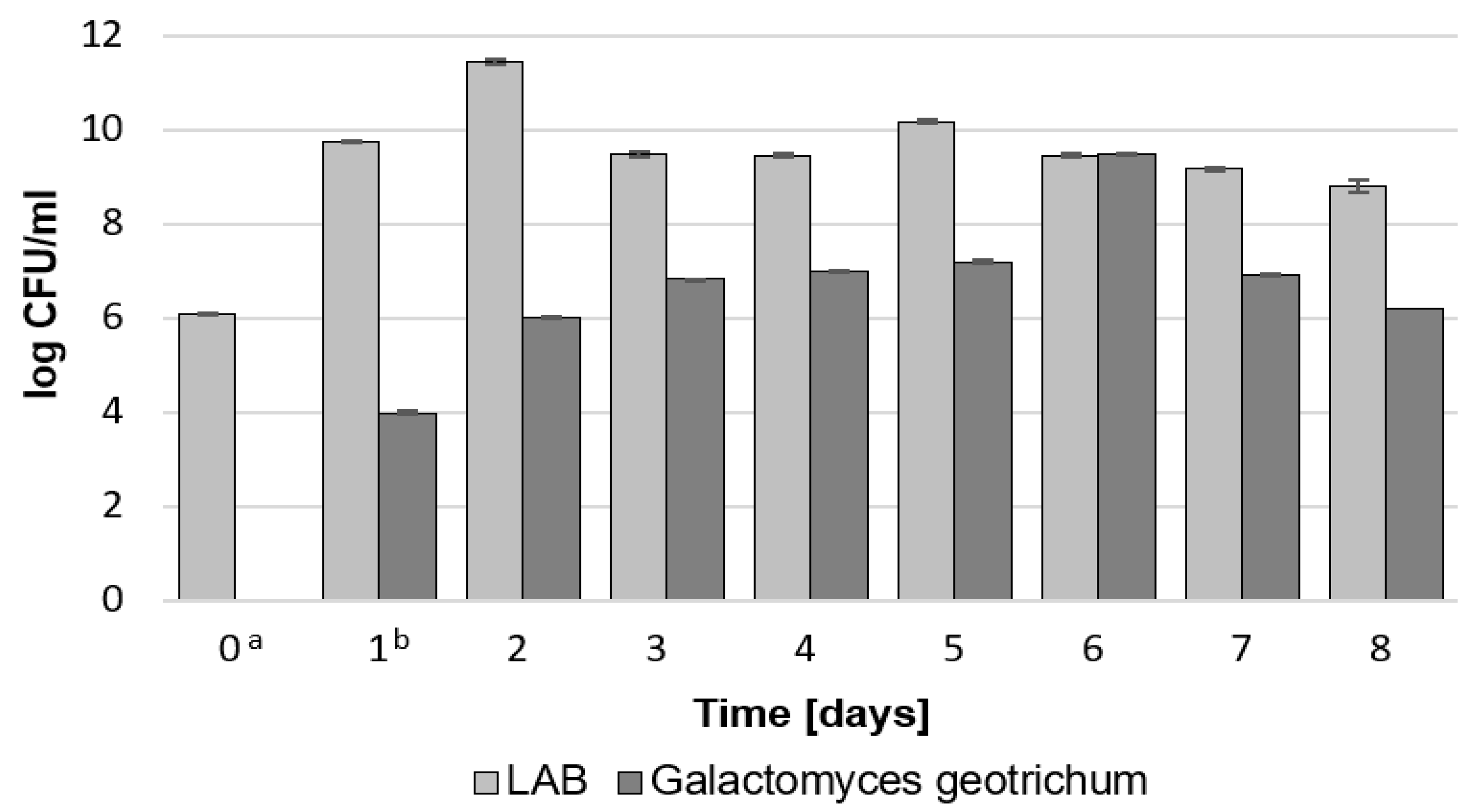
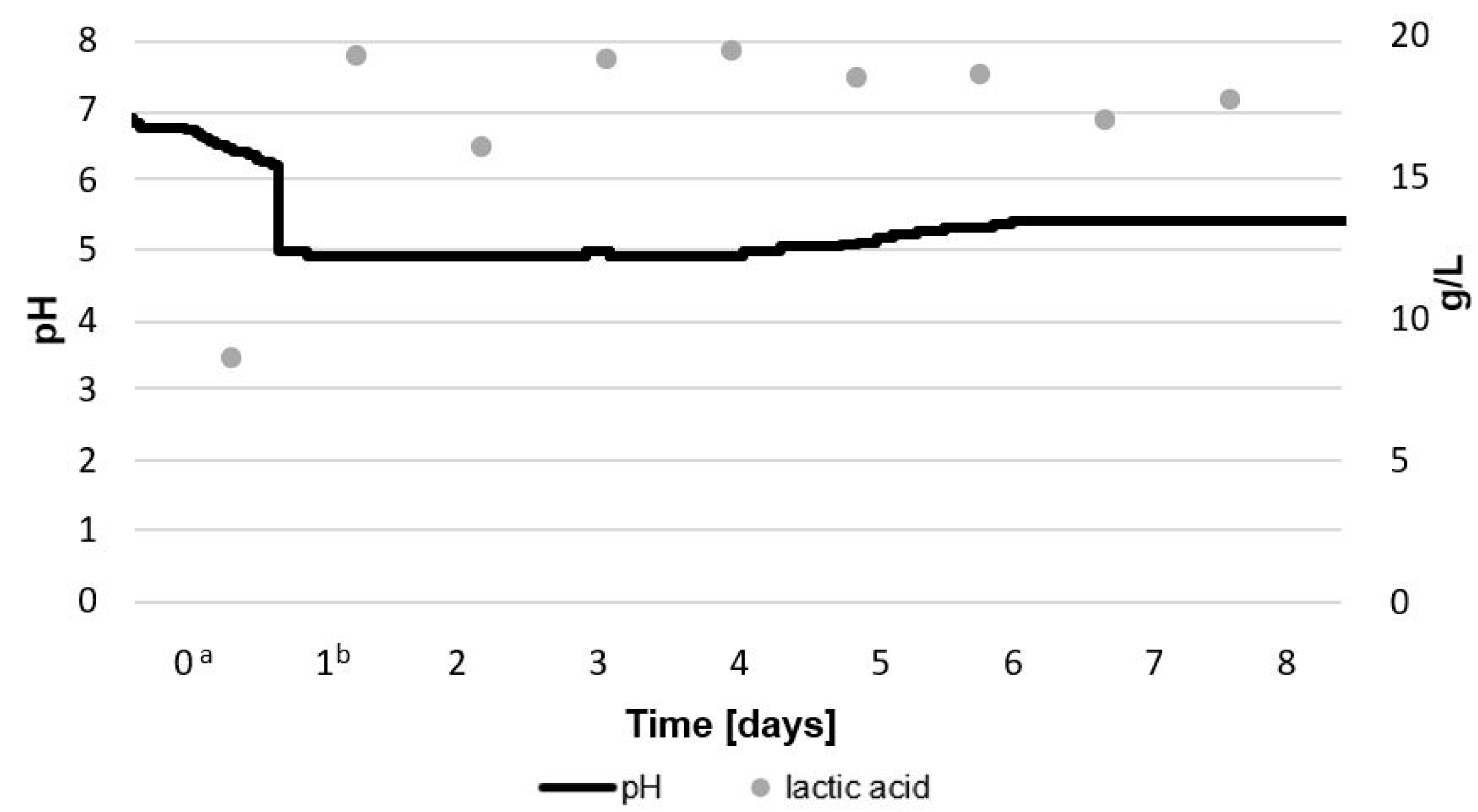
2.1.1. Total Count of LAB and G. geotrichum, Lactic Acid Concentration, and pH during Bioreactor Culture
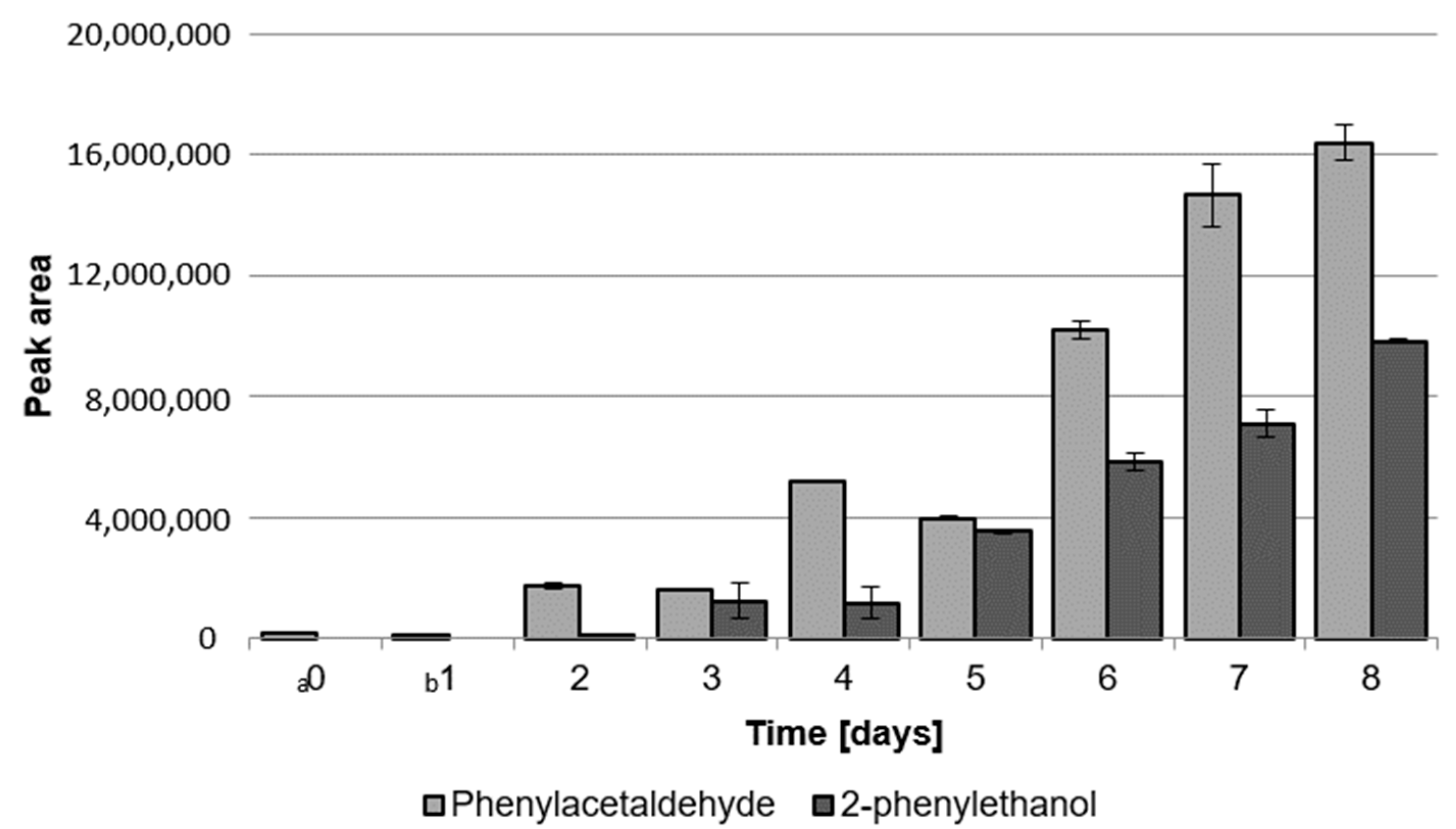
2.1.2. Content of Phenylacetaldehyde and 2-Phenylethanol during Cultivation of LAB and G. geotrichum
2.1.3. Amino Acid Concentration during Biotransformation of Sour Whey Medium by LAB and G. geotrichum
2.2. Sensory Evaluation
2.3. Identification of Key Aroma Compounds in a Post-Fermentation Product Using GC−O Analysis and Calculation of OAVs
3. Materials and Methods
3.1. Chemicals
3.2. Microorganisms
3.3. Bioreactor Culture
3.4. Cultivation Experiments
3.4.1. Total Count of LAB and G. geotrichum
3.4.2. Determination of Lactic Acid Concentration
3.4.3. Analysis of the Amino Acid Profile
3.5. Sensory Evaluation
3.6. Extraction of Odor-Active Compounds Using the Solvent-Assisted Flavor Evaporation (SAFE) Method
3.7. GC-O and GC-GC Analysis
3.7.1. Gas Chromatography–Olfactometry (GC-O)
3.7.2. Comprehensive Two-Dimensional Gas Chromatography (GC-GC)
3.8. Quantitation by Stable Isotope Dilution Assays (SIDAs)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Imarc Group Food Flavors Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2022–2027. Available online: https://www.imarcgroup.com/food-flavors-market (accessed on 10 April 2023).
- Bicas, J.L.; Silva, J.C.; Dionísio, A.P.; Pastore, G.M. Biotechnological production of bioflavors and functional sugars. Ciência E Tecnol. Aliment. 2010, 30, 7–18. [Google Scholar] [CrossRef]
- Sales, A.; Paulino, B.N.; Pastore, G.M.; Bicas, J.L. Biogeneration of aroma compounds. Curr. Opin. Food Sci. 2018, 19, 77–84. [Google Scholar] [CrossRef]
- Paulino, B.N.; Sales, A.; de Oliveira Felipe, L.; Pastore, G.M.; Molina, G.; Bicas, J.L. Recent advances in the microbial and enzymatic production of aroma compounds. Curr. Opin. Food Sci. 2020, 37, 98–106. [Google Scholar] [CrossRef]
- Longo, M.A.; Sanromán, M.A. Production of food aroma compounds: Microbial and enzymatic methodologies. Food Technol. Biotechnol. 2006, 44, 335–353. [Google Scholar] [CrossRef]
- Vilela, A.; Bacelar, E.; Pinto, T.; Anjos, R.; Correia, E.; Gonçalves, B.; Cosme, F. Beverage and Food Fragrance Biotechnology, Novel Applications, Sensory and Sensor Techniques: An Overview. Foods 2019, 8, 643. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, A.; Steinhaus, M.; Kotthoff, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s chemical signatures in human olfaction: A foodborne perspective for future biotechnology. Angew. Chem.—Int. Ed. 2014, 53, 7124–7143. [Google Scholar] [CrossRef] [PubMed]
- Majcher, M.A.; Myszka, K.; Kubiak, J.; Jeleń, H.H. Identification of key odorants of fried cottage cheese and contribution of Galactomyces geotrichum MK017 to the formation of 2-phenylethanol and related rose-like aroma compounds. Int. Dairy J. 2014, 39, 324–329. [Google Scholar] [CrossRef]
- Grygier, A.; Myszka, K.; Juzwa, W.; Białas, W.; Rudzińska, M. Galactomyces geotrichum mold isolated from a traditional fried cottage cheese produced omega-3 fatty acids. Int. J. Food Microbiol. 2020, 319, 108503. [Google Scholar] [CrossRef]
- Tang, H.; Wang, H.; Yu, H.; Piao, C.; Liu, J.; Hu, Y. Galactomyces geotrichum isolated from water kefir: Interaction with Lactobacillus kefir. Ital. J. Food Sci. 2016, 28, 287–297. [Google Scholar]
- Chaves-López, C.; Serio, A.; Rossi, C.; Pepe, A.; Compagnone, E.; Paparella, A. Interaction Between Galactomyces geotrichum KL20B, Lactobacillus plantarum LAT3 and Enterococcus faecalis KE06 During Milk Fermentation. Fermentation 2017, 3, 52. [Google Scholar] [CrossRef]
- Szudera-Kończal, K.; Myszka, K.; Kubiak, P.; Majcher, M.A. The Use of Sour and Sweet Whey in Producing Compositions with Pleasant Aromas Using the Mold Galactomyces geotrichum: Identification of Key Odorants. J. Agric. Food Chem. 2020, 68, 10799–10807. [Google Scholar] [CrossRef] [PubMed]
- Szudera-Kończal, K.; Myszka, K.; Kubiak, P.; Majcher, M.A. Analysis of the ability to produce pleasant aromas on sour whey and buttermilk by-products by mold Galactomyces geotrichum: Identification of key odorants. Molecules 2021, 26, 6239. [Google Scholar] [CrossRef] [PubMed]
- Pollner, G.; Schieberle, P. Characterization of the Key Odorants in Commercial Cold-Pressed Oils from Unpeeled and Peeled Rapeseeds by the Sensomics Approach. J. Agric. Food Chem. 2016, 64, 627–636. [Google Scholar] [CrossRef]
- Sahin, B.; Schieberle, P. Characterization of the Key Aroma Compounds in Yeast Dumplings by Means of the Sensomics Concept. J. Agric. Food Chem. 2019, 67, 2973–2979. [Google Scholar] [CrossRef] [PubMed]
- Khoramnia, A.; Ebrahimpour, A.; Ghanbari, R.; Ajdari, Z.; Lai, O.M. Improvement of Medium Chain Fatty Acid Content and Antimicrobial Activity of Coconut Oil via Solid-State Fermentation Using a Malaysian Geotrichum candidum. Biomed Res. Int. 2013, 2013, 954542. [Google Scholar] [CrossRef] [PubMed]
- Conde-Báez, L.; Castro-Rosas, J.; Villagómez-Ibarra, J.R.; Páez-Lerma, J.B.; Gómez-Aldapa, C. Evaluation of Waste of the Cheese Industry for the Production of Aroma of Roses (Phenylethyl Alcohol). Waste Biomass Valorization 2017, 8, 1343–1350. [Google Scholar] [CrossRef]
- Tian, H.; Shi, Y.; Zhang, Y.; Yu, H.; Mu, H.; Chen, C. Screening of aroma-producing lactic acid bacteria and their application in improving the aromatic profile of yogurt. J. Food Biochem. 2019, 43, e12837. [Google Scholar] [CrossRef]
- Skóra, M.; Witalis, J.; Krzyściak, P.; Macura, A.B. Grzyby z rodzaju Geotrichum jako oportunistyczny patogen człowieka. Postep. Mikrobiol. 2009, 48, 125–132. [Google Scholar]
- Blank, I.; Fay, L.B. Formation of 4-hydroxy-2,5-dimethyl-3(2H)-furanone and 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone through maillard reaction based on pentose sugars. J. Agric. Food Chem. 1996, 44, 531–536. [Google Scholar] [CrossRef]
- Özdemir, N.; Yazıcı, G.; Şimşek, Ö.; Özkal, S.G.; Çon, A.H. The effect of lactic acid bacteria and yeast usage on aroma development during tarhana fermentation. Food Biosci. 2018, 26, 30–37. [Google Scholar] [CrossRef]
- Eliskases-Lechner, F.; Guéguen, M.; Panoff, J.M. Yeasts and Molds|Geotrichum candidum. Encycl. Dairy Sci. Second. Ed. 2011, 765–771. [Google Scholar] [CrossRef]
- Perkins, V.; Vignola, S.; Lessard, M.H.; Plante, P.L.; Corbeil, J.; Dugat-Bony, E.; Frenette, M.; Labrie, S. Phenotypic and Genetic Characterization of the Cheese Ripening Yeast Geotrichum candidum. Front. Microbiol. 2020, 11, 737. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martín, P.; Flórez, A.B.; Hernández-Barranco, A.; Mayo, B. Interaction between dairy yeasts and lactic acid bacteria strains during milk fermentation. Food Control 2008, 19, 62–70. [Google Scholar] [CrossRef]
- Chaves-López, C.; Serio, A.; Paparella, A.; Martuscelli, M.; Corsetti, A.; Tofalo, R.; Suzzi, G. Impact of microbial cultures on proteolysis and release of bioactive peptides in fermented milk. Food Microbiol. 2014, 42, 117–121. [Google Scholar] [CrossRef]
- Etschmann, M.; Bluemke, W.; Sell, D.; Schrader, J. Biotechnological production of 2-phenylethanol. Appl. Microbiol. Biotechnol. 2002, 59, 1–8. [Google Scholar] [CrossRef]
- Ravasio, D.; Wendland, J.; Walther, A. Major contribution of the Ehrlich pathway for 2-phenylethanol/rose flavor production in Ashbya gossypii. FEMS Yeast Res. 2014, 14, 833–844. [Google Scholar] [CrossRef]
- Krings, U.; Berger, R.G. Biotechnological production of flavours and fragrances. Appl. Microbiol. Biotechnol. 1998, 49, 1–8. [Google Scholar] [CrossRef]
- Maoz, I.; Lewinsohn, E.; Gonda, I. Amino acids metabolism as a source for aroma volatiles biosynthesis. Curr. Opin. Plant Biol. 2022, 67, 102221. [Google Scholar] [CrossRef]
- Bilyi, V.; Merzlov, S.; Narizhnyy, S.; Mashkin, Y.; Merzlova, G. Amino Acid Composition of Whey and Cottage Cheese Under Various Rennet Enzymes. Sci. Horiz. 2021, 24, 19–25. [Google Scholar] [CrossRef]
- Greenberg, R.S.; Ledford, R.A. Deamination of Glutamic and Aspartic Acids by Geotrichum candidum. J. Dairy Sci. 1979, 62, 368–372. [Google Scholar] [CrossRef]
- Boutrou, R.; Kerriou, L.; Gassi, J.Y. Contribution of Geotrichum candidum to the proteolysis of soft cheese. Int. Dairy J. 2006, 16, 775–783. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S. Role of lactic acid bacteria on the yogurt flavour: A review. Int. J. Food Prop. 2017, 20, 316–331. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Odour Thresholds; Oliemans Punter & Partners: Utrecht, The Netherlands, 2011; ISBN 9789081089418. [Google Scholar]
- Schuh, C.; Schieberle, P. Characterization of the key aroma compounds in the beverage prepared from Darjeeling black tea: Quantitative differences between tea leaves and infusion. J. Agric. Food Chem. 2006, 54, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Whetstine, M.E.C.; Cadwallader, K.R.; Drake, M.A. Characterization of aroma compounds responsible for the rosy/floral flavor in cheddar cheese. J. Agric. Food Chem. 2005, 53, 3126–3132. [Google Scholar] [CrossRef]
- Gottfried, J.A. Central mechanisms of odour object perception. Nat. Rev. Neurosci. 2010, 11, 628–641. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Pan, H.; Li, X.; Guo, D. Metabolic engineering of Escherichia coli to high efficient synthesis phenylacetic acid from phenylalanine. AMB Express 2017, 7, 105. [Google Scholar] [CrossRef]
- Deetae, P.; Bonnarme, P.; Spinnler, H.E.; Helinck, S. Production of volatile aroma compounds by bacterial strains isolated from different surface-ripened French cheeses. Appl. Microbiol. Biotechnol. 2007, 76, 1161–1171. [Google Scholar] [CrossRef]
- Majcher, M.A.; Myszka, K.; Gracka, A.; Grygier, A.; Jeleń, H.H. Key Odorants of Lazur, a Polish Mold-Ripened Cheese. J. Agric. Food Chem. 2018, 66, 2443–2448. [Google Scholar] [CrossRef]
- Sarhir, S.T.; Amanpour, A.; Bouseta, A.; Selli, S. Key odorants of a Moroccan fermented milk product “Lben” using aroma extract dilution analysis. J. Food Sci. Technol. 2019, 56, 3836–3845. [Google Scholar] [CrossRef]
- Verginer, M.; Leitner, E.; Berg, G. Production of volatile metabolites by grape-associated microorganisms. J. Agric. Food Chem. 2010, 58, 8344–8350. [Google Scholar] [CrossRef]
- Ding, W.; Liu, Y.; Zhao, X.; Peng, C.; Ye, X.; Che, Z.; Liu, Y.; Liu, P.; Lin, H.; Huang, J.; et al. Characterization of volatile compounds of Pixian Douban fermented in closed system of gradient steady-state temperature field. Food Sci. Nutr. 2021, 9, 2862–2876. [Google Scholar] [CrossRef] [PubMed]
- Majcher, M.A.; Olszak-Ossowska, D.; Szudera-Kończal, K.; Jeleń, H.H. Formation of Key Aroma Compounds during Preparation of Pumpernickel Bread. J. Agric. Food Chem. 2019, 68, 10352–10360. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, Y.; Qiu, Y.; Guo, H.; Ju, H.; Wang, Y.; Yuan, Y.; Yue, T. Chemical composition, sensorial properties, and aroma-active compounds of ciders fermented with Hanseniaspora osmophila and Torulaspora quercuum in co- and sequential fermentations. Food Chem. 2020, 306, 125623. [Google Scholar] [CrossRef]
- Nsogning Dongmo, S.; Sacher, B.; Kollmannsberger, H.; Becker, T. Key volatile aroma compounds of lactic acid fermented malt based beverages—Impact of lactic acid bacteria strains. Food Chem. 2017, 229, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.S.; Goddik, L.; Qian, M.C. Aroma compounds in sweet whey powder. J. Dairy Sci. 2004, 87, 4057–4063. [Google Scholar] [CrossRef] [PubMed]
- Grygier, A.; Majcher, M.; Myszka, K. Analysis of the ability to form 2-phenylethyl alcohol by Galactomyces geotrichum MK017. Zywn. Nauk. Technol. Jakosc/Food. Sci. Technol. Qual. 2015, 22, 74–83. [Google Scholar] [CrossRef]
- Stampanoni, C.R. The quantitative flavor profiling technique. Perfect Flavorist 1993, 18, 19–24. [Google Scholar]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent assisted flavour evaporation—A new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur. Food Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]


| Compound a | Odor b | RI-DB 5 c | RI-Wax c | Concentration (µg/kg) d | OT in Water (µg/kg) e | OAV f |
|---|---|---|---|---|---|---|
| 2,3-butanedione | buttery | 670 | 991 | 3500 | 15 | 233 |
| acetic acid | vinegar | 691 | 1445 | 7080 | 99,000 | <1 |
| 3-methyl-1-butanol | fruity | 719 | 1204 | 1269 | 980 | 1.3 |
| methyl butanoate | fruity | 734 | 1052 | 375 | 60 | 6.2 |
| 2,3-butanediol | buttery | 810 | 1583 | 15,464 | 150 | 103 |
| butanoic acid | cheesy | 836 | 1620 | 367 | 1000 | <1 |
| methyl hexanoate | fruity | 923 | 1245 | 164 | 90 | 1.8 |
| dimethyl sulfone | sulfuric | 959 | - | 53,449 * | n.d. | - |
| ethyl hexanoate | pineapple | 988 | 1229 | 17 | 1.2 | 14 |
| hexanoic acid | sweaty | 993 | 1224 | 2823 | 3000 | <1 |
| phenylacetaldehyde | honey | 1080 | 1644 | 9440 | 5.2 | 1815 |
| 2-phenylethanol | rosy | 1124 | 1920 | 5400 | 140 | 39 |
| methyl octanoate | fruity | 1131 | 1378 | 147 | 200 | <1 |
| ethyl octanoate | fruity | 1194 | 1441 | 132 | 8.7 | 15 |
| phenylacetic acid | honey | 1260 | 2568 | 13,400 | 68 | 197 |
| octanoic acid | musty | 1268 | 1420 | 554 | 190 | 2.9 |
| methyl decanoate | sweaty | 1325 | 1590 | <LOD g | 4 | - |
| vanillin | vanilla | 1406 | 2590 | 56 | 53 | 1.1 |
| Compound a | Quant. Ions (m/z) b (m/z) b | Labeled Standards c | Ion IS (m/z) d |
|---|---|---|---|
| 2,3-butanedione | 86 | [13C4] 2,3-butanedione | 90 |
| acetic acid | 60 | [2H3] acetic acid | 63 |
| 3-methyl-1-butanol | 70 | [2H2] 3-methyl-1-butanol | 72 |
| methyl butanoate | 102 | [2H9] ethyl 3-methylbutanoate | 139 |
| 2,3-butanediol | 90 | [13C4] 2,3-butanedione | 90 |
| butanoic acid | 73 | [2H7] butanoic acid | 77 |
| methyl hexanoate | 130 | [2H9] ethyl 3-methylbutanoate | 139 |
| dimethyl sulfone | 94 | [2H8] naphthalene | 131 |
| ethyl hexanoate | 144 | [2H9] ethyl 3-methylbutanoate | 139 |
| hexanoic acid | 116 | [2H7] butanoic acid | 77 |
| phenylacetaldehyde | 120 | [2H5] phenylacetaldehyde | 125 |
| 2-phenylethanol | 122 | [2H5] 2-phenylethanol | 127 |
| methyl octanoate | 158 | [2H9] ethyl 3-methylbutanoate | 139 |
| ethyl octanoate | 172 | [2H9] ethyl 3-methylbutanoate | 139 |
| phenylacetic acid | 136 | [2H5] 2-phenylethanol | 127 |
| octanoic acid | 122 | [2H7] butanoic acid | 77 |
| methyl decanoate | 186 | [2H9] ethyl 3-methylbutanoate | 139 |
| vanillin | 152 | [2H3] vanillin | 155 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szudera-Kończal, K.; Myszka, K.; Kubiak, P.; Drabińska, N.; Majcher, M.A. The Combined Effect of Lactic Acid Bacteria and Galactomyces geotrichum Fermentation on the Aroma Composition of Sour Whey. Molecules 2023, 28, 4308. https://doi.org/10.3390/molecules28114308
Szudera-Kończal K, Myszka K, Kubiak P, Drabińska N, Majcher MA. The Combined Effect of Lactic Acid Bacteria and Galactomyces geotrichum Fermentation on the Aroma Composition of Sour Whey. Molecules. 2023; 28(11):4308. https://doi.org/10.3390/molecules28114308
Chicago/Turabian StyleSzudera-Kończal, Kamila, Kamila Myszka, Piotr Kubiak, Natalia Drabińska, and Małgorzata Anna Majcher. 2023. "The Combined Effect of Lactic Acid Bacteria and Galactomyces geotrichum Fermentation on the Aroma Composition of Sour Whey" Molecules 28, no. 11: 4308. https://doi.org/10.3390/molecules28114308
APA StyleSzudera-Kończal, K., Myszka, K., Kubiak, P., Drabińska, N., & Majcher, M. A. (2023). The Combined Effect of Lactic Acid Bacteria and Galactomyces geotrichum Fermentation on the Aroma Composition of Sour Whey. Molecules, 28(11), 4308. https://doi.org/10.3390/molecules28114308