A Simple Method for Synthesis of Chitosan Nanoparticles with Ionic Gelation and Homogenization
Abstract
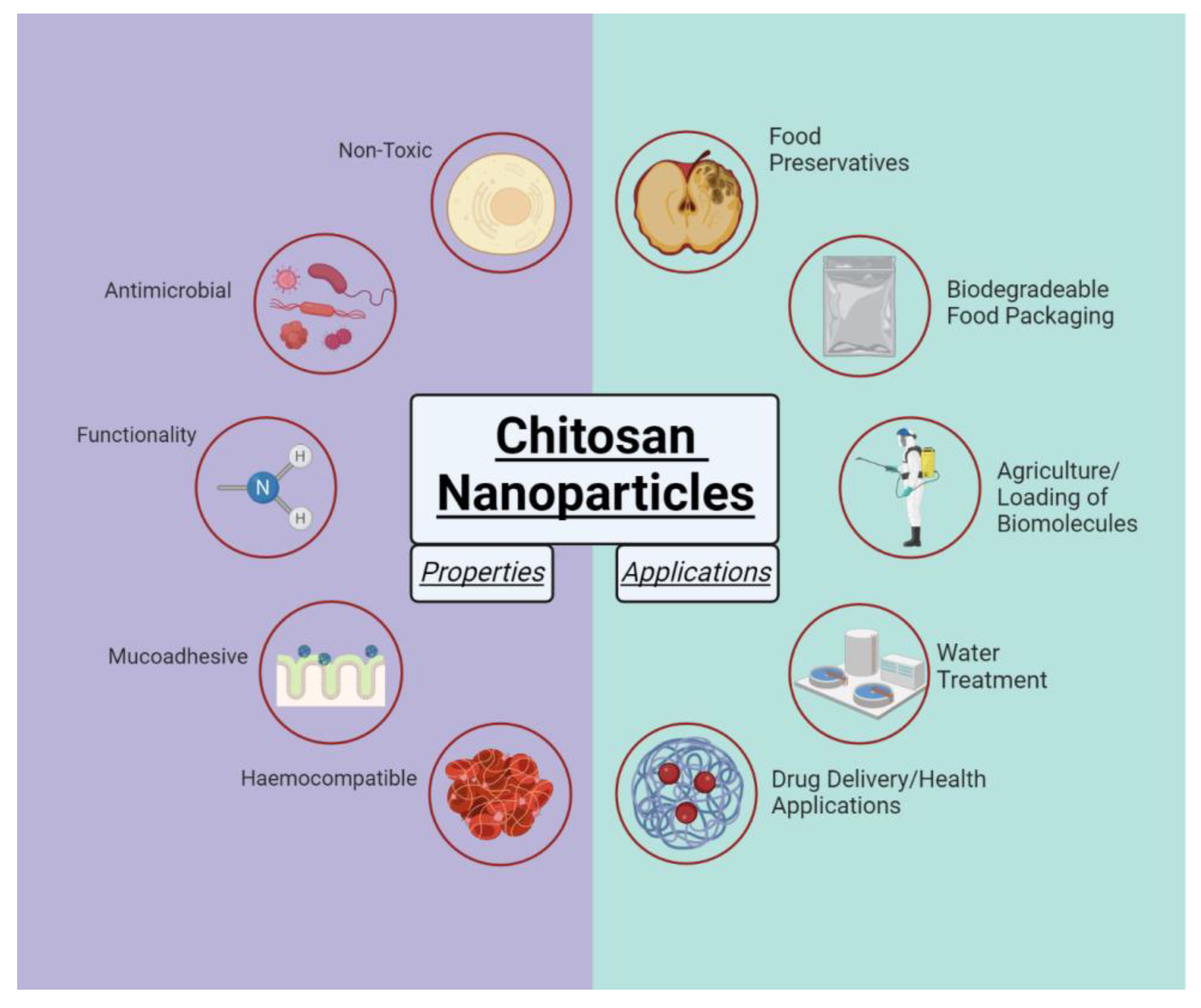
1. Introduction
2. Results and Discussion
2.1. Chitosan Nanoparticle Synthesis
2.1.1. Characteristics of Chitosan Nanoparticles under Ionic Strength Control
2.1.2. Characteristics of Chitosan Nanoparticles under pH Control
2.1.3. Characteristics of Chitosan Nanoparticles with a Homogenizer
2.2. Purification and Collection of Chitosan Nanoparticles
2.2.1. Purification of Chitosan Nanoparticles with Ultracentrifugation
2.2.2. Purification of Chitosan Nanoparticles with Size Exclusion Chromatography
2.2.3. Purification of Chitosan Nanoparticles Using Syringe Filtration
2.3. Characterization of Chitosan Nanoparticles with Scanning Electron Microscopy
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Chitosan Nanoparticles
3.3. Purification of Chitosan Nanoparticles
3.4. Synthesis of Chitosan Nanoparticles with Ionic Strength Control
3.5. Synthesis of Chitosan Nanoparticles with pH Control
3.6. Characterization of Chitosan Nanoparticles
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-Based Nanomaterials: A State-of-the-Art Review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Tang, Q.; Qiu, P.; Gou, D.; Zhao, J. Chitosan-Based Materials: An Overview of Potential Applications in Food Packaging. J. Foods 2022, 11, 1490. [Google Scholar] [CrossRef]
- Al-Manhel, A.J.; Al-Hilphy, A.R.S.; Niamah, A.K. Extraction of Chitosan, Characterisation and Its Use for Water Purification. J. Saudi Soc. Agric. Sci. 2018, 17, 186–190. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Sillanpää, M. Applications of Chitin- and Chitosan-Derivatives for the Detoxification of Water and Wastewater—A Short Review. Adv. Colloid Interface Sci. 2009, 152, 26–38. [Google Scholar] [CrossRef]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A Review of Bio-Based Materials for Oil Spill Treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Wei, C.; Liao, X.; Huang, Y.; Zhou, F. Effects of Chitosan and Licorice Antioxidants on Skin Color of Duck Meat Refrigerated and the Number of Micro-Organisms on the Surface. J. Food Sci. Technol. 2020, 45, 280–285. [Google Scholar]
- Yanat, M.; Schroën, K. Preparation Methods and Applications of Chitosan Nanoparticles; with an Outlook toward Reinforcement of Biodegradable Packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Saad, G.R.; Sayed, A.M. Mechanical, Thermal, and Dielectric Properties of Poly(Lactic Acid)/Chitosan Nanocomposites. Polym. Eng. Sci. 2016, 56, 987–994. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan: Drug Delivery and Biomedical Applications; Jana, S., Ed.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Másson, M. Antimicrobial Properties of Chitosan and Its Derivatives. In Chitosan for Biomaterials III. Advances in Polymer Science; Jayakumar, R., Prabaharan, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 131–168. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as A Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Al-Zahrani, S.S.; Bora, R.S.; Al-Garni, S.M. Antimicrobial Activity of Chitosan Nanoparticles. Biotechnol. Biotechnol. Equip. 2021, 35, 1874–1880. [Google Scholar] [CrossRef]
- Felipe, V.; Breser, M.L.; Bohl, L.P.; Rodrigues da Silva, E.; Morgante, C.A.; Correa, S.G.; Porporatto, C. Chitosan Disrupts Biofilm Formation and Promotes Biofilm Eradication in Staphylococcus Species Isolated from Bovine Mastitis. Int. J. Biol. Macromol. 2019, 126, 60–67. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Deshmukh, P.R.; Joshi, A.; Vikhar, C.; Khdabadi, S.S.; Tawar, M. Current Applications of Chitosan Nanoparticles. Syst. Rev. Pharm. 2022, 13, 685–693. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of Chitosan Nanoparticles to Enhance Absorption and Bioavailability of Tea Polyphenols: A Review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Grillo, R.; Pereira, A.E.S.; Nishisaka, C.S.; De Lima, R.; Oehlke, K.; Greiner, R.; Fraceto, L.F. Chitosan/Tripolyphosphate Nanoparticles Loaded with Paraquat Herbicide: An Environmentally Safer Alternative for Weed Control. J. Hazard. Mater. 2014, 278, 163–171. [Google Scholar] [CrossRef]
- Thinh, N.N.; Hanh, P.T.B.; Ha, L.T.T.; Anh, L.N.; Hoang, T.V.; Hoang, V.D.; Dang, L.H.; Van Khoi, N.; Lam, T.D. Magnetic Chitosan Nanoparticles for Removal of Cr(VI) from Aqueous Solution. Mater. Sci. Eng. C 2013, 33, 1214–1218. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, L.; Chen, C.; Shi, X.; Cao, Q.; Gao, L. In Situ Preparation of Magnetic Fe3O4/Chitosan Nanoparticles via a Novel Reduction-Precipitation Method and Their Application in Adsorption of Reactive Azo Dye. Powder Technol. 2014, 260, 90–97. [Google Scholar] [CrossRef]
- Kunjachan, S.; Jose, S. Understanding the Mechanism of Ionic Gelation for Synthesis of Chitosan Nanoparticles Using Qualitative Techniques. Asian J. Pharm. 2010, 4, 148–153. [Google Scholar] [CrossRef]
- Sawtarie, N.; Cai, Y.; Lapitsky, Y. Preparation of Chitosan/Tripolyphosphate Nanoparticles with Highly Tunable Size and Low Polydispersity. Colloids Surf. B Biointerfaces 2017, 157, 110–117. [Google Scholar] [CrossRef]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors Determining the Stability, Size Distribution, and Cellular Accumulation of Small, Monodisperse Chitosan Nanoparticles as Candidate Vectors for Anticancer Drug Delivery: Application to the Passive Encapsulation of [14 C]-Doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef]
- Dolai, J.; Mandal, K.; Jana, N.R. Nanoparticle Size Effects in Biomedical Applications. ACS Appl. Nano Mater. 2021, 4, 6471–6496. [Google Scholar] [CrossRef]
- Mozaffari, S.; Li, W.; Dixit, M.; Seifert, S.; Lee, B.; Kovarik, L.; Mpourmpakis, G.; Karim, A.M. The Role of Nanoparticle Size and Ligand Coverage in Size Focusing of Colloidal Metal Nanoparticles. Nanoscale Adv. 2019, 1, 4052–4066. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on in Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Nunes, R.; Sousa, Â.; Simaite, A.; Aido, A.; Buzgo, M. Sub-100 Nm Chitosan-Triphosphate-DNA Nanoparticles for Delivery of DNA Vaccines. Proceedings 2021, 78, 12. [Google Scholar] [CrossRef]
- Pang, L.; Farkas, K.; Bennett, G.; Varsani, A.; Easingwood, R.; Tilley, R.; Nowostawska, U.; Lin, S. Mimicking Filtration and Transport of Rotavirus and Adenovirus in Sand Media Using DNA-Labeled, Protein-Coated Silica Nanoparticles. Water Res. 2014, 62, 167–179. [Google Scholar] [CrossRef]
- Farkas, K.; Varsani, A.; Pang, L. Adsorption of Rotavirus, MS2 Bacteriophage and Surface-Modified Silica Nanoparticles to Hydrophobic Matter. Food Environ. Virol. 2015, 7, 261–268. [Google Scholar] [CrossRef]
- Clemens, H.; Pang, L.; Morgan, L.K.; Weaver, L. Attenuation of Rotavirus, MS2 Bacteriophage and Biomolecule-Modified Silica Nanoparticles in Undisturbed Silt Loam over Gravels Dosed with Onsite Wastewater. Water Res. 2020, 169, 115272. [Google Scholar] [CrossRef]
- Alonso, M.J.; Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L. Novel Hydrophilic Chitosan-Polyethylene Oxide Nanoparticles as Protein Carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Rampino, A.; Borgogna, M.; Bellich, B.; Blasi, P.; Virgilio, F.; Cesàro, A. Chitosan-Pectin Hybrid Nanoparticles Prepared by Coating and Blending Techniques. Eur. J. Pharm. Sci. 2016, 84, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Tammam, S.N.; Azzazy, H.M.E.; Breitinger, H.G.; Lamprecht, A. Chitosan Nanoparticles for Nuclear Targeting: The Effect of Nanoparticle Size and Nuclear Localization Sequence Density. Mol. Pharm. 2015, 12, 4277–4289. [Google Scholar] [CrossRef] [PubMed]
- Kamat, V.; Bodas, D.; Paknikar, K. Chitosan Nanoparticles Synthesis Caught in Action Using Microdroplet Reactions. Sci. Rep. 2016, 6, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Zhang, Y.; Wang, W.; Zhao, X.; Han, X.; Wang, K.; Ge, Y. Preparation and in Vitro/in Vivo Evaluation of Resveratrol-Loaded Carboxymethyl Chitosan Nanoparticles. Drug Deliv. 2016, 23, 981–991. [Google Scholar] [CrossRef]
- Budi, S.; Asih Suliasih, B.; Rahmawati, I.; Erdawati. Size-Controlled Chitosan Nanoparticles Prepared Using Ionotropic Gelation. ScienceAsia 2020, 46, 457–461. [Google Scholar] [CrossRef]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rivera-Rodriguez, G.R. Parameters Influencing the Size of Chitosan-TPP Nano- and Microparticles. Sci. Rep. 2018, 8, 4695. [Google Scholar] [CrossRef]
- Huang, Y.; Lapitsky, Y. On the Kinetics of Chitosan/Tripolyphosphate Micro- and Nanogel Aggregation and Their Effects on Particle Polydispersity. J. Colloid Interface Sci. 2017, 486, 27–37. [Google Scholar] [CrossRef]
- Jonassen, H.; Kjøniksen, A.L.; Hiorth, M. Effects of Ionic Strength on the Size and Compactness of Chitosan Nanoparticles. Colloid Polym. Sci. 2012, 290, 919–929. [Google Scholar] [CrossRef]
- Mattu, C.; Li, R.; Ciardelli, G. Chitosan Nanoparticles as Therapeutic Protein Nanocarriers: The Effect of Ph on Particle Formation and Encapsulation Efficiency. Polym. Compos. 2013, 34, 1538–1545. [Google Scholar] [CrossRef]
- Katas, H.; Alpar, H.O. Development and Characterisation of Chitosan Nanoparticles for SiRNA Delivery. J. Control. Release 2006, 115, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.K.; Boateng, J.S. Comparison and Process Optimization of PLGA, Chitosan and Silica Nanoparticles for Potential Oral Vaccine Delivery. Ther. Deliv. 2019, 10, 493–514. [Google Scholar] [CrossRef] [PubMed]
- Iswanti, F.C.; Nurulita, I.; Djauzi, S.; Sadikin, M.; Witarto, A.B.; Yamazaki, T. Preparation, Characterization, and Evaluation of Chitosan-Based Nanoparticles as CpG ODN Carriers. Biotechnol. Biotechnol. Equip. 2019, 33, 390–396. [Google Scholar] [CrossRef]
- Pang, L.; Abeysekera, G.; Hanning, K.; Premaratne, A.; Robson, B.; Abraham, P.; Sutton, R.; Hanson, C.; Hadfield, J.; Heiligenthal, L.; et al. Water Tracking in Surface Water, Groundwater and Soils Using Free and Alginate-Chitosan Encapsulated Synthetic DNA Tracers. Water Res. 2020, 184, 116192. [Google Scholar] [CrossRef] [PubMed]








| SEC Fraction | Major Species Hydrodynamic Diameter (nm) | Z-Average (nm) |
|---|---|---|
| Fraction 1 | 62.12 | 146.7 |
| Fraction 2 | 75.42 | 60.15 |
| SEC Pooled Fractions | Z-Average (nm) | PDI |
| 1, 2 Pooled | 103 | 0.218 |
| 3, 4, 5, 6, 7 Pooled | 95.18 | 0.399 |
| Not Subjected | 83.36 | 0.301 |
| Facility/Equipment | Size (nm) | PDI | Zeta Potential (mV) | Particle Concentration (Particle/mL) |
|---|---|---|---|---|
| University of Calgary/Malvern ZetaSizer Nano ZS | 73 | 0.344 | 10.17 ± 3.35 | - |
| University of Calgary/Malvern ZetaSizer Nano ZS | 68 | 0.330 | 21.00 ± 0.70 | - |
| University of Calgary/Malvern ZetaSizer Nano ZS | 77 | 0.319 | 21.10 ± 1.98 | - |
| IZON Science (Christchurch, New Zealand) TRPS with Exoid | 72 | 0.227 * | - | 3.57 × 109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Bavel, N.; Issler, T.; Pang, L.; Anikovskiy, M.; Prenner, E.J. A Simple Method for Synthesis of Chitosan Nanoparticles with Ionic Gelation and Homogenization. Molecules 2023, 28, 4328. https://doi.org/10.3390/molecules28114328
Van Bavel N, Issler T, Pang L, Anikovskiy M, Prenner EJ. A Simple Method for Synthesis of Chitosan Nanoparticles with Ionic Gelation and Homogenization. Molecules. 2023; 28(11):4328. https://doi.org/10.3390/molecules28114328
Chicago/Turabian StyleVan Bavel, Nicolas, Travis Issler, Liping Pang, Max Anikovskiy, and Elmar J. Prenner. 2023. "A Simple Method for Synthesis of Chitosan Nanoparticles with Ionic Gelation and Homogenization" Molecules 28, no. 11: 4328. https://doi.org/10.3390/molecules28114328
APA StyleVan Bavel, N., Issler, T., Pang, L., Anikovskiy, M., & Prenner, E. J. (2023). A Simple Method for Synthesis of Chitosan Nanoparticles with Ionic Gelation and Homogenization. Molecules, 28(11), 4328. https://doi.org/10.3390/molecules28114328






