Efficient and Controllable Synthesis of 1-Aminoanthraquinone via High-Temperature Ammonolysis Using Continuous-Flow Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. Reaction Behavior Investigation
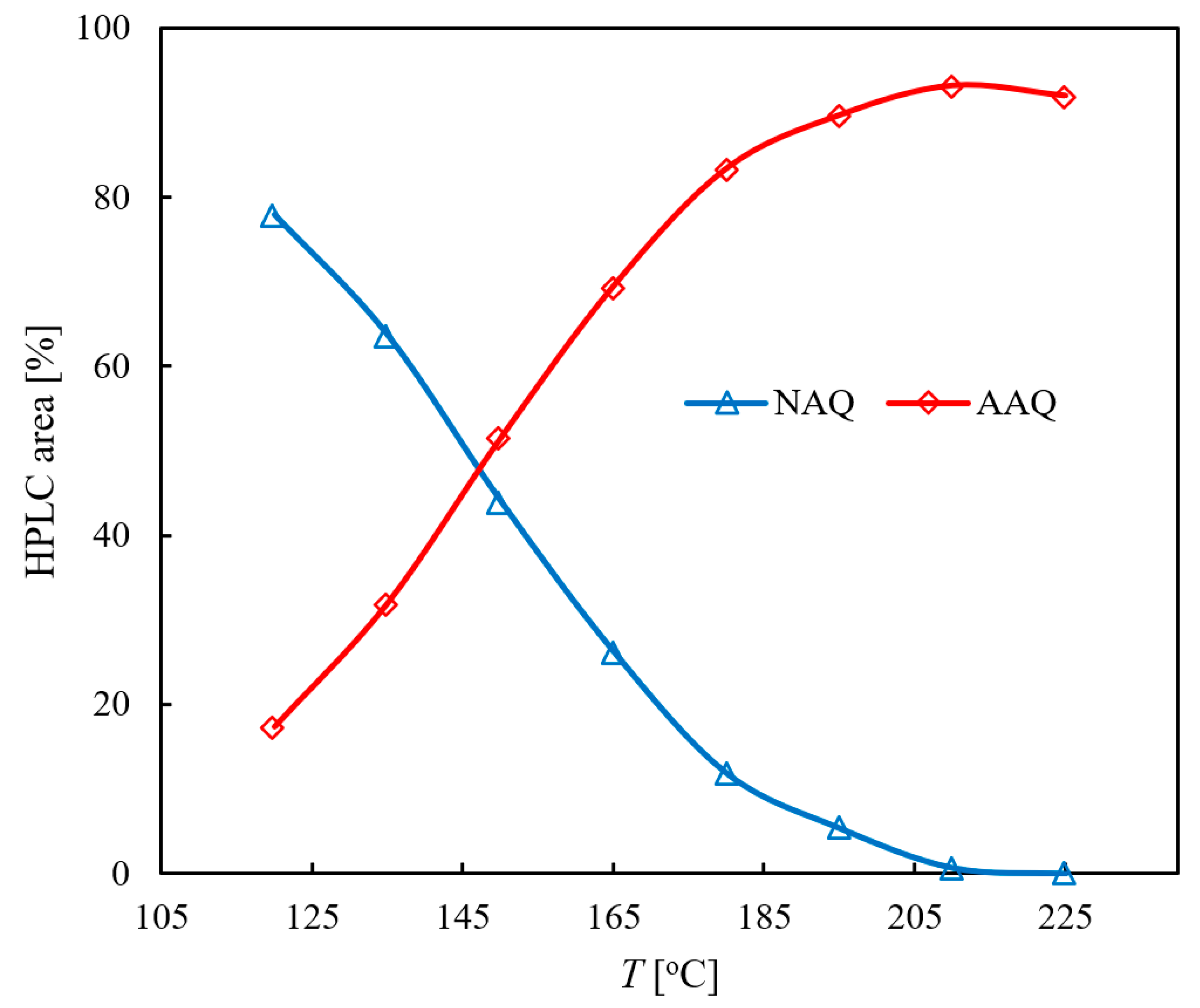
2.1.1. Influence of the Reaction Temperature
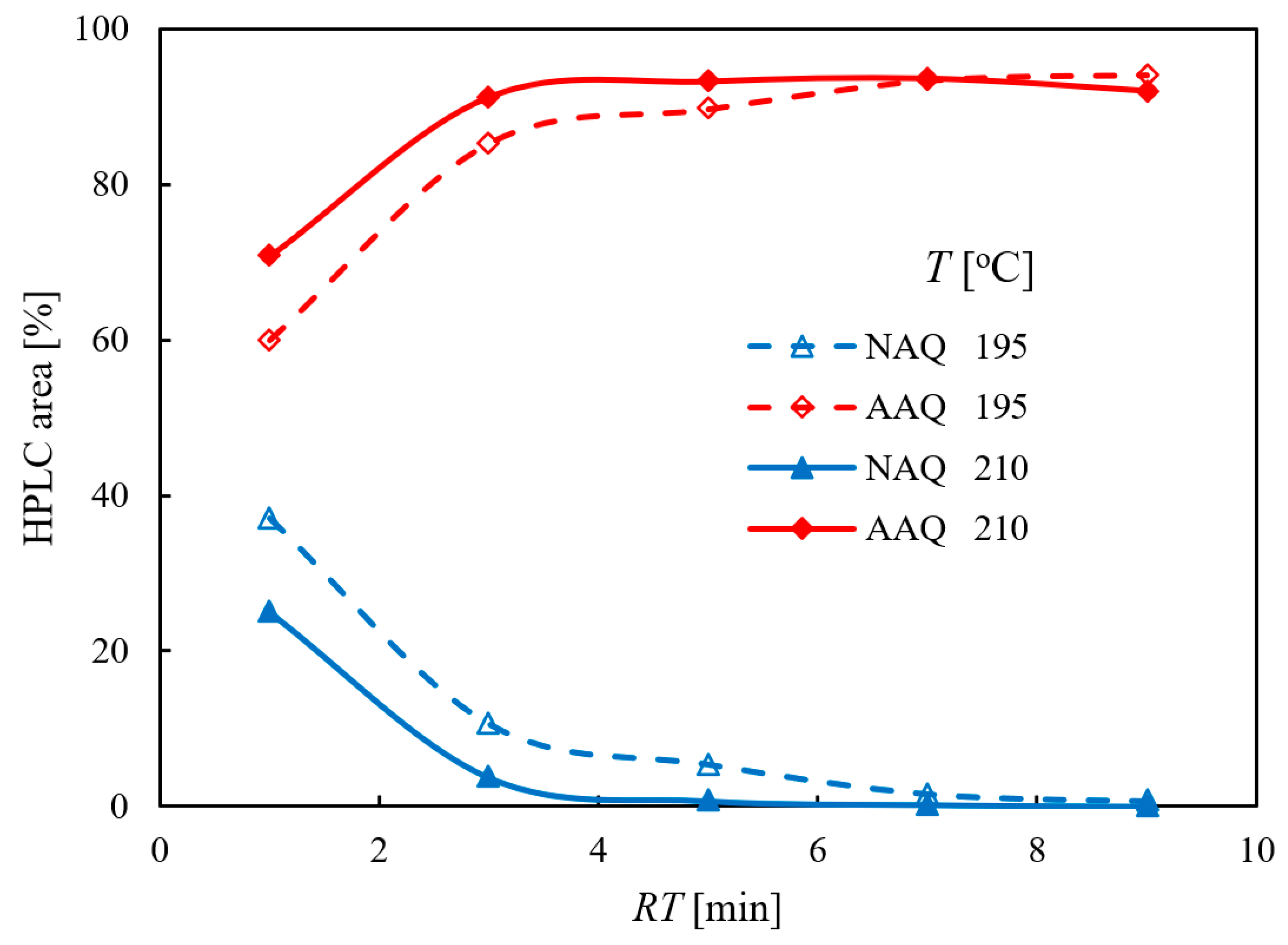
2.1.2. Influence of the Residence Time
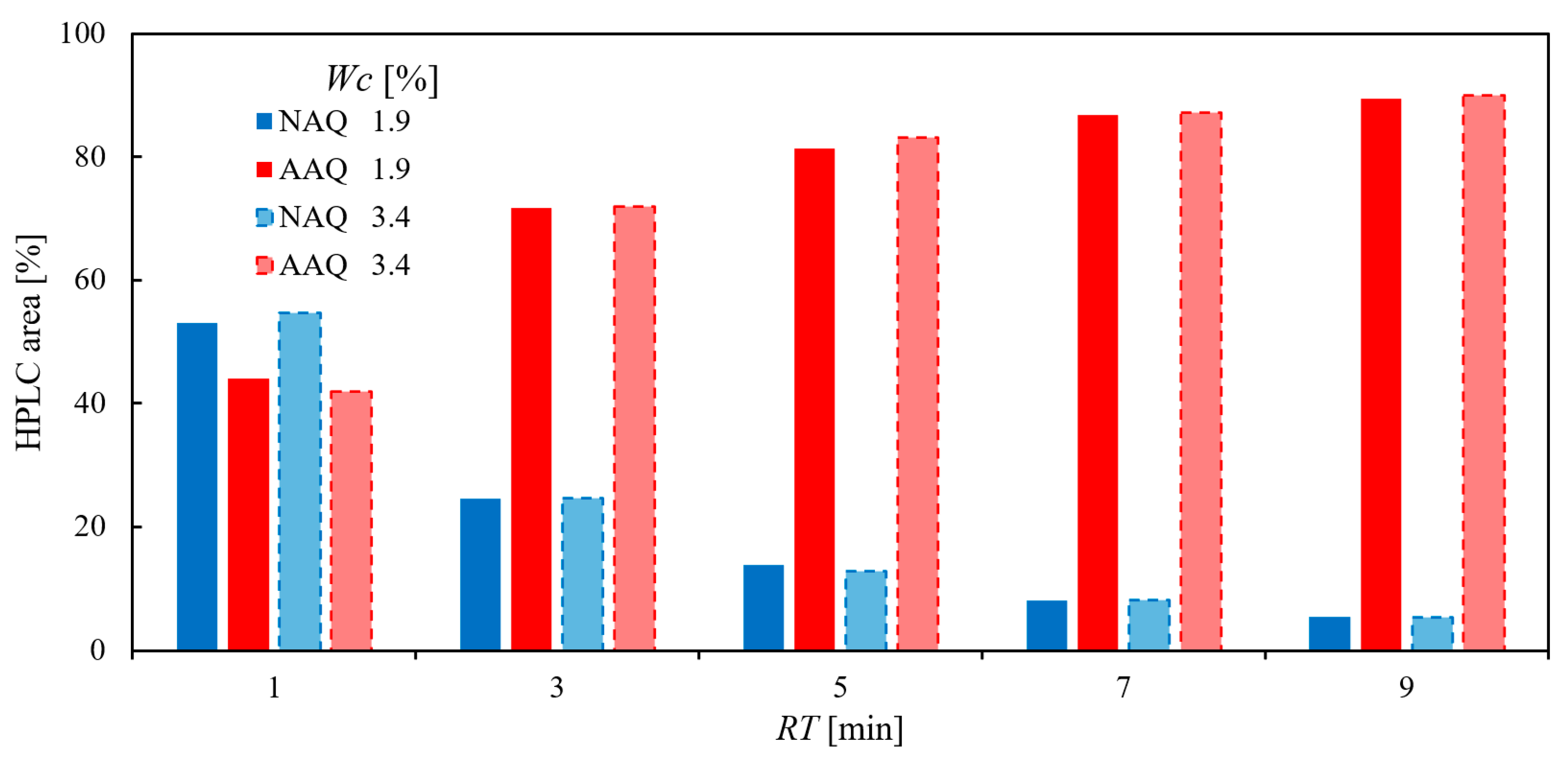
2.1.3. Effect of the Water Content
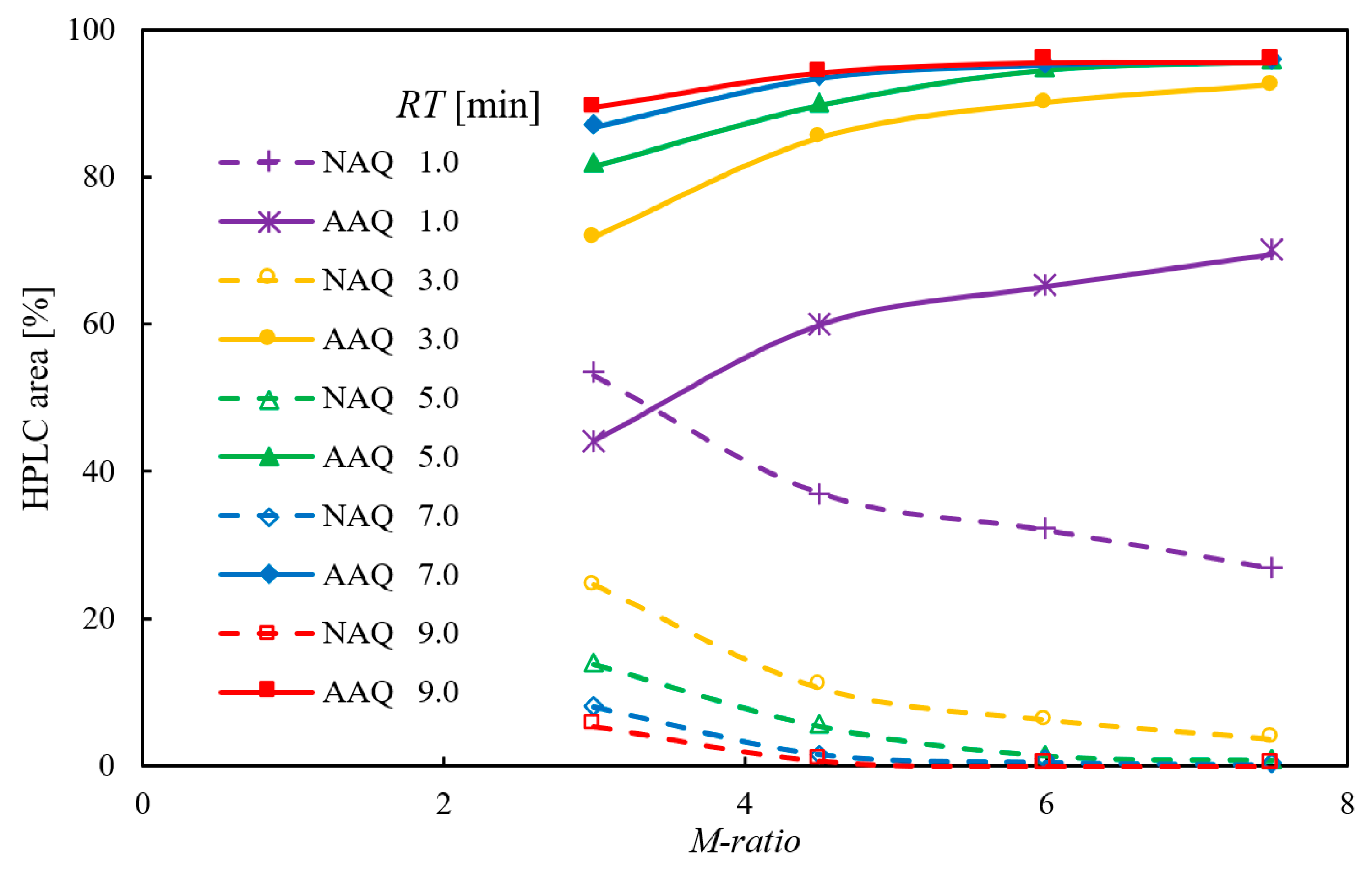
2.1.4. Screening of the M-Ratio
2.2. Optimized Ammonolysis Process for the Preparation of AAQ
2.2.1. Detailed Optimization Design of the Continuous Process
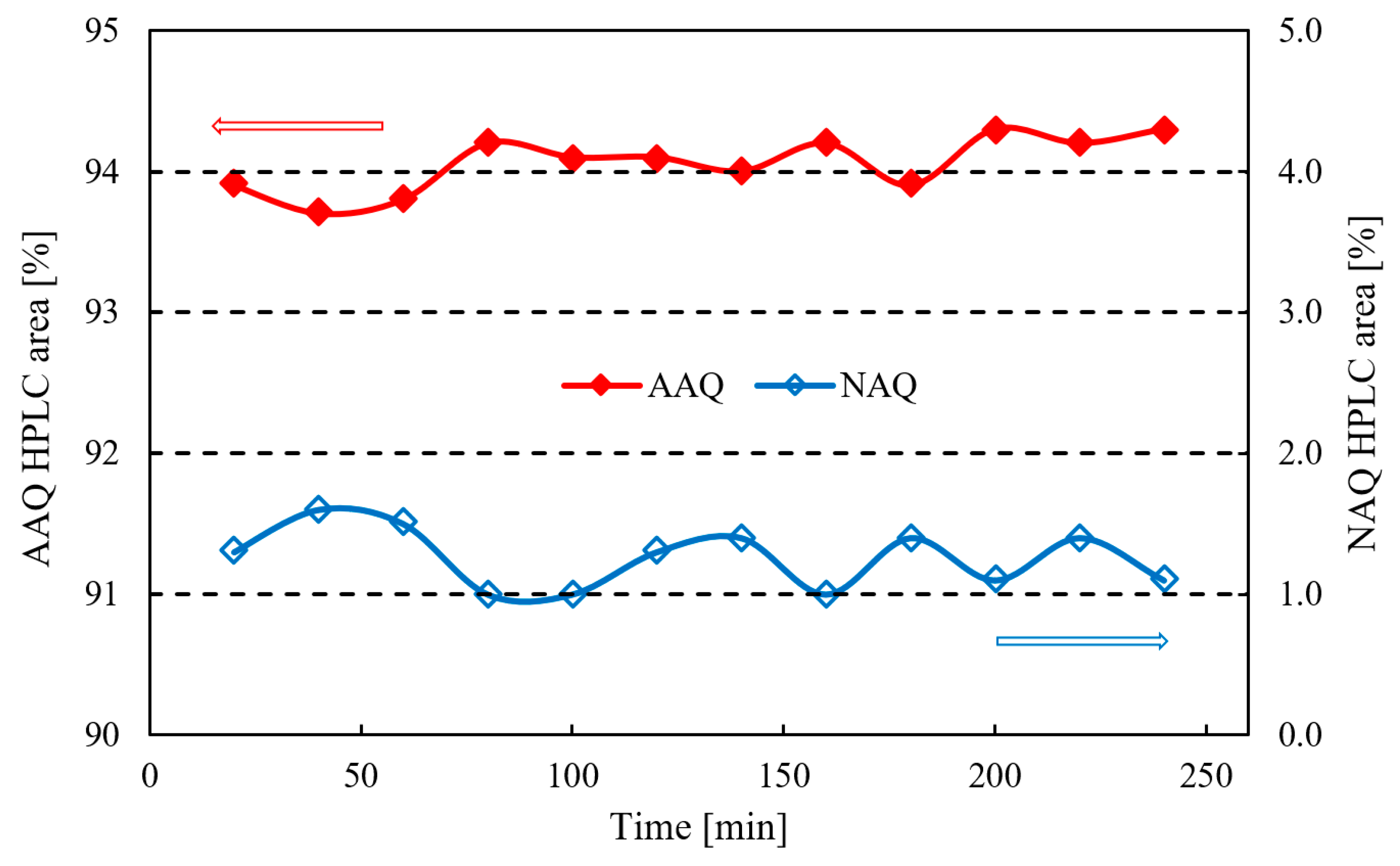
2.2.2. Optimized Process Reliability Evaluation
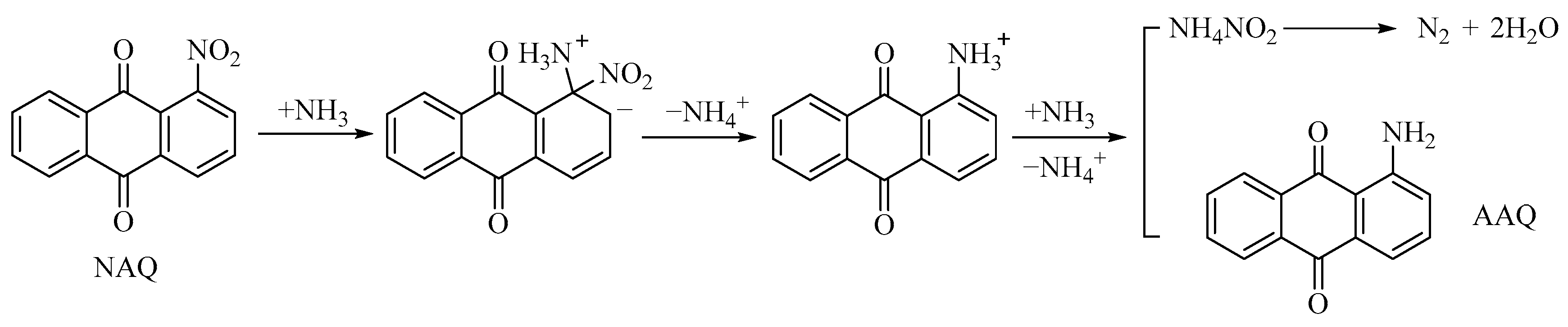
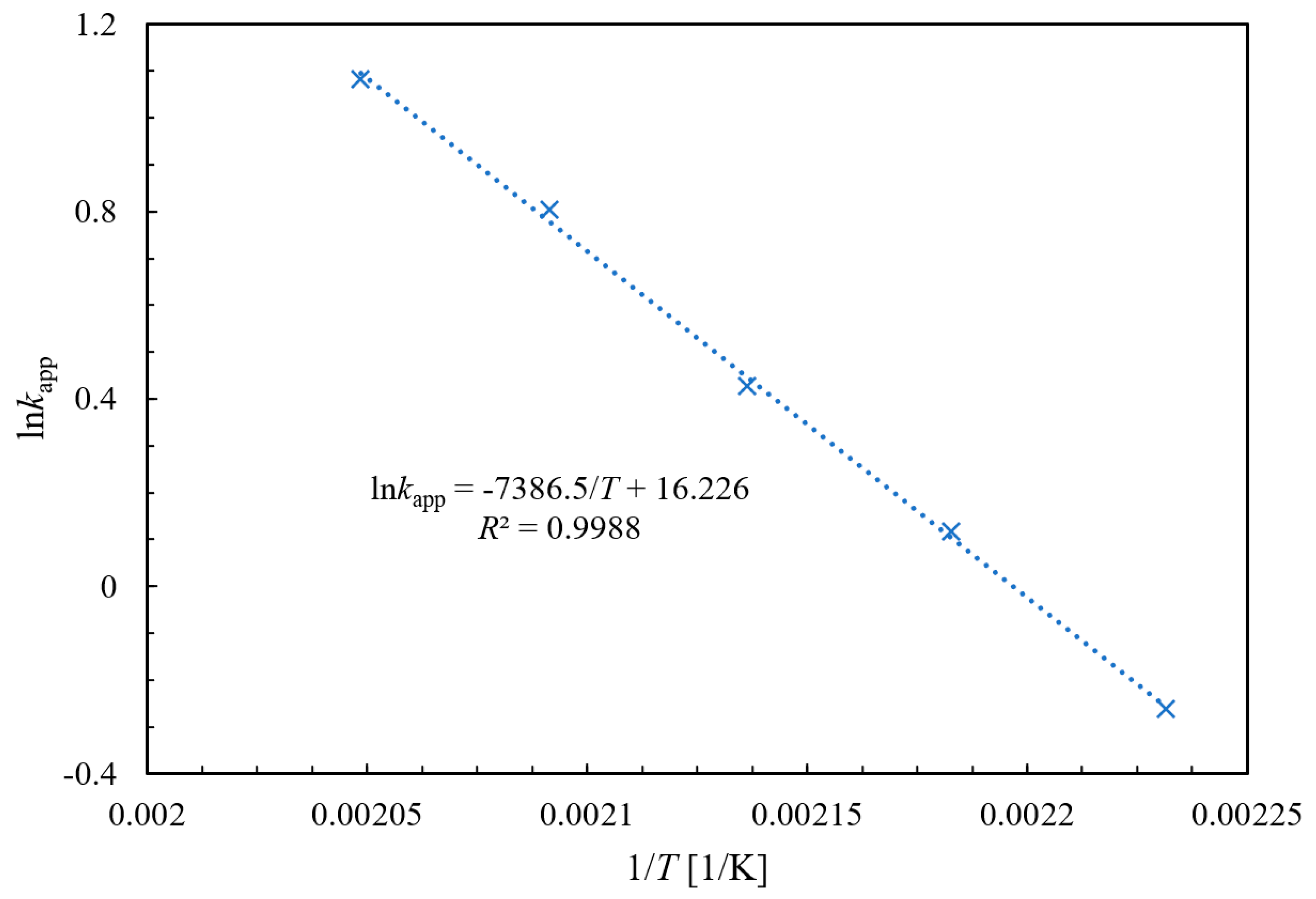
2.3. Kinetic Behavior Study for the Ammonolysis of NAQ
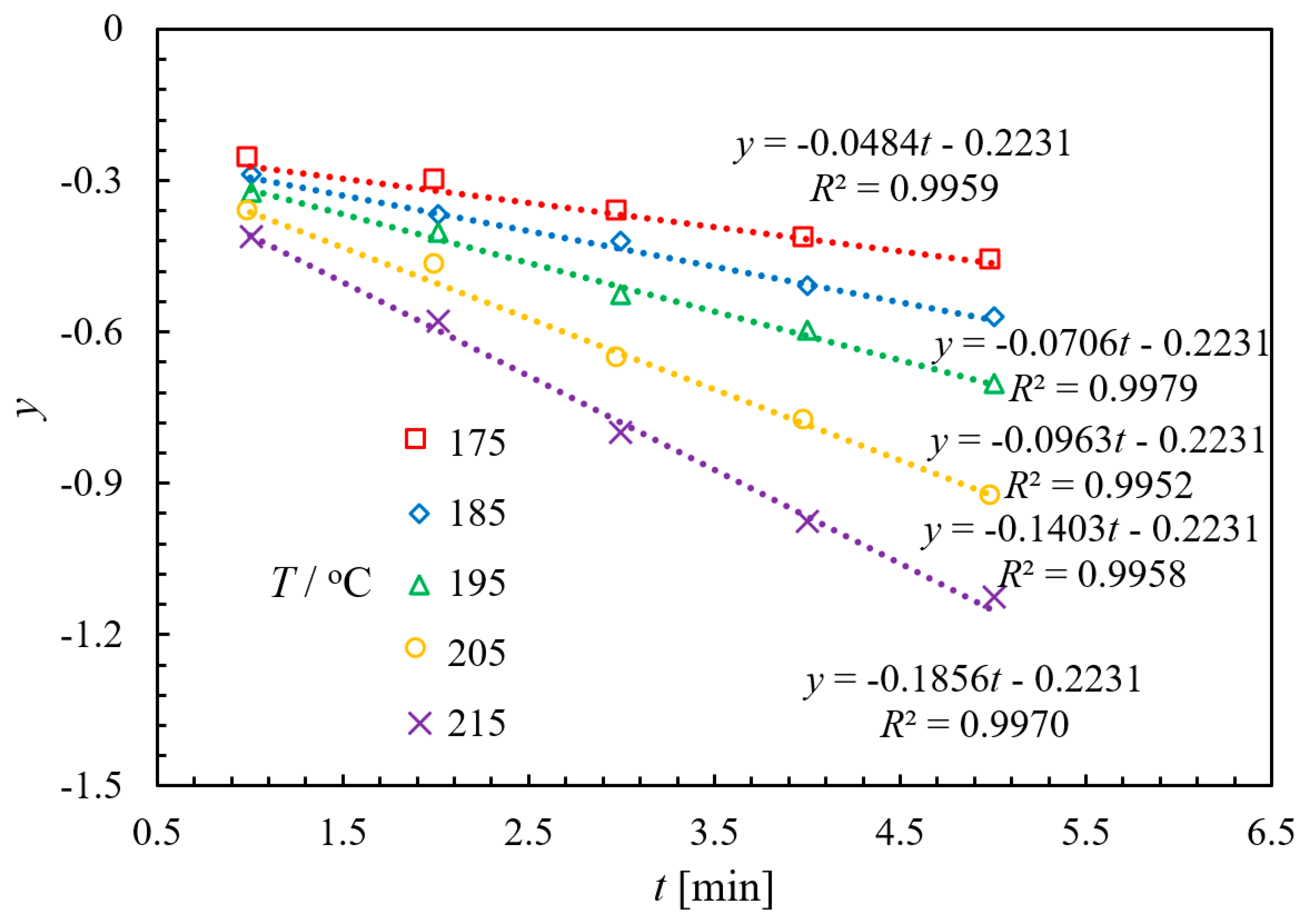
2.3.1. Experimental and Kinetic Modeling Investigation
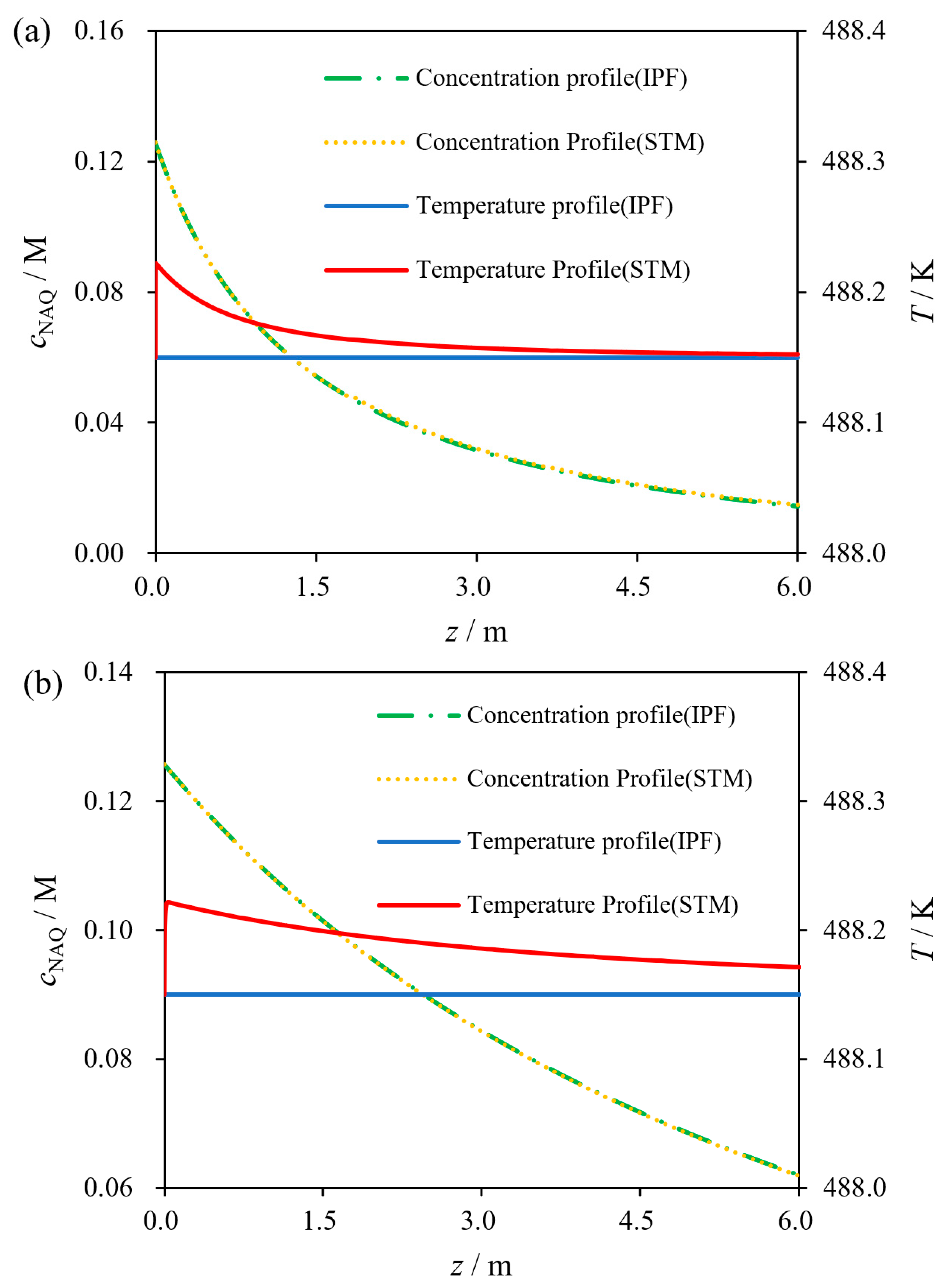
2.3.2. Validation Verification of Kinetic Experiments
3. Experimental Section
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Freeman, H.S.; Mock, G.N. Dye Application, Manufacture of Dye Intermediates and Dyes. In Handbook of Industrial Chemistry and Biotechnology; Kent, J.A., Ed.; Springer: Boston, MA, USA, 2012; pp. 475–548. [Google Scholar]
- Bien, H.S.; Stawitz, J.; Wunderlich, K. Anthraquinone dyes and intermediates. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd.: Weinheim, Germay, 2011. [Google Scholar]
- Ministry of Emergency Management of the People’s Republic of China. Notice on the Announcement of the First Batch of Catalogue of Hazardous Chemical Processes under Key Supervision. 12 June 2009. Available online: https://www.mem.gov.cn/gk/gwgg/agwzlfl/tz_01/200906/t20090615_408946.shtml (accessed on 15 June 2009).
- Gangopadhyay, R.K.; Das, S.K. Ammonia leakage from refrigeration plant and the management practice. Process Saf. Prog. 2008, 27, 15–20. [Google Scholar] [CrossRef]
- Liang, B.; Gao, W.; Zhang, K.; Li, Y.C. Ammonia-air combustion and explosion characteristics at elevated temperature and elevated pressure. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Sun, L.; He, P.; Xu, B.; Xu, X.; Wang, X. Promoting the catalytic efficiency of a catalyst by a solvothermal method. RSC Adv. 2013, 3, 5819–5823. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, X.; Zhang, H.; Jiang, Y.; Ma, D. CuI/4-Hydro-l-proline as a more effective catalytic system for coupling of aryl bromides with N-boc hydrazine and aqueous ammonia. J. Org. Chem. 2009, 74, 4542–4546. [Google Scholar] [CrossRef] [PubMed]
- Havasi, F.; Ghorbani-Choghamarani, A.; Nikpour, F. Pd-grafted functionalized mesoporous MCM-41: A novel, green and heterogeneous nanocatalyst for the selective synthesis of phenols and anilines from aryl halides in water. New J. Chem. 2015, 39, 6504–6512. [Google Scholar] [CrossRef]
- Jiang, S.; Dong, X.; Qiu, Y.; Chen, D.; Wu, X.; Jiang, S. A new ligand for copper-catalyzed amination of aryl halides to primary (hetero) aryl amines. Tetrahedron Lett. 2020, 61, 151683. [Google Scholar] [CrossRef]
- Li, Y.; Shi, R.; Lin, W.; Cheng, H.; Zhang, C.; Arai, M.; Zhao, F. A green and recyclable ligand-free copper (I) catalysis system for amination of halonitrobenzenes in aqueous ammonia solution. Mol. Catal. 2019, 475, 110462. [Google Scholar] [CrossRef]
- Zhu, Y.; Wei, Y. A simple and efficient copper-catalyzed amination of aryl halides by aqueous ammonia in water. Can. J. Chem. 2011, 89, 645–649. [Google Scholar] [CrossRef]
- Baumann, M.; Moody, T.S.; Smyth, M.; Wharry, S. A perspective on continuous flow chemistry in the pharmaceutical industry. Org. Process Res. Dev. 2020, 24, 1802–1813. [Google Scholar] [CrossRef]
- Hartman, R.L. Flow chemistry remains an opportunity for chemists and chemical engineers. Curr. Opin. Chem. Eng. 2020, 29, 42–50. [Google Scholar] [CrossRef]
- Naber, J.R.; Kappe, C.O.; Pesti, J.A. Flow Chemistry Enabling Efficient Synthesis. Org. Process Res. Dev. 2020, 24, 1779–1780. [Google Scholar] [CrossRef]
- Berton, M.; de Souza, J.M.; Abdiaj, I.; McQuade, D.T.; Snead, D.R. Scaling continuous API synthesis from milligram to kilogram: Extending the enabling benefits of micro to the plant. J. Flow Chem. 2020, 10, 73–92. [Google Scholar] [CrossRef]
- Donnelly, K.; Baumann, M. Scalability of photochemical reactions in continuous flow mode. J. Flow Chem. 2021, 11, 223–241. [Google Scholar] [CrossRef]
- Rogers, L.; Jensen, K.F. Continuous manufacturing-the Green Chemistry promise? Green Chem. 2019, 21, 3481–3498. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, H.C.; Wen, Z.H.; Zhang, B.Y.; Chen, G.W. Toward the efficient synthesis of pseudoionone from citral in a continuous-flow microreactor. Ind. Eng. Chem. Res. 2018, 57, 11288–11298. [Google Scholar] [CrossRef]
- Cui, Y.; Song, J.; Du, C.; Deng, J.; Luo, G. Determination of the kinetics of chlorobenzene nitration using a homogeneously continuous microflow. AIChE J. 2022, 68, e17564. [Google Scholar] [CrossRef]
- Zhang, J.; Teixeira, A.R.; Zhang, H.; Jensen, K.F. Determination of fast gas-liquid reaction kinetics in flow. React. Chem. Eng. 2020, 5, 51–57. [Google Scholar] [CrossRef]
- Zhang, Z. Fine Organic Synthesis Unit Reaction, 2nd ed.; East China University of Science and Technology Press: Shanghai, China, 2003. [Google Scholar]
- Auge, W.; Thiem, K.W.; Neeff, R.; Losacker, P.; Braden, R. Process for Preparing 1-Amino Anthraquinone. U.S. Patent 4,003,924, 5 March 1974. [Google Scholar]
- Kotwica, K.; Bujak, P.; Wamil, D.; Materna, M.; Skorka, L.; Gunka, P.A.; Nowakowski, B.; Golec, B.; Luszczynska, B.; Zagorskaa, M.; et al. Indanthrone dye revisited after sixty years. Chem. Commun. 2014, 50, 11543–11546. [Google Scholar] [CrossRef] [PubMed]
- Sharghi, H.; Tamaddon, F.; Rezaie, R. Synthesis of 1-Hydroxy-2-(Prop-2′-Enyl) 9-Antrone. Iran. J. Chem. Chem. Eng. 1993, 12, 1–7. [Google Scholar]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2016. [Google Scholar]
- Sen, S.; Mondal, U.; Singh, G. Dual optimization in phase transfer catalyzed synthesis of dibenzyl sulfide using response surface methodology. Org. Process Res. Dev. 2016, 20, 1765–1773. [Google Scholar] [CrossRef]
- McMurry, J.E.; Learning, C. Organic Chemistry, 9th ed.; Cengage Learning: Boston, MA, USA, 2015. [Google Scholar]
- Bergman, T.L.; Bergman, T.L.; Incropera, F.P.; Dewitt, D.P.; Lavine, A.S. Fundamentals of Heat and Mass Transfer; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Davis, M.E.; Davis, R.J. Fundamentals of Chemical Reaction Engineering; Courier Corporation: New York, NY, USA, 2012. [Google Scholar]
- Taylor, G.I. Dispersion of soluble matter in solvent flowing slowly through a tube. Proc. R. Soc. Lond. Ser. A 1953, 219, 186. [Google Scholar]
- Aris, R. On the dispersion of a solute in a fluid flowing through a tube. Proc. R. Soc. Lond. Ser. A 1956, 235, 67. [Google Scholar]
- Schwolow, S.; Ko, J.Y.; Kockmann, N.; Röder, T. Enhanced heat transfer by exothermic reactions in laminar flow capillary reactors. Chem. Eng. Sci. 2016, 141, 356–362. [Google Scholar] [CrossRef]
- Yaws, C.L. Yaws’ Handbook of Thermodynamic and Physical Properties of Chemical Compounds; Knovel: New York, NY, USA, 2003. [Google Scholar]
- Ma, P.S.; Li, Y.H.; Yang, C.S. Chemical Engineering Thermodynamics, 2nd ed.; Chemical Industry Press: Beijing, China, 2009. [Google Scholar]
- Ma, P.S. Chemical Engineering Data; China Petrochemical Press: Beijing, China, 2003. [Google Scholar]
- Poling, B.E.; Prausnitz, J.M.; O’connell, J.P. Properties of Gases and Liquids; McGraw-Hill Education: New York, NY, USA, 2001. [Google Scholar]
- Ma, P.S.; Xu, W.; Liu, Y.S. Estimation of enthalpy of vaporization at boiling point with functional group method. Petrochem. Technol. 1992, 21, 613–617. [Google Scholar]
- Speight, J. Lange’s Handbook of Chemistry; McGraw-Hill Education: New York, NY, USA, 2005. [Google Scholar]
- Westerterp, K.R.; van Swaaij, W.P.M.; Beenackers, A.A.C.M.; Kramers, H. Chemical Reactor Design and Operation; Wiley: Chichester, UK, 1984. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering; Wiley: New York, NY, USA, 1999. [Google Scholar]

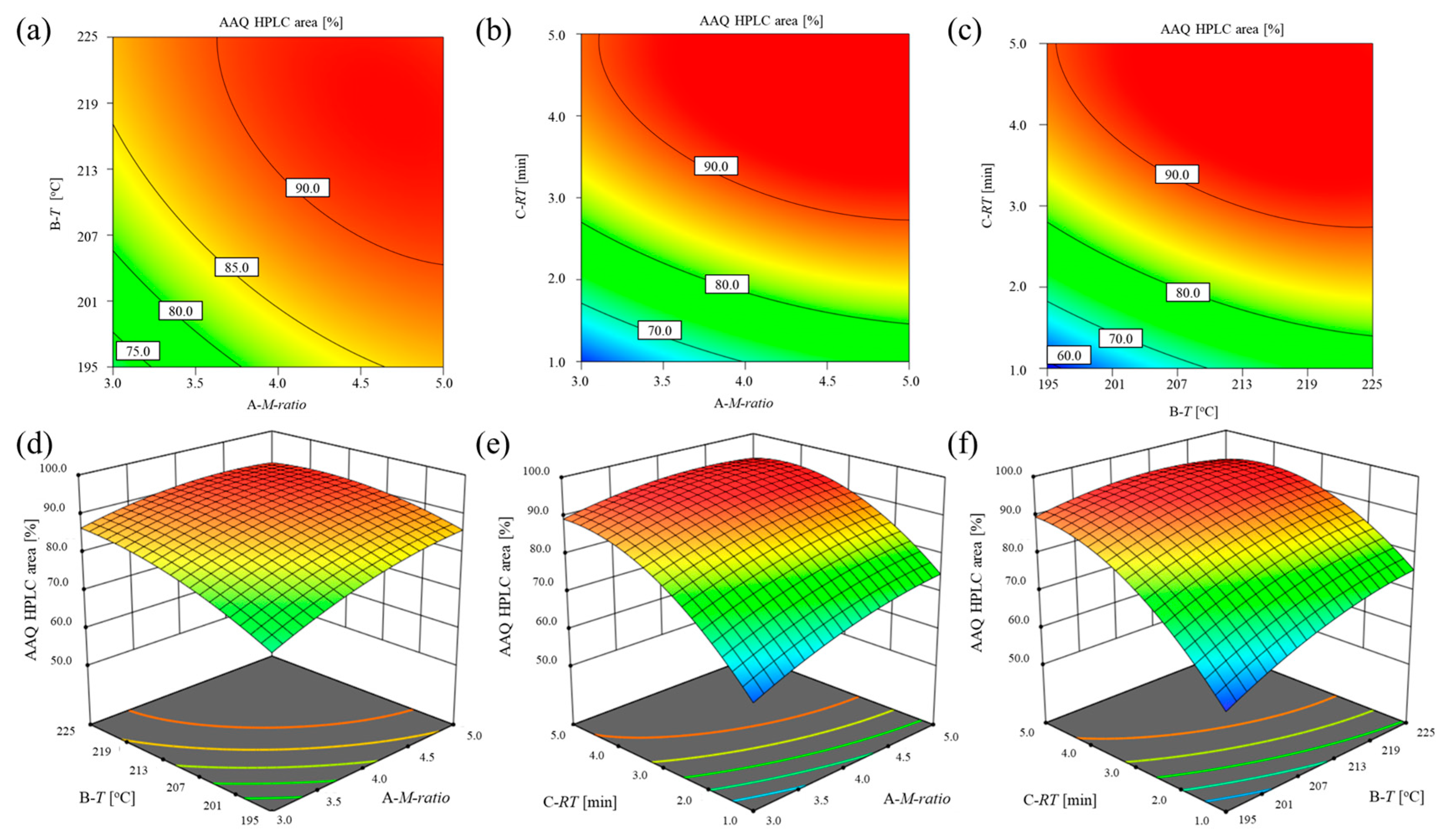


| Level | Coded Level | Uncoded Level | ||
|---|---|---|---|---|
| A: M-Ratio | B: T [°C] | C: RT [min] | ||
| low | −1 | 3 | 195 | 1 |
| mid | 0 | 4 | 210 | 3 |
| high | 1 | 5 | 225 | 5 |
| Run | Actual Level of Variables | AAQ HPLC Area [%] | |||
|---|---|---|---|---|---|
| M-Ratio | T [°C] | RT [min] | Observed | Predicted | |
| 1 | 4.0 | 210 | 3.0 | 88.8 | 89.2 |
| 2 | 5.0 | 210 | 5.0 | 92.5 | 93.1 |
| 3 | 4.0 | 225 | 5.0 | 91.7 | 91.8 |
| 4 | 3.0 | 210 | 1.0 | 61.1 | 60.6 |
| 5 | 4.0 | 225 | 1.0 | 75.4 | 75.8 |
| 6 | 3.0 | 195 | 3.0 | 71.8 | 72.5 |
| 7 | 3.0 | 210 | 5.0 | 89.6 | 89.3 |
| 8 | 5.0 | 225 | 3.0 | 91.7 | 91.1 |
| 9 | 4.0 | 210 | 3.0 | 89.6 | 89.2 |
| 10 | 4.0 | 210 | 3.0 | 89.8 | 89.2 |
| 11 | 5.0 | 195 | 3.0 | 86.2 | 86.0 |
| 12 | 4.0 | 195 | 1.0 | 58.9 | 58.8 |
| 13 | 3.0 | 225 | 3.0 | 86.3 | 86.5 |
| 14 | 4.0 | 210 | 3.0 | 89.4 | 89.2 |
| 15 | 4.0 | 195 | 5.0 | 90.0 | 89.7 |
| 16 | 5.0 | 210 | 1.0 | 74.6 | 74.9 |
| 17 | 4.0 | 210 | 3.0 | 88.6 | 89.2 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 1860.74 | 9 | 206.75 | 474.97 | <0.0001 | significant |
| A-M-ratio | 163.81 | 1 | 163.81 | 376.32 | <0.0001 | |
| B-T | 182.40 | 1 | 182.40 | 419.05 | <0.0001 | |
| C-RT | 1099.81 | 1 | 1099.81 | 2526.63 | <0.0001 | |
| AB | 20.25 | 1 | 20.25 | 46.52 | 0.0002 | |
| AC | 28.09 | 1 | 28.09 | 64.53 | <0.0001 | |
| BC | 54.76 | 1 | 54.76 | 125.80 | <0.0001 | |
| A2 | 24.15 | 1 | 24.15 | 55.48 | 0.0001 | |
| B2 | 34.08 | 1 | 34.08 | 78.29 | <0.0001 | |
| C2 | 230.26 | 1 | 230.26 | 528.98 | <0.0001 | |
| Residual | 3.05 | 7 | 0.4353 | |||
| Lack of Fit | 1.98 | 3 | 0.6583 | 2.46 | 0.2028 | not significant |
| Pure Error | 1.07 | 4 | 0.2680 | |||
| Cor Total | 1863.79 | 16 |
| Operation Conditions | Results | ||||||
|---|---|---|---|---|---|---|---|
| HPLC Area | Stage 1 | Stage 2 | |||||
| M-ratio = 4.5 T = 213 °C RT = 4.3 min | Experimental NAQ | Experimental AAQ | Predicted AAQ | Conversion of NAQ | Yield of AAQ | Conversion of NAQ | Yield of AAQ |
| 1.3% (Average) | 94.1% (Average) | 94.7% | 98.4% | 88.3% | 98.4% | 87.9% | |
| T/°C | 175 | 185 | 195 | 205 | 215 |
|---|---|---|---|---|---|
| kapp/L·mol−1·min−1 | 0.7701 | 1.1233 | 1.5322 | 2.2323 | 2.9531 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.; Cai, L.; Ye, W.; Zhu, K.; Li, J.; Li, Y.; Xu, W.; Wang, P.; Duanmu, C. Efficient and Controllable Synthesis of 1-Aminoanthraquinone via High-Temperature Ammonolysis Using Continuous-Flow Method. Molecules 2023, 28, 4314. https://doi.org/10.3390/molecules28114314
Zhou F, Cai L, Ye W, Zhu K, Li J, Li Y, Xu W, Wang P, Duanmu C. Efficient and Controllable Synthesis of 1-Aminoanthraquinone via High-Temperature Ammonolysis Using Continuous-Flow Method. Molecules. 2023; 28(11):4314. https://doi.org/10.3390/molecules28114314
Chicago/Turabian StyleZhou, Feng, Lei Cai, Wenjie Ye, Kai Zhu, Jin Li, Yanxing Li, Weichuan Xu, Pan Wang, and Chuansong Duanmu. 2023. "Efficient and Controllable Synthesis of 1-Aminoanthraquinone via High-Temperature Ammonolysis Using Continuous-Flow Method" Molecules 28, no. 11: 4314. https://doi.org/10.3390/molecules28114314
APA StyleZhou, F., Cai, L., Ye, W., Zhu, K., Li, J., Li, Y., Xu, W., Wang, P., & Duanmu, C. (2023). Efficient and Controllable Synthesis of 1-Aminoanthraquinone via High-Temperature Ammonolysis Using Continuous-Flow Method. Molecules, 28(11), 4314. https://doi.org/10.3390/molecules28114314






