Schisandra henryi—A Rare Species with High Medicinal Potential
Abstract
1. Introduction
2. Methodology
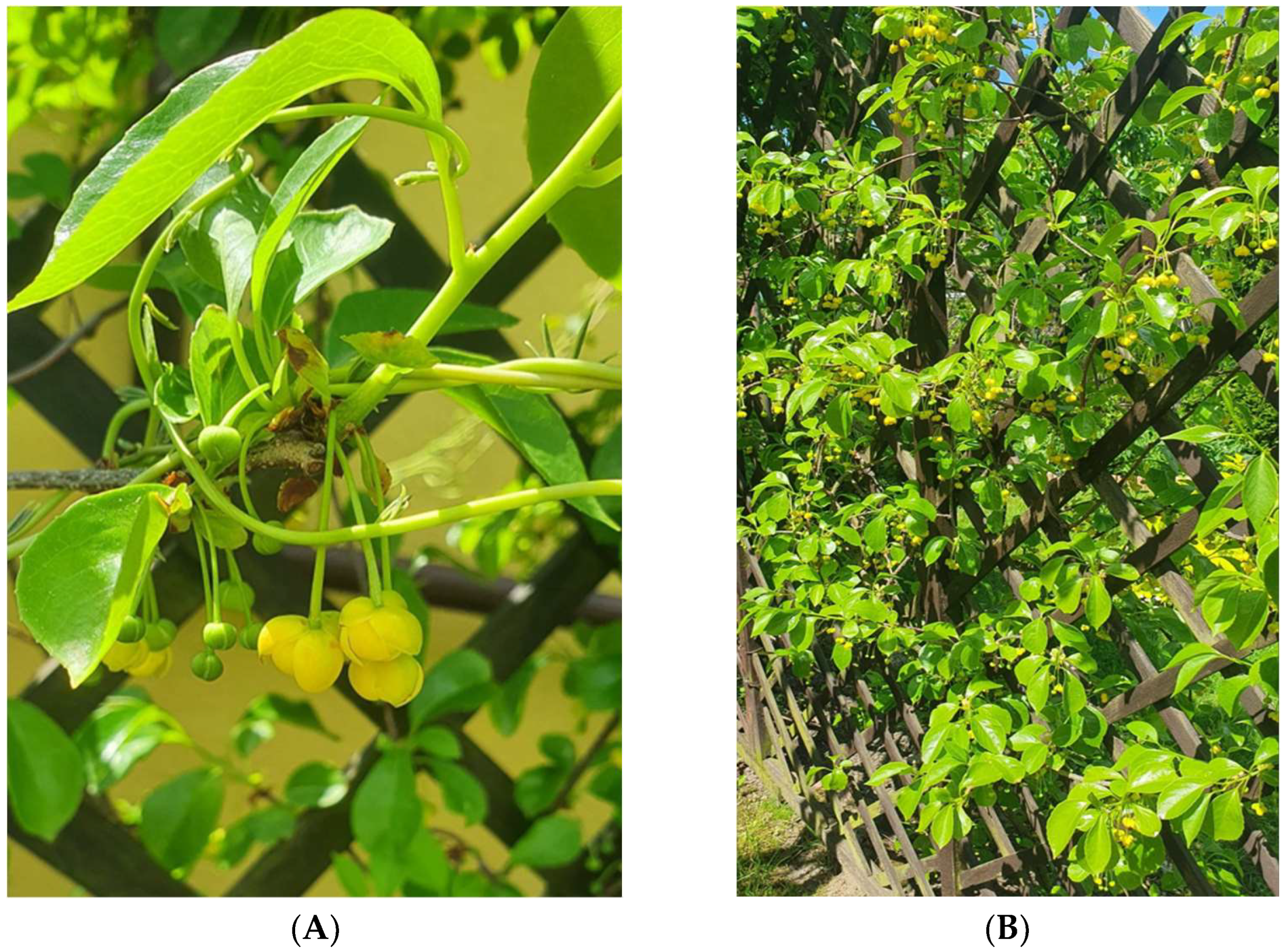
3. Morphology and Natural Habitats
4. Chemical Composition
5. Reports on the Biological Activities
6. Biological Activity of Chosen Dibenzocyclooctadiene Lignans
6.1. Antioxidant Activity
6.2. Anti-Inflammatory Activity
6.3. Anticancer Activity
6.4. Antiviral Activity
6.5. Neuroprotective Activity
6.6. Hepatoprotective and Hepatoregenerative Activity
6.7. Cardioprotective Activity
6.8. Supportive Activity in the Treatment of Intestinal Dysfunction
6.9. Anti-Osteoporotic Activity
7. Plant Biotechnology Research
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saunders, R.M.K. Monograph of Schisandra (Schisandraceae). Am. Soc. Plant Taxon. 2000, 58, 1–146. [Google Scholar] [CrossRef]
- Saunders, R.M.K. Species Plantarum Flora of the World Part 4. Schisandraceae. In Australian Biological Resources Study; Australian Biological Resources Study: Canberra, Australia, 2001; pp. 1–68. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Chemical Industry Press: Beijing, China, 2005. [Google Scholar]
- Committee of the Japanese Pharmacopoeia Evaluation and Licensing Division Pharmaceuticals and Food Safety. Japanese Pharmacopoeia. Labour and Welfare; Bureau Ministry of Health: Tokyo, Japan, 2006. [Google Scholar]
- Central Pharmaceutical Affairs Council of Korea. Korean Pharmacopoeia; Central Pharmaceutical Affairs Council of Korea: Seoul, Korea, 2002. [Google Scholar]
- European Directorate for the Quality of Medicine. European Pharmacopoeia 10.0; European Directorate for the Quality of Medicine: Strasbourg, France, 2010. [Google Scholar]
- Upton, R.; Graff, A.; Jolliffe, G.; Länger, R.; Williamson, E. American Herbal Pharmacopoeia: Botanical Pharmacognosy—Microscopic Characterization of Botanical Medicines; CRC Press: Boca Raton, FL, USA, 2011; ISBN 1420073281. [Google Scholar]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal Plants of the Russian Pharmacopoeia; Their History and Applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef] [PubMed]
- WHO Monographs on Selected Medicinal Plants. Volume 2; Department of Essential Drugs and Medicines Policy—World Health Organization: Geneva, Switzerland, 2004.
- Kam Ming, K.; Jun, Y.; Chuixin, Q. Schisandra Chinensis: An Herb of North Eastern China Origin; World Scientific Publishing: Singapore, 2015. [Google Scholar]
- Tian, Z. Characterization and Genetic Analysis of the Complete Chloroplast Genome of Schisandra chinensis (Magnoliaceae: Schisandra), an Herbal Medicine from China. Mitochondrial DNA B Resour. 2019, 4, 2428–2430. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.L.; Liu, B.Y.; Ma, K.W. Traditional Chinese Medicine. Lancet 2008, 372, 1938–1940. [Google Scholar] [CrossRef]
- Jin, J.; Bi, H.; Hu, J.; Zeng, H.; Zhong, G.; Zhao, L.; Huang, Z.; Huang, M. Effect of Wuzhi Tablet (Schisandra sphenanthera Extract) on the Pharmacokinetics of Paclitaxel in Rats. Phytother. Res. 2011, 25, 1250–1253. [Google Scholar] [CrossRef]
- Xue, X.P.; Qin, X.L.; Xu, C.; Zhong, G.P.; Wang, Y.; Huang, M.; Bi, H.C. Effect of Wuzhi Tablet (Schisandra sphenanthera Extract) on the Pharmacokinetics of Cyclosporin A in Rats. Phytother. Res. 2013, 27, 1255–1259. [Google Scholar] [CrossRef]
- Bi, H.; Li, F.; Krausz, K.W.; Qu, A.; Johnson, C.H.; Gonzalez, F.J. Targeted Metabolomics of Serum Acylcarnitines Evaluates Hepatoprotective Effect of Wuzhi Tablet (Schisandra sphenanthera Extract) against Acute Acetaminophen Toxicity. Evid. Based Complement. Altern. Med. 2013, 2013, 985257. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, D.F. Analysis of Schisandra chinensis and Schisandra sphenanthera. J. Chromatogr. A 2009, 1216, 1980–1990. [Google Scholar] [CrossRef]
- Szopa, A.; Ekiert, H. Lignans in Schisandra chinensis in Vitro Cultures. Pharmazie 2011, 66, 633–634. [Google Scholar]
- Jiang, P.; Lu, Y.; Chen, D. Authentication of Schisandra chinensis and Schisandra sphenanthera in Chinese Patent Medicines. J. Pharm. Biomed. Anal. 2016, 131, 263–271. [Google Scholar] [CrossRef]
- Szopa, A.; Barnaś, M.; Ekiert, H. Phytochemical Studies and Biological Activity of Three Chinese Schisandra Species (Schisandra sphenanthera, Schisandra henryi and Schisandra rubriflora): Current Findings and Future Applications. Phytochem. Rev. 2019, 18, 109–128. [Google Scholar] [CrossRef]
- Szopa, A.; Dziurka, M.; Granica, S.; Klimek-Szczykutowicz, M.; Kubica, P.; Warzecha, A.; Jafernik, K.; Ekiert, H. Schisandra Rubriflora Plant Material and in Vitro Microshoot Cultures as Rich Sources of Natural Phenolic Antioxidants. Antioxidants 2020, 9, 488. [Google Scholar] [CrossRef]
- Xiao, W.L.; Gong, Y.Q.; Wang, R.R.; Weng, Z.Y.; Luo, X.; Li, X.N.; Yang, G.Y.; He, F.; Pu, J.X.; Yang, L.M.; et al. Bioactive Nortriterpenoids from Schisandra grandiflora. J. Nat. Prod. 2009, 72, 1678–1681. [Google Scholar] [CrossRef]
- Poornima, B.; Kumar, D.A.; Siva, B.; Venkanna, A.; Vadaparthi, P.R.R.; Kumar, K.; Tiwari, A.K.; Babu, K.S. Advanced Glycation End-Products Inhibitors Isolated from Schisandra grandiflora. Nat. Prod. Res. 2016, 30, 493–496. [Google Scholar] [CrossRef]
- Shi, W.; Liu, H.W.; Guo, X.; Hou, L.; Gao, J.M. Triterpenoids from the Stems of Schisandra grandiflora and Their Biological Activity. J. Asian Nat. Prod. Res. 2016, 18, 711–718. [Google Scholar] [CrossRef]
- Poornima, B.; Siva, B.; Shankaraiah, G.; Venkanna, A.; Nayak, V.L.; Ramakrishna, S.; Venkat Rao, C.; Babu, K.S. Novel Sesquiterpenes from Schisandra grandiflora: Isolation, Cytotoxic Activity and Synthesis of Their Triazole Derivatives Using “Clickg” Reaction. Eur. J. Med. Chem. 2015, 92, 449–458. [Google Scholar] [CrossRef]
- Lei, C.; Huang, S.X.; Chen, J.J.; Yang, L.B.; Xiao, W.L.; Chang, Y.; Lu, Y.; Huang, H.; Pu, J.X.; Sun, H.D. Propindilactones E-J, Schiartane Nortriterpenoids from Schisandra propinqua Var. propinqua. J. Nat. Prod. 2008, 71, 1228–1232. [Google Scholar] [CrossRef]
- Lei, C.; Huang, S.X.; Chen, J.J.; Pu, J.X.; Yang, L.B.; Zhao, Y.; Liu, J.P.; Gao, X.M.; Xiao, W.L.; Sun, H.D. Lignans from Schisandra propinqua Var. propinqua. Chem. Pharm. Bull. 2007, 55, 1281–1283. [Google Scholar] [CrossRef]
- Ding, W.P.; Hu, K.; Liu, M.; Li, X.R.; Chen, R.; Li, X.N.; Du, X.; Puno, P.T.; Sun, H.D. Five New Schinortriterpenoids from Schisandra propinqua Var. propinqua. Fitoterapia 2018, 127, 193–200. [Google Scholar] [CrossRef]
- Lei, C.; Huang, S.X.; Xiao, W.L.; Ma, Y.B.; Chen, J.J.; Pu, J.X.; Sun, H.D. Bisnortriterpenoids Possessing an 18-Nor-Schiartane Skeleton from Schisandra propinqua Var. propinqua. Planta Med. 2010, 76, 1611–1615. [Google Scholar] [CrossRef]
- Liu, J.S.; Huang, M.F.; Ayer, W.A.; Nakashima, T.T. Structure of Enshicine from Schisandra henryi. Phytochemistry 1984, 23, 1143–1145. [Google Scholar] [CrossRef]
- Iu, H.L.; Li-jia, X.U.; Eng, Y.P.; Ang, X.Y.; Iao, P.X. Two New Lignans from Schisandra henryi. Chem. Pharm. Bull. 2009, 57, 405–407. [Google Scholar] [CrossRef]
- Schisandraceae. Flora China 2008, 7, 39–43.
- Jafernik, K.; Szopa, A.; Barnaś, M.; Dziurka, M.; Ekiert, H. Schisandra henryi C. B. Clarke in Vitro Cultures: A Promising Tool for the Production of Lignans and Phenolic Compounds. Plant Cell Tissue Organ Cult. 2020, 143, 45–60. [Google Scholar] [CrossRef]
- Yuan, L.C.; Luo, Y.B.; Thien, L.B.; Fan, J.H.; Xu, H.L.; Chen, Z.D. Pollination of Schisandra henryi (Schisandraceae) by Female, Pollen-Eating Megommata Species (Cecidomyiidae, Diptera) in South-Central China. Ann. Bot. 2007, 99, 451–460. [Google Scholar] [CrossRef]
- Lian-Niang, L.; Hong, X. Henricine, a New Tetrahydrofuran Lignan from Schisandra henryi. Planta Med. 1986, 52, 493–494. [Google Scholar] [CrossRef]
- Umezawa, T. Diversity in Lignan Biosynthesis. Phytochem. Rev. 2003, 2, 371–390. [Google Scholar] [CrossRef]
- Kochetkov, N.; Khorlin, A.; Chizhov, O.; Al, E. Schizandrin—Lignan of Unusual Structure. Tetrahedron Lett. 1961, 2, 730–734. [Google Scholar] [CrossRef]
- Whiting, D.A. Lignans, Neolignans, and Related Compounds. Nat. Prod. Rep. 1990, 7, 349–364. [Google Scholar] [CrossRef]
- Liu, G. Bicyclol: A Novel Drug for Treating Chronic Viral Hepatitis B and C. Med. Chem. 2009, 5, 29–43. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, H.; Zhao, X.; Qin, Y.; Gong, X. Bicyclol Induces Cell Cycle Arrest and Autophagy in HepG2 Human Hepatocellular Carcinoma Cells through the PI3K/AKT and Ras/Raf/MEK/ERK Pathways. BMC Cancer 2016, 16, 742. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Dong, Y.; Zongile, Z. Bicyclol Medical Composition and Preparation Method Thereof. CN103242286A, 14 August 2013. [Google Scholar]
- Hu, W.; Li, Y.; Zhang, C.Z. Enantioseparation of Racemic Anti-Hepatitis New Drug Bicyclol with Crystallization. Chin. Chem. Lett. 2005, 16, 1471–1473. [Google Scholar]
- Yang, X.Y.; Zhuo, Q.; Wu, T.X.; Liu, G.J. Bicyclol for Chronic Hepatitis C. Cochrane Database Syst. Rev. 2007, 1. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yu, L.; Wei, H.; Liu, G. A Novel Antihepatitis Drug, Bicyclol, Prevents Liver Carcinogenesis in Diethylnitrosamine-Initiated and Phenobarbital-Promoted Mice Tumor Model. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.N.; Chen, H.; Li, Y. Effect of Bicyclol on Cisplatin-Induced Hepatotoxicity in the Hepatocarcinoma 22 Tumour-Bearing Mice. Basic Clin. Pharmacol. Toxicol. 2009, 104, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-S.; Tao, Y.; Huang, M.-F. Studies on the Constituents of Schisandra Henryi. V. The Structures of Wulignan A1, A2, Epiwulignan A1 and Epischisandrone. J. Chin. Chem. Soc. 1988, 46, 483–488. [Google Scholar]
- Yue, J.-m.; Chen, Y.-z.; Huang, S.-m.; Cheng, J.-l.; Cui, Y.-x. Ganschisandrine, a Lignan from Schisandra sphenanthera. Phytochemistry 1989, 28, 1774–1776. [Google Scholar] [CrossRef]
- Yamada, Y.; Hsu, C.; Iguchi, K.; Suzuki, S.; Hsu, H.Y.; Chen, Y.P. Structure of kadsuric acid. A new seco-triterpenoid from Kadsura japonica Dunal. Chem. Lett. 1976, 5, 1307–1310. [Google Scholar] [CrossRef]
- Xue, Y.B.; Yang, J.H.; Li, X.N.; Du, X.; Pu, J.X.; Xiao, W.L.; Su, J.; Zhao, W.; Li, Y.; Sun, H.D. Henrischinins A–C: Three New Triterpenoids from Schisandra henryi. Org. Lett. 2011, 13, 1564–1567. [Google Scholar] [CrossRef]
- Li, R.; Shen, Y.; Xiang, W.; Sun, H. Four Novel Nortriterpenoids Isolated from Schisandra henryi Var. yunnanensis. Eur. J. Org. Chem. 2004, 2004, 807–811. [Google Scholar] [CrossRef]
- He, T.B.; Yan, B.C.; Hu, K.; Li, X.N.; Sun, H.D.; Puno, P.T. Neuroprotective Schinortriterpenoids with Diverse Scaffolds from Schisandra henryi. Bioorg. Chem. 2020, 105, 104353. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-S.; Huang, M.-F.; Gao, Y.-L. Studies on the Constituents of Schisandra henryi Clarke. H. The Structures of Schisanhenrin and Schisanhenric Acid. Acta Chim. Sin. 1980, 38, 363–370. [Google Scholar]
- Chen, Y.G.; Wu, Z.C.; Lv, Y.P.; Gui, S.H.; Wen, J.; Liao, X.R.; Yuan, L.M.; Halaweish, F. Triterpenoids from Schisandra henryi with Cytotoxic Effect on Leukemia and Hela Cells in Vitro. Arch. Pharm. Res. 2003, 26, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.-L.; Wang, R.-R.; Zhao, W.; Tian, R.-R.; Shang, S.-Z.; Yang, L.-M.; Yang, J.-H.; Pu, J.-X.; Zheng, Y.-T.; Sun, H.-D. Anti-HIV-1 activity of lignans from the fruits of Schisandra rubriflora. Arch. Pharm. Res. 2010, 33, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.; Jeong, K.; Han, S.; Jeong, T.; Yum, M. Antioxidant Activity and Inhibition of MMP-1 Expression of Schizandrae Fructus (Schizandra Chinensis) Extract. Korean J. Pharmacogn. 2013, 1, 47–52. [Google Scholar]
- Yan, Z.; Guo, J.; Tian, F.; Mao, X.; Li, Y.; Li, C. Active Compounds from Schisandra chinensis Exhibiting Tyrosinase Activity and Melanin Content Inhibition in B16 Melanoma Cells. Biotechnol. Bioprocess Eng. 2015, 4, 814–823. [Google Scholar] [CrossRef]
- Chiu, P.Y.; Lam, P.Y.; Yan, C.W.; Ko, K.M. Schisandrin B Protects against Solar Irradiation-Induced Oxidative Injury in BJ Human Fibroblasts. Fitoterapia 2011, 82, 682–691. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kim, Y.H.; Bae, D.S.; Um, B.H.; Pan, C.H.; Kim, C.Y.; Lee, H.J.; Lee, J.K. Anti-Inflammatory Effects of Gomisin N, Gomisin J, and Schisandrin C Isolated from the Fruit of Schisandra chinensis. Biosci. Biotechnol. Biochem. 2010, 74, 285–291. [Google Scholar] [CrossRef]
- Casarin, E.; Dall’Acqua, S.; Šmejkal, K.; Šlapetová, T.; Innocenti, G.; Carrara, M. Molecular Mechanisms of Antiproliferative Effects Induced by Schisandra-Derived Dibenzocyclooctadiene Lignans (+)-Deoxyschisandrin and (-)-Gomisin N in Human Tumour Cell Lines. Fitoterapia 2014, 98, 241–247. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, K.; Hu, Y.; Su, F.; Gao, Z.; Hu, T.; Yang, Y.; Cao, X.; Qian, F. Schisantherin A Induces Cell Apoptosis through ROS/JNK Signaling Pathway in Human Gastric Cancer Cells. Biochem. Pharmacol. 2020, 173, 113673. [Google Scholar] [CrossRef]
- Sa, F.; Zhang, L.Q.; Chong, C.M.; Guo, B.J.; Li, S.; Zhang, Z.J.; Zheng, Y.; Hoi, P.M.; Lee, S.S.S.M.Y. Discovery of Novel Anti-Parkinsonian Effect of Schisantherin A in in Vitro and in Vivo. Neurosci. Lett. 2015, 593, 7–12. [Google Scholar] [CrossRef]
- Caichompoo, W.; Zhang, Q.-Y.; Hou, T.-T.; Gao, H.-J.; Qin, L.-P.; Zhou, X.-J. Optimization of Extraction and Purification of Active Fractions from Schisandra chinensis (Turcz.) and Its Osteoblastic Proliferation Stimulating Activity. Phytother. Res. 2009, 23, 289–292. [Google Scholar] [CrossRef]
- Lam, P.; Yan, C.; Chiu, P.; Leung, H.; Ko, K. Schisandrin B Protects against Solar Irradiation-Induced Oxidative Stress in Rat Skin Tissue. Fitoterapia 2011, 82, 393–400. [Google Scholar] [CrossRef]
- Xu, L.; Grandi, N.; Del Vecchio, C.; Al, E. From the Traditional Chinese Medicine Plant Schisandra chinensis New Scaffolds Effective on HIV-1 Reverse Transcriptase Resistant to Non-Nucleoside Inhibitors. J. Microbiol. 2015, 53, 288–293. [Google Scholar] [CrossRef]
- Chang, R.; Li, Y.; Yang, X.; Yue, Y.; Dou, L.; Wang, Y.; Zhang, W.; Li, X. Protective Role of Deoxyschizandrin and Schisantherin A against Myocardial Ischemia-Reperfusion Injury in Rats. PLoS ONE 2013, 8, 2–11. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, M.; Dou, D.; Kang, T.; Li, F. Effects of Deoxyschisandrin on Visceral Sensitivity of Mice with Inflammatory Bowel Disease. Evid. -Based Complement. Altern. Med. 2019, 29, 86–97. [Google Scholar] [CrossRef]
- Ci, X.; Ren, R.; UXu, K.; Li, H.; Yu, Q.; Song, Y.; Wang, D.; Li, R.; Deng, X. Schisantherin A Exhibits Anti-Inflammatory Properties by down-Regulating NF-KappaB and MAPK Signaling Pathways in Lipopolysaccharide-Treated RAW 264.7 Cells. Inflammation 2010, 33, 126–136. [Google Scholar] [CrossRef]
- Liao, S.; Zhou, K.; Li, D.; Xie, X.; Jun, F.; Wang, J. Schisantherin A Suppresses Interleukin-1β-Induced Inflammation in Human Chondrocytes via Inhibition of NF-ΚB and MAPKs Activation. Eur. J. Pharmacol. 2016, 780, 65–70. [Google Scholar] [CrossRef]
- Li, C.; Chen, T.; Zhou, H.; Zhang, C.; Feng, Y.; Tang, F.; Hoi, M.P.M.; He, C.; Zheng, Y.; Lee, S.M.Y. Schisantherin a Attenuates Neuroinflammation in Activated Microglia: Role of Nrf2 Activation through ERK Phosphorylation. Cell. Physiol. Biochem. 2018, 47, 1769–1784. [Google Scholar] [CrossRef]
- Zhou, E.; Li, Y.; Wei, Z.; Fu, Y.; Lei, H.; Zhang, N.; Yang, Z.; Xie, G. Schisantherin A Protects Lipopolysaccharide-Induced Acute Respiratory Distress Syndrome in Mice through Inhibiting NF-??B and MAPKs Signaling Pathways. Int. Immunopharmacol. 2014, 22, 133–140. [Google Scholar] [CrossRef]
- Chen, Y.G.; Wu, Z.C.; Gui, S.H.; Lv, Y.P.; Liao, X.R.; Halaweish, F. Lignans from Schisandra hernyi with DNA Cleaving Activity and Cytotoxic Effect on Leukemia and Hela Cells in Vitro. Fitoterapia 2005, 76, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, X.; Xu, X.; Mao, X.; Zhi, L.; Li, H.; Guo, L.; Kaishun, B.; Jia, Y. Schisantherin A Recovers Aβ-Induced Neurodegeneration with Cognitive Decline in Mice. Physiol. Behav. 2014, 132, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Sa, F.; Chong, C.M.; Ying, W.; Zhou, Y.Z.; Chang, R.C.C.; Chan, S.W.; Hoi, P.M.; Lee, S.M.Y. Schisantherin A Protects against 6-OHDA-Induced Dopaminergic Neuron Damage in Zebrafish and Cytotoxicity in SH-SY5Y Cells through the ROS/NO and AKT/GSK3β Pathways. J. Ethnopharmacol. 2015, 170, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Liu, F.; Lu, H.; Zhan, Y.; Zhang, M.; Guo, W.; Ding, G. Schisantherin A Protects against Liver Ischemia-Reperfusion Injury via Inhibition of Mitogen-Activated Protein Kinase Pathway. Int. Immunopharmacol. 2017, 47, 28–37. [Google Scholar] [CrossRef]
- Wang, H.; Che, J.; Cui, K.; Zhuang, W.; Li, H.; Sun, J.; Chen, J.; Wang, C. Schisantherin A Ameliorates Liver Fibrosis through TGF-Β1mediated Activation of TAK1/MAPK and NF-ΚB Pathways in Vitro and in Vivo. Phytomedicine 2021, 88. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Bernatoniene, J. Antioxidant Effects of Schisandra chinensis Fruits and Their Active Constituents. Antioxidants 2021, 10, 620. [Google Scholar] [CrossRef]
- Lu, H.; Liu, G.T. Anti-Oxidant Activity of Dibenzocyclooctene Lignans Isolated from Schisandraceae. Planta Med. 1992, 58, 311–313. [Google Scholar] [CrossRef]
- Maharjan, S.; Park, B.K.; Lee, S.I.; Lim, Y.; Lee, K.; Kwon, H.J. Gomisin G Inhibits the Growth of Triple-Negative Breast Cancer Cells by Suppressing AKT Phosphorylation and Decreasing Cyclin D1. Biomol. Ther. 2018, 26, 322–327, Erratum in Biomol. Ther. 2018, 26, 520. [Google Scholar] [CrossRef]
- Maharjan, S.; Park, B.K.; Lee, S.I.; Lim, Y.; Lee, K.; Lee, Y.; Kwon, H.J. Gomisin g Suppresses the Growth of Colon Cancer Cells by Attenuation of Akt Phosphorylation and Arrest of Cell Cycle Progression. Biomol. Ther. 2019, 27, 210–215. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, S.; Xie, L.J.X.; Xie, J.; Chen, K.; Kashiwada, Y.; Zhou, B.; Wang, P.; Cosentino, L.; Lee, K. Anti-AIDS Agents—XXVI. Structure-Activity Correlations of Gomisin-G-Related Anti-HIV Lignans from Kadsura Interior and of Related Synthetic Analogues. Bioorg. Med. Chem. 1997, 5, 1715–1723. [Google Scholar] [CrossRef]






| Compound Extracted from Raw Material | Extraction Condition | Analysis Method | References |
|---|---|---|---|
| Enshicine from fruit |
| column chromatography on silica gel (gradient mode: benzene, benzene–ethyl acetate (10:1), benzene–ethyl acetate (4:1), and ethyl acetate) | [29] |
| Triterpenoids, lignans from leaves |
| UHPLC-MS/MS with triple quadrupole mass filter (QQQ) (analytical column: Kinetex C18 150 × 4.6 mm, 2.6 µm, gradient mode: 50% methanol in water (A), 100% methanol (B) with 1% formic acid) | [32] |
| Phenolic acids and flavonoids from leaves |
| HPLC-DAD (analytical column: Purospher RP-18, mobile phase: methanol and 0.5% acetic acid (A), methanol (B)) | [32] |
| Triterpenoids from leaves and stems |
| column chromatography on silica gel, (chloroform–acetone (1:0 to 0:1), semi-preparative HPLC (analytical column: Agilent 1100 HPLC; Zorbax SB-C-18, Agilent, 9.4 mm 25 cm, gradient mode: methanol–water (65:35) | [48] |
| Nortriterpenoids form stems and leaves |
| RP-HPLC (55% methanol/water) column chromatography on silica gel (gradient systems: chloroform-Me2CO 1:0 0:1), repeated column chromatography (silica gel, petroleum ether/Me2CO, 9:1 and petroleum ether/ethyl acetate 4:1), RP-HPLC (gradient mode: 55% methanol/water) | [49] |
| Triterpenoids from stems |
| column chromatography on silica gel (petroleum ether–ethyl acetate 4:1), repeated column chromatography on silica gel | [52] |
| Schinortriterpenoids from stems and leaves |
| column chromatography with silica gel (chloroform/acetone 1:0, 9:1, 7:3, 3:2, 1:1 and 0:1), semi-preparative HPLC (analytical column; RP-18, Sephadex LH-20-methanol/water) | [50] |
| Lignan | Chemical Structure of Compound | Maximal Content [mg/100 g DM ± SD] | Action | Mode of Action | Reference | |
|---|---|---|---|---|---|---|
| Microshoot Cultures | Leaves of the Parent Plant | |||||
| Schisandrin (schizandrin, schizandrol A, schisandrol A) |  | 61.24 ± 0.23 | 8.62 ± 0.95 | Antioxidant |
| [54] |
| Anti-osteoporotic |
| [60] | ||||
| Schisandrin C (wuweizisu C) |  | 28.61 ± 0.23 | 1.06 ± 0.38 | Antioxidant |
| [57,62] |
| Anti-inflammatory |
| [57] | ||||
| Deoxyschisandrin (schisandrin A, schizandrin A, deoxyschizandrin) |  | 3.63 ± 0.27 | 1.70 ± 0.55 | Anticancer |
| [54,57,60,63] |
| Antiviral |
| [63,64] | ||||
| Cardioprotective |
| |||||
| Supportive treatment in intestinal dysfunction |
| [65] | ||||
| Anti-osteoporotic |
| [61] | ||||
| Schisantherin A (gomisin C, gomisin) |  | 143.74 ± 0.43 | 4.75 ± 0.54 | Anti-inflammatory |
| [66,67,68,69] |
| Anticancer |
| [59,70] | ||||
| Neuroprotective |
| [60,71,72] | ||||
| Hepatoprotective |
| [73,74] | ||||
| Cardioprotective |
| [64] | ||||
| Schisantherin B (Gomisin B, Schisandrer, Wuweizi ester B) |  | 622.59 ± 0.57 | 48.99 ± 4.73 | Antioxidant |
| [75,76] |
| Gomisin G |  | 18.20 ± 0.18 | 1.62 ± 0.51 | Anticancer |
| [70,77,78] |
| Antiviral |
| [79] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafernik, K.; Ekiert, H.; Szopa, A. Schisandra henryi—A Rare Species with High Medicinal Potential. Molecules 2023, 28, 4333. https://doi.org/10.3390/molecules28114333
Jafernik K, Ekiert H, Szopa A. Schisandra henryi—A Rare Species with High Medicinal Potential. Molecules. 2023; 28(11):4333. https://doi.org/10.3390/molecules28114333
Chicago/Turabian StyleJafernik, Karolina, Halina Ekiert, and Agnieszka Szopa. 2023. "Schisandra henryi—A Rare Species with High Medicinal Potential" Molecules 28, no. 11: 4333. https://doi.org/10.3390/molecules28114333
APA StyleJafernik, K., Ekiert, H., & Szopa, A. (2023). Schisandra henryi—A Rare Species with High Medicinal Potential. Molecules, 28(11), 4333. https://doi.org/10.3390/molecules28114333









