The Study of Interfacial Adsorption Behavior for Hydroxyl-Substituted Alkylbenzene Sulfonates by Interfacial Tension Relaxation Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. Full-Frequency Spectrum of Interfacial Dilational Elasticity of Adsorption Film
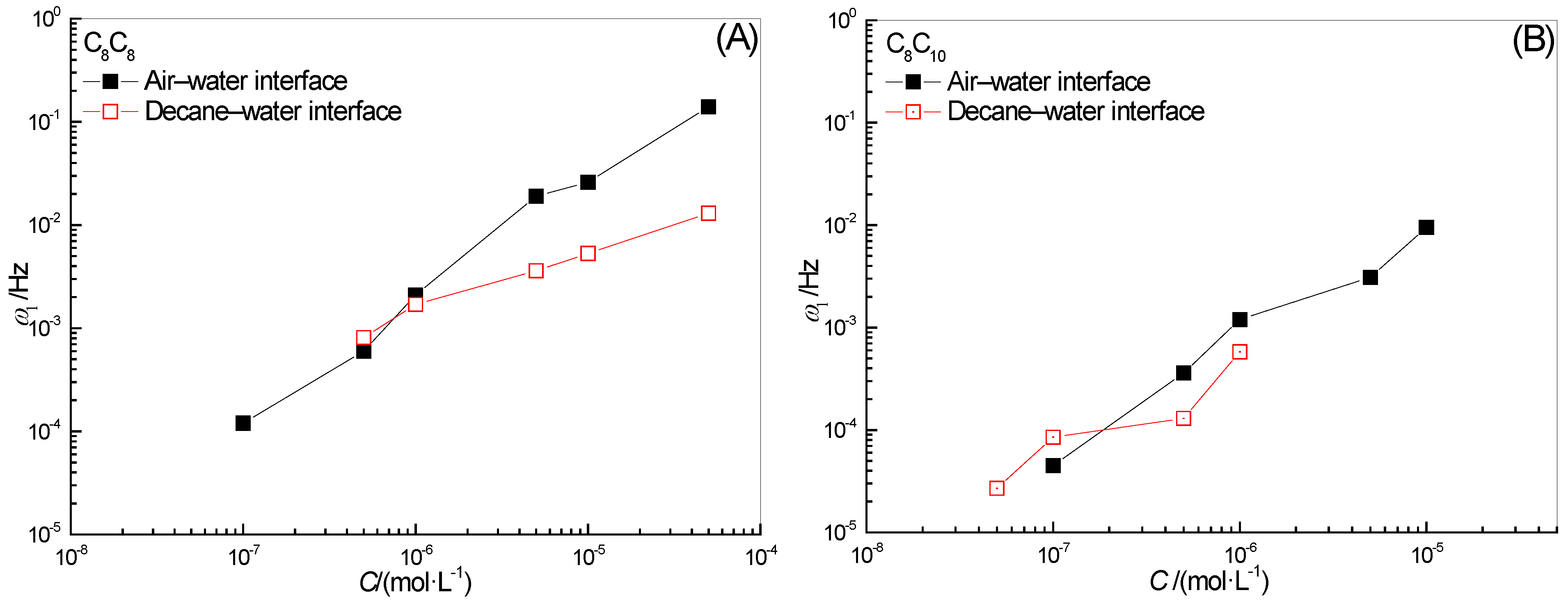
2.2. Effect of Surfactant Concentration on ω1
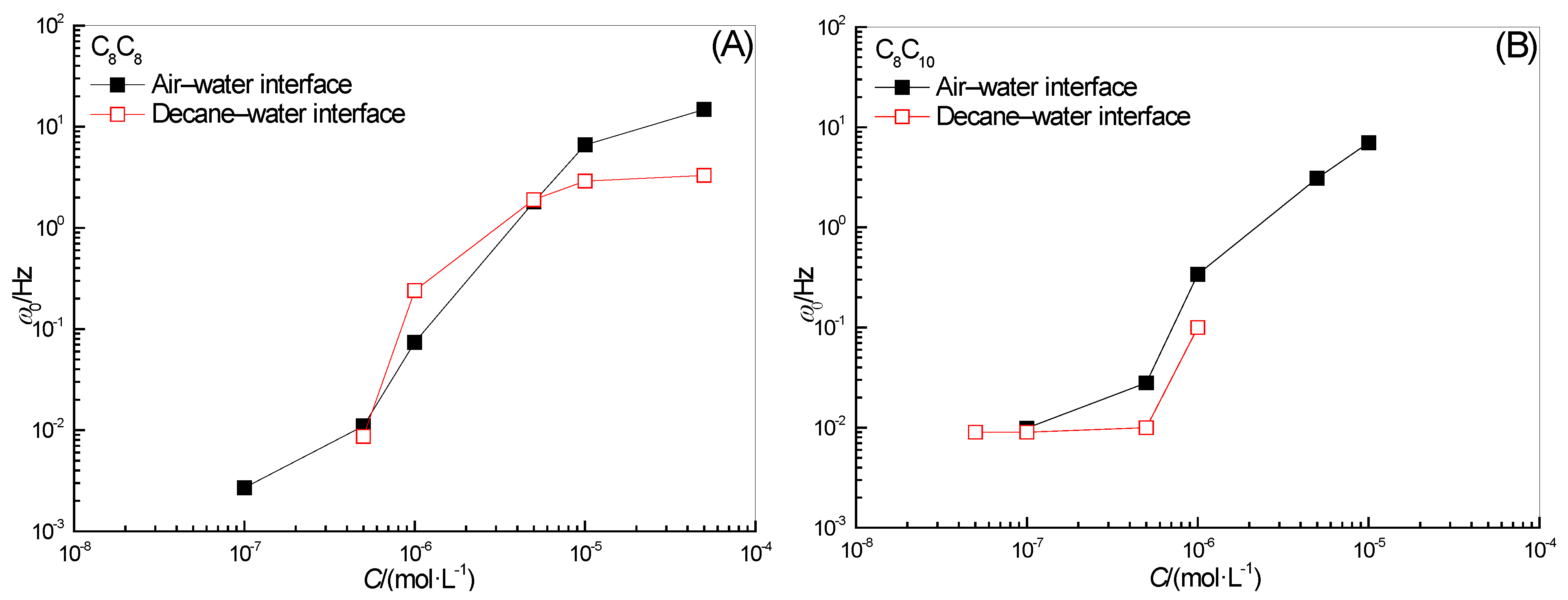
2.3. Effect of Surfactant Concentration on ω0
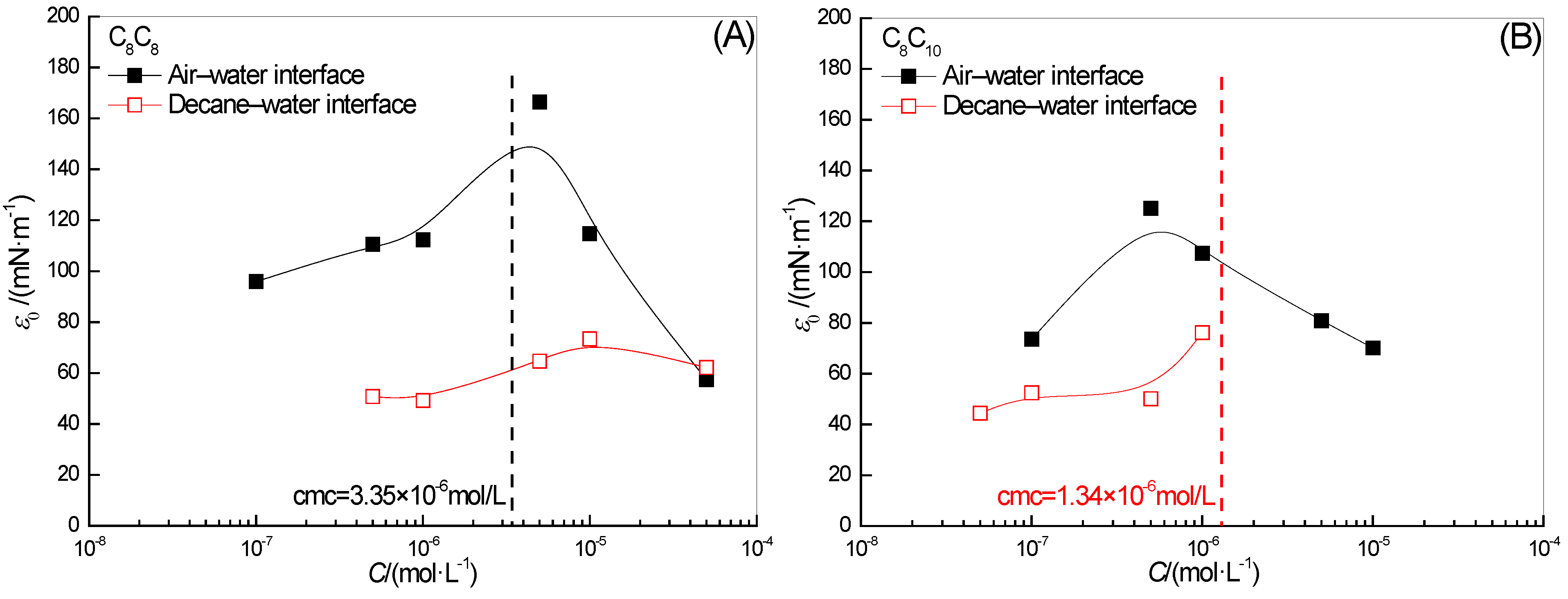
2.4. Effect of Surfactant Concentration on ε0
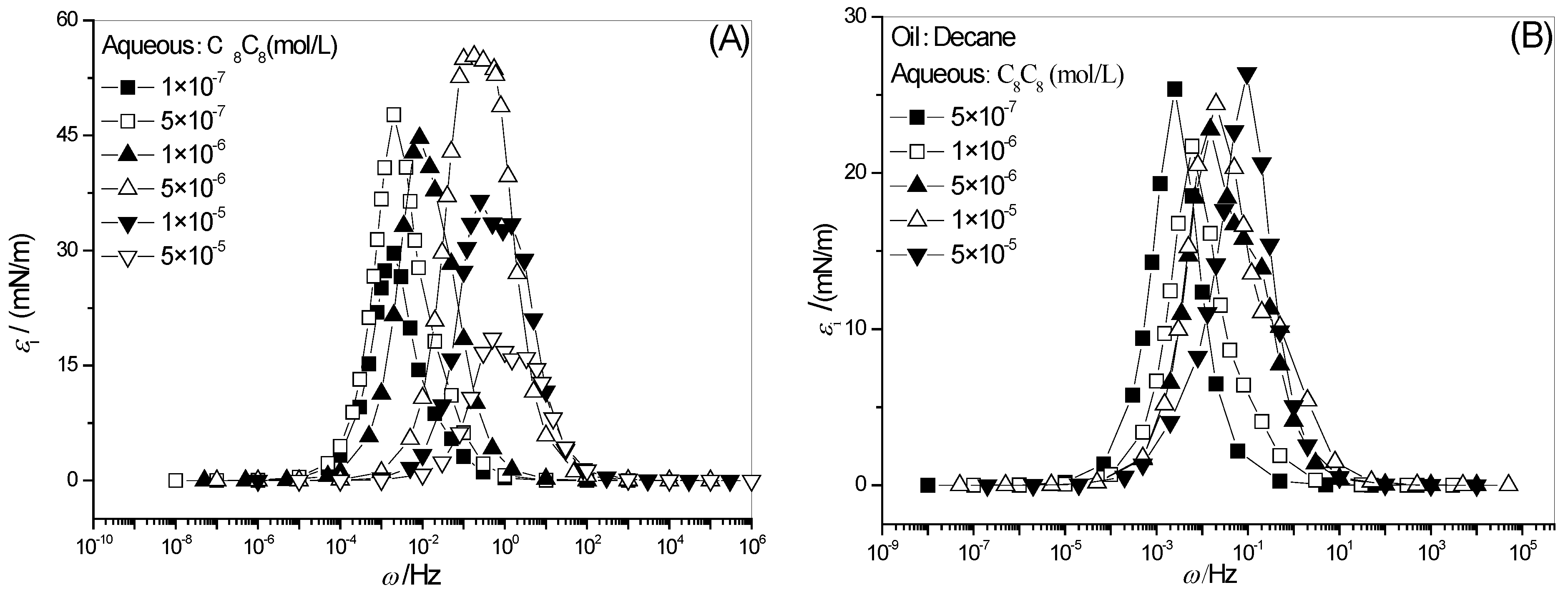
2.5. Full-Frequency Spectrum of Interfacial Dilational Viscosity of Adsorption Film
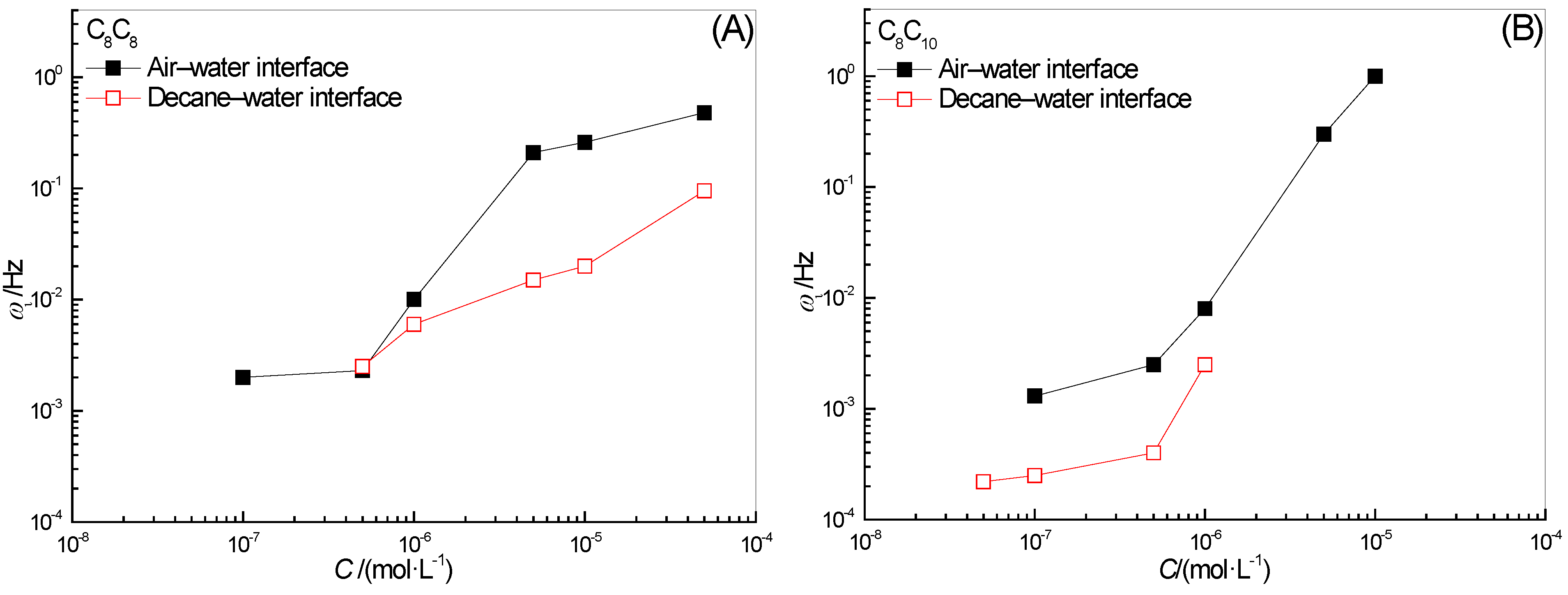
2.6. Effect of Surfactant Concentration on ωi
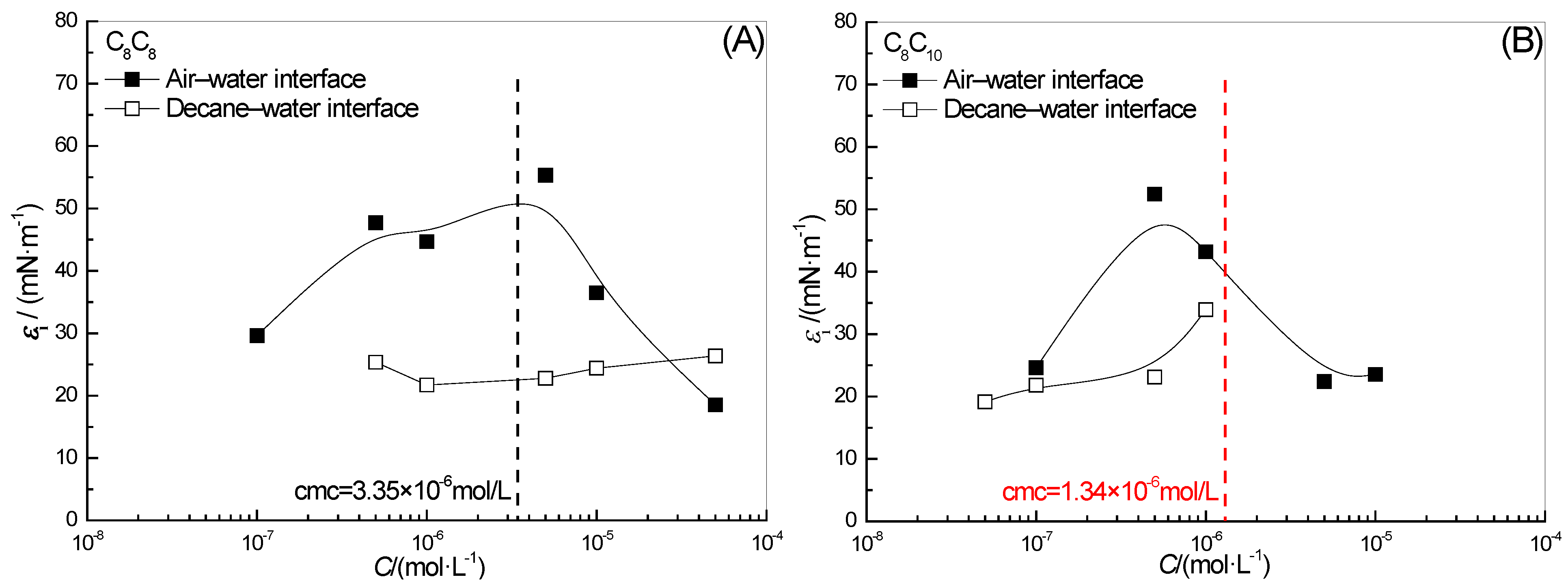
2.7. Effect of Surfactant Concentration on εi0
3. Experiment Section
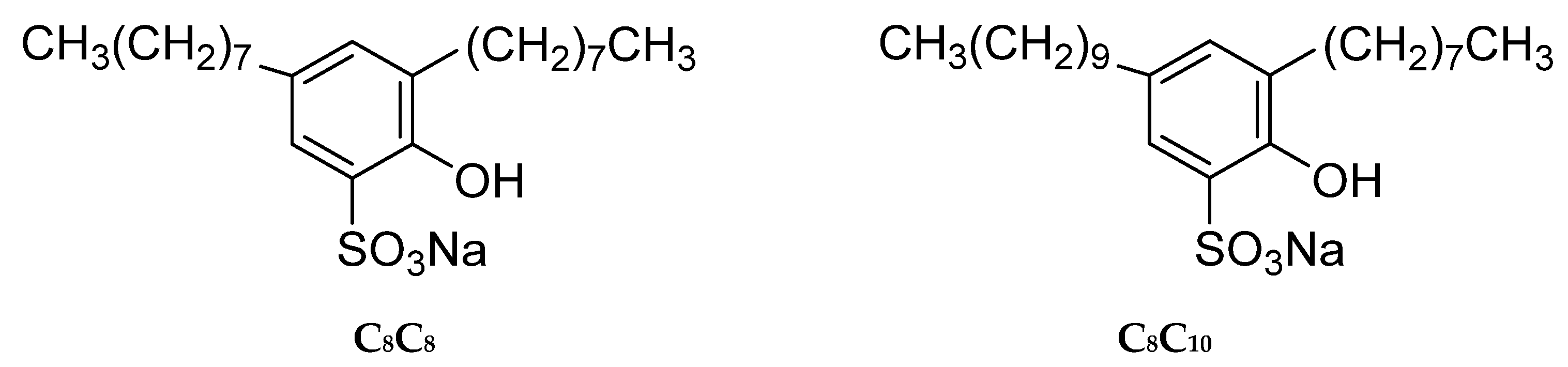
3.1. Materials
3.2. Experimental Method
3.3. Theoretical Background
4. Conclusions
- (1)
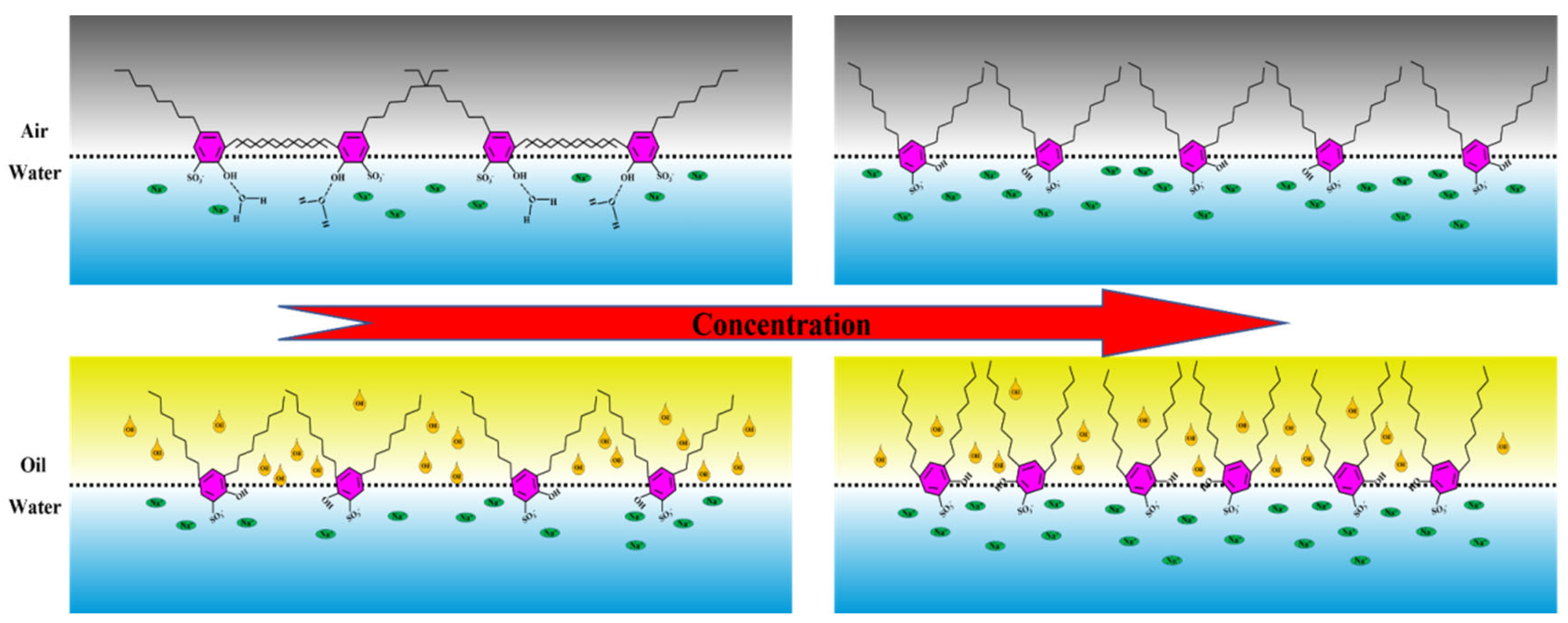
- The sulfonic acid group and hydroxyl group on the benzene ring all interact with the water phase, which will have a certain “positioning” effect on the interfacial configuration of the surfactant molecules. The long-chain alkyl groups adjacent to the hydroxyl group tend to extend along the interface, showing strong intermolecular interaction; while the long-chain alkyl at the para position of the hydroxyl group tends to extend into the air. Therefore, the length of the hydroxyl ortho alkyl chain is the main controlling factor of the film properties and has little to do with the length of the para-alkyl chain.
- (2)
- For the gas–liquid interface, at first, due to the interaction between alkyl chains, the properties of the interfacial film are mainly controlled by the slow relaxation process of interfacial rearrangement. With the further increase in surfactant concentration, the adjacent alkyl groups and hydroxyl groups begin to extend to the air, and the interfacial film begins to be controlled by the fast relaxation process that is dominated by diffusion exchange.
- (3)
- For the oil–water interface, the insertion of oil molecules will destroy the interaction between alkyl chains. The properties of the interfacial film have always been controlled by the fast relaxation process that is dominated by diffusion exchange. With the further increase in surfactant concentration, the interaction between alkyl chains and oil phase is enhanced, and the characteristic frequency of the fast relaxation process will be slightly reduced.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, J.; Hu, X.Y.; Fang, Y.; Jin, G.Y.; Xia, Y.M. What dominates the interfacial properties of extended surfactants: Amphipathicity or surfactant shape? J. Colloid Interface Sci. 2019, 547, 190–198. [Google Scholar] [CrossRef]
- Bardoula, V.; Leclercq, L.; Hoogenboom, R.; Nardello-Rataj, V. Amphiphilic nonionic block and gradient copoly(2-oxazoline)s based on 2-methyl-2-oxazoline and 2-phenyl-2-oxazoline as efficient stabilizers for the formulation of tailor-made emulsions. J. Colloid Interface Sci. 2023, 632, 223–236. [Google Scholar] [CrossRef]
- Grigoryev, A.; Tokarev, I.; Kornev, K.G.; Luzinov, I.; Minko, S. Superomniphobic magnetic microtextures with remote wetting control. J. Am. Chem. Soc. 2012, 134, 12916–12919. [Google Scholar] [CrossRef]
- Ma, R.; Fakudze, S.; Liu, S.; Wei, Y.; Chen, J.; Han, J.; Wang, C.; Wang, J. Surfactants/citric acid catalyzed hydrothermal carbonization of pomelo peel for solid fuels: Conversion mechanism and combustion performance. Fuel 2023, 342, 127762–127772. [Google Scholar] [CrossRef]
- Kroll, P.; Sadowski, G.; Brandenbusch, C. Solubilization of Aldehydes and Amines in Aqueous C(i)E(j) Surfactant Aggregates: Solubilization Capacity and Aggregate Properties. Langmuir 2022, 38, 10022–10031. [Google Scholar] [CrossRef]
- Yu, S.; Jing, Y.; Fan, Y.; Xiong, L.; Wang, H.; Lei, J.; Zhang, Y.; Liu, J.; Wang, S.; Chen, X.; et al. Ultrahigh efficient emulsification with drag-reducing liquid gating interfacial behavior. Proc. Natl. Acad. Sci. USA 2022, 119, e2206462119. [Google Scholar] [CrossRef]
- Fan, Y.; Sheng, Z.; Chen, J.; Pan, H.; Chen, B.; Wu, F.; Wang, S.; Chen, X.; Hou, X. Visual Chemical Detection Mechanism by a Liquid Gating System with Dipole-Induced Interfacial Molecular Reconfiguration. Angew. Chem. Int. Ed. 2019, 58, 3967–3971. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, L.; Zhang, X.-X.; Du, F.-P. Effect of gum arabic on the surface tension and surface dilational rheology of trisiloxane surfactant. Food Hydrocolloids 2013, 30, 456–462. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, Z.-H.; Zhang, Q.; Zhang, F.; Ma, G.-Y.; Zhang, L.; Zhang, L. Effect of Electrolyte on Synergism for Reducing Interfacial Tension between Betaine and Petroleum Sulfonate. Energy Fuels 2020, 34, 3188–3198. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, F.T.; Han, L.; Zhu, X.Y.; Zhang, F.; Ma, G.Y.; Zhang, L.; Zhou, Z.H.; Zhang, L. The Synergistic Effects between Sulfobetaine and Hydrophobically Modified Polyacrylamide on Properties Related to Enhanced Oil Recovery. Molecules 2023, 28, 1787. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.C.; Gong, Q.T.; Zhang, L.; Luo, L.; Zhao, S.; Yu, J.Y. Interfacial dilational properties of tri-substituted alkyl benzene sulfonates at air/water and decane/water interfaces. J. Colloid Interface Sci. 2008, 327, 451–458. [Google Scholar] [CrossRef]
- Zhu, Y.-W.; Zhang, L.; Song, X.-W.; Luo, L.; Zhang, L.; Zhao, S.; Yu, J.-Y. Effect of electrolyte on interfacial dilational properties of chemical flooding systems by relaxation measurements. Fuel 2011, 90, 3172–3178. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, R.-H.; Zhang, L.; Xu, Z.-C.; Jin, Z.-Q.; Luo, L.; Zhang, L.; Zhao, S. Effect of Electrolyte and Temperature on Interfacial Tensions of Alkylbenzene Sulfonate Solutions. Energy Fuels 2012, 26, 2175–2181. [Google Scholar] [CrossRef]
- Zhao, R.-H.; Zhang, L.; Zhang, L.; Zhao, S.; Yu, J.-Y. Effect of the Hydrophilic−Lipophilic Ability on Dynamic Interfacial Tensions of Alkylbenzene Sulfonates. Energy Fuels 2010, 24, 5048–5052. [Google Scholar] [CrossRef]
- Chen, X.-F.; Xu, Z.-C.; Gong, Q.-T.; Wu, D.-h.; Zhang, L.; Zhang, L.; Zhao, S. Adsorption of extended anionic surfactants at the water- polymethylmethacrylate interface: The effect of polyoxyethylene groups. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130395. [Google Scholar] [CrossRef]
- Hu, S.S.; Zhou, Z.H.; Zhang, L.; Xu, Z.C.; Gong, Q.T.; Jin, Z.Q.; Zhang, L.; Zhao, S. Adsorption behaviors of novel betaines on the wettability of the quartz surface. Soft Matter 2015, 11, 7960–7968. [Google Scholar] [CrossRef]
- Hu, S.-S.; Zhang, L.; Xu, Z.-C.; Gong, Q.-T.; Jin, Z.-Q.; Luo, L.; Zhang, L.; Zhao, S. Wettability alteration by novel betaines at polymer–aqueous solution interfaces. Appl. Surf. Sci. 2015, 355, 868–877. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, Z.-H.; Shangguan, Y.-N.; Han, L.; Wang, L.-L.; Fan, W.; Zhang, Q.; Zhang, L.; Ma, G.-Y.; Zhang, L. Effect of bivalent cations on the interfacial tensions of extended anionic surfactant solutions. J. Mol. Liq. 2022, 349, 118162. [Google Scholar] [CrossRef]
- Wang, Z.-S.; Zhou, Z.-H.; Han, L.; Chen, X.; He, H.-J.; Zhang, Q.; Xu, Z.-C.; Gong, Q.-T.; Zhang, L.; Ma, G.-Y.; et al. The mechanism for lowering interfacial tension by extended surfactant containing ethylene oxide and propylene oxide groups. J. Mol. Liq. 2022, 359, 119364. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, L.; Luan, H.; Ren, J.; Xu, C.; Li, G.; Xiao, H.; Zhou, Z.; Zhang, L. Synergism for lowering interfacial tensions between betaines and extended surfactants: The role of self-regulating molecular size. J. Mol. Liq. 2023, 378, 121605. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Zhang, L.; Zhou, J.; Wang, J.; Yan, H. Dilational properties of novel amphiphilic dendrimers at water-air and water-heptane interfaces. J. Phys. Chem. B 2012, 116, 12760–12768. [Google Scholar] [CrossRef]
- Tang, L.; Fang, H.-B.; Zong, H.; Zhang, L.; Jin, Z.-Q.; Xu, Z.-C.; Zhang, L.; Zhao, S. Dilational rheological properties of interfacial films containing Branch-Preformed particle gel and crude oil fractions. J. Appl. Polym. Sci. 2015, 132, 41337–41344. [Google Scholar] [CrossRef]
- Zhao, F.J.; Yuan, F.Q.; Pan, B.L.; Xu, Z.C.; Gong, Q.T.; Zhang, L.; Hou, J.; Zhang, L. Dilational Rheological Properties of Surfactants at the Crude Oil-Water Interface: The Effect of Branch-Preformed Particle Gels and Polymers. ACS Omega 2022, 7, 24871–24880. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Jin, Y.-X.; Wang, T.; Xu, Z.-C.; Gong, Q.-T.; Jin, Z.-Q.; Zhang, L.; Zhang, L. Effect of branched chain and polyoxyethylene group on surface dilational rheology of cationic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 249–256. [Google Scholar] [CrossRef]
- Sun, H.-Q.; Zhang, L.; Li, Z.-Q.; Zhang, L.; Luo, L.; Zhao, S. Interfacial dilational rheology related to enhance oil recovery. Soft Matter 2011, 7, 7601–7611. [Google Scholar] [CrossRef]
- Feng, J.; Liu, X.P.; Zhang, L.; Zhao, S.; Yu, J.Y. Dilational properties of anionic gemini surfactants with polyoxyethylene spacers at water-air and water-decane interfaces. Langmuir 2010, 26, 11907–11914. [Google Scholar] [CrossRef]
- Llamas, S.; Santini, E.; Liggieri, L.; Salerni, F.; Orsi, D.; Cristofolini, L.; Ravera, F. Adsorption of Sodium Dodecyl Sulfate at Water-Dodecane Interface in Relation to the Oil in Water Emulsion Properties. Langmuir 2018, 34, 5978–5989. [Google Scholar] [CrossRef]
- Fainerman, V.B.; Aksenenko, E.V.; Lylyk, S.V.; Makievski, A.V.; Ravera, F.; Petkov, J.T.; Yorke, J.; Miller, R. Adsorption layer characteristics of Tritons surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2009, 334, 16–21. [Google Scholar] [CrossRef]
- Fainerman, V.B.; Kovalchuk, V.I.; Aksenenko, E.V.; Nikolenko, M.V.; Miller, R. Dilational surface visco-elasticity of CnEOm solutions under dynamic conditions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 557, 131–136. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Dai, Y.H.; Zhang, L.; Tang, K.; Luo, L.; Gong, Q.; Zhao, S.; Li, M.Z.; Wang, E.J.; Yu, J.Y. The interfacial dilational properties of hydrophobically modified associating polyacrylamide studied by the interfacial tension relaxation method at an oil-water interface. J. Colloid Interface Sci. 2004, 280, 76–82. [Google Scholar] [CrossRef]
- Cui, X.-H.; Zhang, L.; Luo, L.; Zhang, L.; Zhao, S.; Yu, J.-Y. Interfacial dilational properties of model oil and chemical flooding systems by relaxation measurements. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 106–112. [Google Scholar] [CrossRef]
- Ma, B.-D.; Zhang, L.; Gao, B.-Y.; Zhang, L.; Zhao, S.; Yu, J.-Y. Interfacial dilational rheological property and lamella stability of branched alkyl benzene sulfonates solutions. Colloid Polym. Sci. 2011, 289, 911–918. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Li, Z.-Q.; Zhang, L.; Huang, H.-Y.; Zhang, L.; Zhao, S.; Yu, J.-Y. Dilational Properties of Sodium 2,5-Dialkyl Benzene Sulfonates at Air−Water and Decane−Water Interfaces. J. Chem. Eng. Data 2011, 56, 2393–2398. [Google Scholar] [CrossRef]
- Zhang, J.-C.; Guo, L.-L.; Zhang, L.; Dong, L.-F.; Zhang, L.; Luo, L.; Zhao, S. Surface Dilational Properties of Sodium 4-(1-methyl)-Alkyl Benzene Sulfonates: Effect of Alkyl Chain Length. Z. Für Phys. Chem. 2013, 227, 429–440. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, Q.; Gong, Q.-T.; Liu, Z.-Y.; Liu, M.; Zhang, L.; Zhang, L.; Zhao, S. Interfacial dilational properties of di-substituted alkyl benzene sulfonates at kerosene/water and crude oil/water interfaces. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 561–569. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.-C.; Gong, Q.-T.; Luo, L.; Zhang, L.; Zhao, S.; Yong-Yu, J. Effect of Electrolyte on the Interfacial Dilational Properties of Sodium 4,5-Diheptyl-2-propylbenzene Sulfonate at the Oil–Water Interface. J. Dispers. Sci. Technol. 2009, 30, 217–221. [Google Scholar] [CrossRef]
- Song, X.-W.; Zhang, L.; Cao, X.-L.; Li, Z.-Q.; Zhang, L.; Luo, L.; Zhao, S. Surface dilational rheological and lamella properties of branched alkyl benzene sulfonate solutions. Colloids Surf. A Physicochem. Eng. Asp. 2012, 412, 64–71. [Google Scholar] [CrossRef]
- Wu, X.-L.; Chu, Y.-P.; Huang, Y.-P.; Xu, Z.-C.; Zhang, L.; Zhang, L.; Zhao, S. Effect of Alkyl Chain Length on the Interfacial Dilational Properties of Hydroxy-Substituted Alkyl Benzenesulfonates. J. Dispers. Sci. Technol. 2016, 37, 1738–1744. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Zhang, L.; Zhang, L.; Luo, L.; Zhao, S.; Yu, J.-Y. Dynamic Interfacial Dilational Properties of Hydroxy-Substituted Alkyl Benzenesulfonates. J. Phys. Chem. B 2007, 111, 5640–5647. [Google Scholar] [CrossRef]
- Zhang, J.-C.; Zhang, L.; Wang, X.-C.; Zhang, L.; Zhao, S.; Yu, J.-Y. A Surface Rheological Study of Polyoxyethylene Alkyl Ether Carboxylic Salts and the Stability of Corresponding Foam. J. Dispers. Sci. Technol. 2011, 32, 372–379. [Google Scholar] [CrossRef]
- Wang, C.; Cao, X.-L.; Guo, L.-L.; Xu, Z.-C.; Zhang, L.; Gong, Q.-T.; Zhang, L.; Zhao, S. Effect of molecular structure of catanionic surfactant mixtures on their interfacial properties. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 601–612. [Google Scholar] [CrossRef]
- Zhou, Z.-H.; Ma, D.-S.; Zhang, Q.; Wang, H.-Z.; Zhang, L.; Luan, H.-x.; Zhu, Y.; Zhang, L. Surface dilational rheology of betaine surfactants: Effect of molecular structures. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 739–747. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Z.-H.; Dong, L.-F.; Wang, H.-Z.; Cai, H.-Y.; Zhang, F.; Zhang, L.; Zhang, L.; Zhao, S. Dilational rheological properties of sulphobetaines at the water–decane interface: Effect of hydrophobic group. Colloids Surf. A Physicochem. Eng. Asp. 2014, 455, 97–103. [Google Scholar] [CrossRef]
- Sun, H.-Q.; Guo, Z.-Y.; Cao, X.-L.; Zhu, Y.-W.; Pan, B.-L.; Liu, M.; Zhang, L.; Zhang, L. Interfacial interactions between oleic acid and betaine molecules at decane-water interface: A study of dilational rheology. J. Mol. Liq. 2020, 316, 113784. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, L.; Wang, Y.; Peng, B.; Zhao, S.; Li, M.; Yu, J. Dynamic Dilational Properties of Oil–Water Interfacial Films Containing Surface Active Fractions from Crude Oil. J. Dispers. Sci. Technol. 2003, 24, 699–707. [Google Scholar] [CrossRef]
- Yu-Ping, H.; Lei, Z.; Lan, L.; Lu, Z.; Sui, Z.; Jia-Yong, Y. Dilational Properties of Hydroxy-substituted SodiumAlkyl Benzene Sulfonate at Surface and Water-decane Interface. Acta Phys.-Chim. Sin. 2007, 23, 12–15. [Google Scholar] [CrossRef]
- Felix, M.; Romero, A.; Carrera-Sanchez, C.; Guerrero, A. Assessment of interfacial viscoelastic properties of Faba bean (Vicia faba) protein-adsorbed O/W layers as a function of pH. Food Hydrocolloids 2019, 90, 353–359. [Google Scholar] [CrossRef]
- RıBo, O.I.D.; Neumann, A.W. Axisymmetric Drop Shape Analysis: Computational Methods for the Measurement of Interfacial Properties from the Shape and Dimensions of Pendant and Sessile Drops. J. Colloid Interface Sci. 1997, 196, 136–147. [Google Scholar]
- Yan, F.; Zhang, L.; Zhao, R.-H.; Huang, H.-Y.; Dong, L.-F.; Zhang, L.; Zhao, S.; Yu, J.-Y. Surface dilational rheological and foam properties of aromatic side chained N-acyltaurate amphiphiles. Colloids Surf. A Physicochem. Eng. Asp. 2012, 396, 317–327. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Xu, Z.; Gong, Q.; Ma, W.; Jin, Z.; Zhang, L.; Zhang, L. The Study of Interfacial Adsorption Behavior for Hydroxyl-Substituted Alkylbenzene Sulfonates by Interfacial Tension Relaxation Method. Molecules 2023, 28, 4318. https://doi.org/10.3390/molecules28114318
Sun Q, Xu Z, Gong Q, Ma W, Jin Z, Zhang L, Zhang L. The Study of Interfacial Adsorption Behavior for Hydroxyl-Substituted Alkylbenzene Sulfonates by Interfacial Tension Relaxation Method. Molecules. 2023; 28(11):4318. https://doi.org/10.3390/molecules28114318
Chicago/Turabian StyleSun, Qi, Zhicheng Xu, Qingtao Gong, Wangjing Ma, Zhiqiang Jin, Lei Zhang, and Lu Zhang. 2023. "The Study of Interfacial Adsorption Behavior for Hydroxyl-Substituted Alkylbenzene Sulfonates by Interfacial Tension Relaxation Method" Molecules 28, no. 11: 4318. https://doi.org/10.3390/molecules28114318
APA StyleSun, Q., Xu, Z., Gong, Q., Ma, W., Jin, Z., Zhang, L., & Zhang, L. (2023). The Study of Interfacial Adsorption Behavior for Hydroxyl-Substituted Alkylbenzene Sulfonates by Interfacial Tension Relaxation Method. Molecules, 28(11), 4318. https://doi.org/10.3390/molecules28114318






