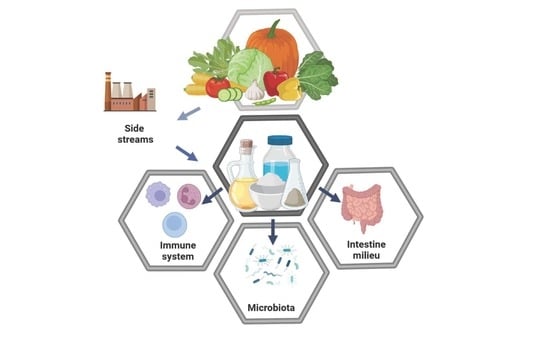
Side Streams of Vegetable Processing and Its Bioactive Compounds Support Microbiota, Intestine Milieu, and Immune System
Abstract
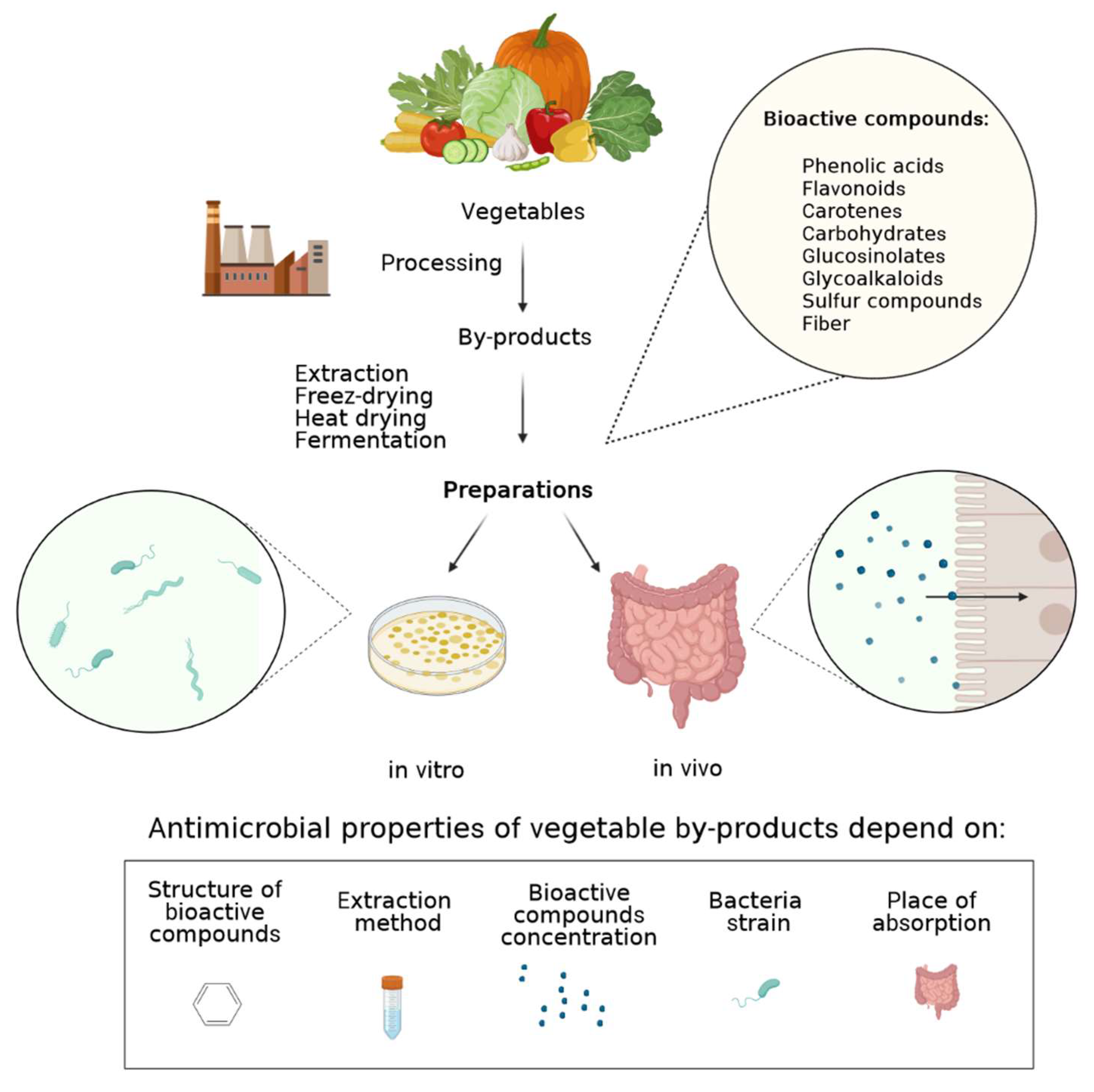
:1. Introduction
2. Modulation of the Intestine Milieu and Microbiota
2.1. The Effect on Intestinal Microbiota
2.2. Antimicrobial Activity toward Microbial Pathogens
2.3. Helth Benefits of Fibre from Vegetable Waste Sources
2.4. Intestine and Inflammatory Bowel Diseases
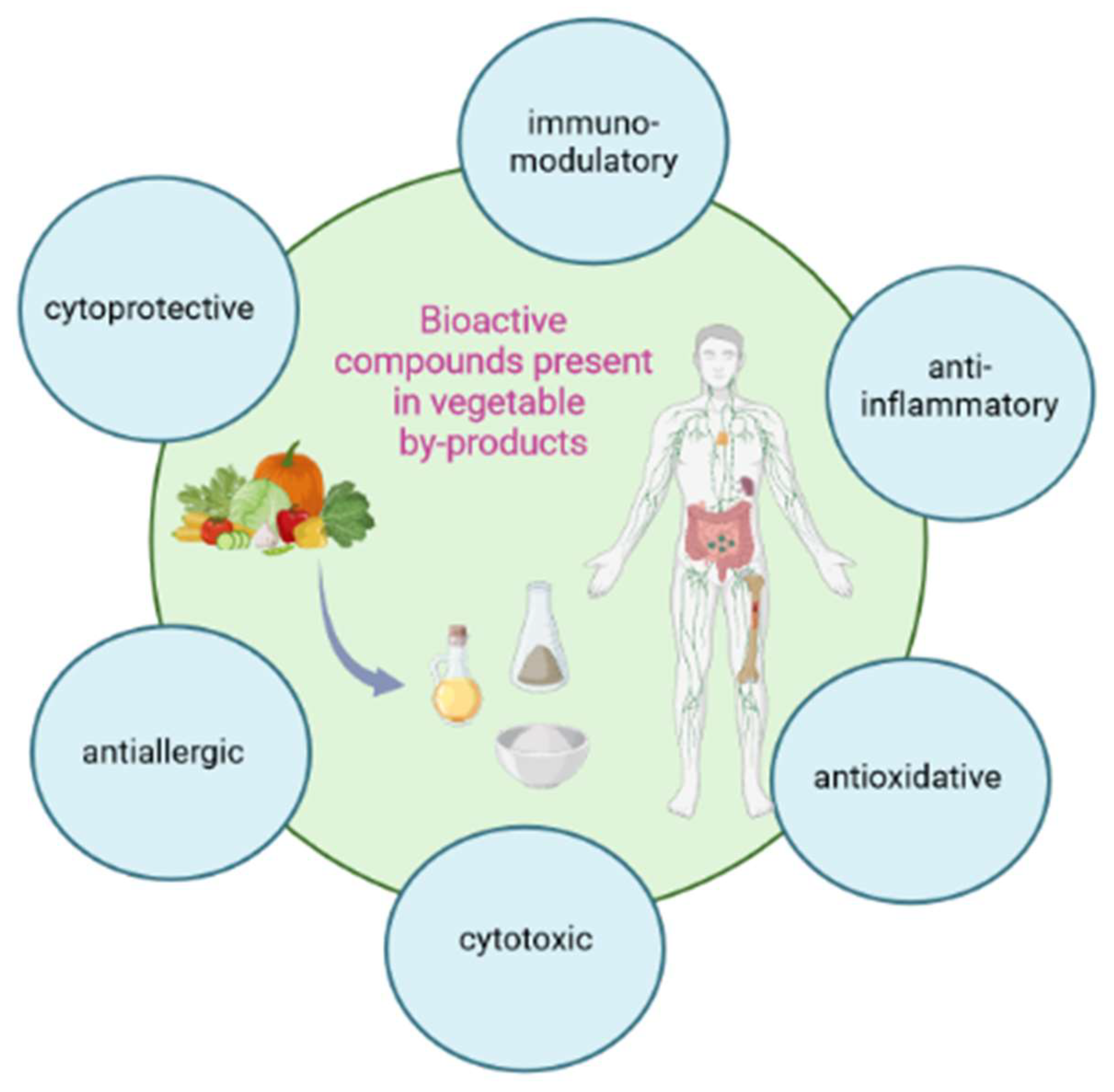
3. Modulation of the Immune Parameters
3.1. Anti-Inflammatory, Antioxidant and Cytoprotective Effect
3.2. Anti-Allergic and Innate Immunity Inducing Effect
3.3. Impact on Various Aspects of Livestock Immunity
3.4. Immunoactive Properties of Liquid Post-Production Wastes
4. Final Remarks
5. Study Design
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LAB | lactic acid bacteria |
| L. | Lactobacillus |
| L. casei | new taxonomic name Lacticaseibacillus casei |
| L. brevis | new taxonomic name Levilactobacillus brevis |
| L. plantarum | new taxonomic name Lactiplantibacillus plantarum |
| E. | Escherichia |
| S. | Staphylococcus |
| B. | Bacillus |
| DF | dietary fibre |
| FOS | fructooligosaccharides |
| MRS | Mann Rogosa Sharpe broth |
| TSB | Tryptone Soya Broth |
| G+ | Gram-positive |
| G− | Gram-negative |
| SCFA | short-chain fatty acids |
| MIC | minimum inhibitory concentration |
| MBC | minimum bactericidal concentration |
| MFC | minimum fungicidal concentration |
| DIZ | diameters of the inhibition zone |
| IZ | inhibition zone |
| BGL | β-Glucosidase |
| GUS | β-glucuronidase |
| GC | gas chromatography |
| TNBS | trinitrobenzenesulfonic acid |
| LDL | low-density lipoprotein |
References
- Blakeney, M. Food loss and food waste and food security. In Food Loss and Food Waste: Causes and Solutions; Edward Elgar Publishing: Northampton, MA, USA, 2011; pp. 1–26. [Google Scholar] [CrossRef]
- Kabir, F.; Tow, W.W.; Hamauzu, Y.; Katayama, S.; Tanaka, S.; Nakamura, S. Antioxidant and cytoprotective activities of extracts prepared from fruit and vegetable wastes and by-products. Food Chem. 2015, 167, 358–362. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive Potential of Fruit and Vegetable Wastes. Adv. Food Nutr. Res. 2020, 91, 157–225. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.Á.; Casquete, R.; Martín, A.; Córdoba, M.D.G.; Aranda, E.; Benito, M.J. Strategies to Increase the Biological and Biotechnological Value of Polysaccharides from Agricultural Waste for Application in Healthy Nutrition. Int. J. Environ. Res. Public Health 2021, 18, 5937. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.T.; Khong, N.M.H.; Lim, S.S.; Hee, Y.Y.; Sim, B.I.; Lau, K.Y.; Lai, O.M. A Review: Modified Agricultural by-Products for the Development and Fortification of Food Products and Nutraceuticals. Trends Food Sci. Technol. 2017, 59, 148–160. [Google Scholar] [CrossRef]
- Li, M.; Zhu, X.; Yang, H.; Xie, X.; Zhu, Y.; Xu, G.; Hu, X.; Jin, Z.; Hu, Y.; Hai, Z.; et al. Treatment of Potato Starch Wastewater by Dual Natural Flocculants of Chitosan and Poly-Glutamic Acid. J. Clean. Prod. 2020, 264, 121641. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The Gut Microbiome in Health and in Disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Jandhyala, S.M. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Molloy, M.J.; Bouladoux, N.; Belkaid, Y. Intestinal Microbiota: Shaping Local and Systemic Immune Responses. Semin. Immunol. 2012, 24, 58–66. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Sig. Transduct. Target Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Khalili, S.; Saeidi Asl, M.R.; Khavarpour, M.; Vahdat, S.M.; Mohammadi, M. Comparative Study on the Effect of Extraction Solvent on Total Phenol, Flavonoid Content, Antioxidant and Antimicrobial Properties of Red Onion (Allium cepa). J. Food Meas. Charact. 2022, 16, 3578–3588. [Google Scholar] [CrossRef]
- Nguyen, T.P. Extracting, Evaluating Biological Activities of Phenolic Compounds from Yellow Onion Peels (Allium cepa L.) and Their Applicability for Fish Preservation. IOP Conf. Ser. Earth Environ. Sci. 2021, 947, 012042. [Google Scholar] [CrossRef]
- Roldán-Marín, E.; Krath, B.N.; Poulsen, M.; Binderup, M.L.; Nielsen, T.H.; Hansen, M.; Barri, T.; Langkilde, S.; Pilar Cano, M.; Sánchez-Moreno, C.; et al. Effects of an Onion by-Product on Bioactivity and Safety Markers in Healthy Rats. Brit. J. Nutr. 2009, 102, 1574–1582. [Google Scholar] [CrossRef]
- Kim, W.J.; Lee, K.A.; Kim, K.-T.; Chung, M.-S.; Cho, S.W.; Paik, H.-D. Antimicrobial Effects of Onion (Allium cepa L.) Peel Extracts Produced via Subcritical Water Extraction against Bacillus Cereus Strains as Compared with Ethanolic and Hot Water Extraction. Food Sci. Biotechnol. 2011, 20, 1101–1106. [Google Scholar] [CrossRef]
- Lee, K.A.; Kim, K.-T.; Nah, S.-Y.; Chung, M.-S.; Cho, S.; Paik, H.-D. Antimicrobial and Antioxidative Effects of Onion Peel Extracted by the Subcritical Water. Food Sci. Biotechnol. 2011, 20, 543–548. [Google Scholar] [CrossRef]
- Żary-Sikorska, E.; Fotschki, B.; Fotschki, J.; Wiczkowski, W.; Juśkiewicz, J. Preparations from Purple Carrots Containing Anthocyanins Improved Intestine Microbial Activity, Serum Lipid Profile and Antioxidant Status in Rats. J. Funct. Foods 2019, 60, 103442. [Google Scholar] [CrossRef]
- Cheaib, D.; Raafat, K.; El Darra, N. Evaluation of phenolic content, antiradical and antibacterial activities of orange and carrot pomace extracts. BAU J. Health Well-Being 2019, 1, 1–8. [Google Scholar] [CrossRef]
- Shen, X.; Xu, Y.; Yin, L.; Cheng, J.; Yin, D.; Zhao, R.; Dai, Y.; Hu, X.; Hou, H.; Qian, K.; et al. Tofu Whey Wastewater as a Beneficial Supplement to Poultry Farming: Improving Production Performance and Protecting against Salmonella Infection. Foods 2022, 12, 79. [Google Scholar] [CrossRef]
- Corpuz, H.M.; Arimura, M.; Chawalitpong, S.; Miyazaki, K.; Sawaguchi, M.; Nakamura, S.; Katayama, S. Oral Administration of Okara Soybean By-Product Attenuates Cognitive Impairment in a Mouse Model of Accelerated Aging. Nutrients 2019, 11, 2939. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Gómez, V.; González-Barrio, R.; Baenas, N.; Moreno, D.A.; Periago, M.J. Dietary-Fibre-Rich Fractions Isolated from Broccoli Stalks as a Potential Functional Ingredient with Phenolic Compounds and Glucosinolates. Int. J. Mol. Sci. 2022, 23, 13309. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.Á.; Benito, M.J.; Martín, A.; Córdoba, M.D.G.; Ruíz-Moyano, S.; Casquete, R. Improve the Functional Properties of Dietary Fibre Isolated from Broccoli By-Products by Using Different Technologies. Innov. Food Sci. Emerg. Technol. 2022, 80, 103075. [Google Scholar] [CrossRef]
- Prandi, B.; Baldassarre, S.; Babbar, N.; Bancalari, E.; Vandezande, P.; Hermans, D.; Bruggeman, G.; Gatti, M.; Elst, K.; Sforza, S. Pectin Oligosaccharides from Sugar Beet Pulp: Molecular Characterization and Potential Prebiotic Activity. Food Funct. 2018, 9, 1557–1569. [Google Scholar] [CrossRef]
- Park, S.Y.; Yoon, K.Y. Enzymatic Production of Soluble Dietary Fiber from the Cellulose Fraction of Chinese Cabbage Waste and Potential Use as a Functional Food Source. Food Sci. Biotechnol. 2015, 24, 529–535. [Google Scholar] [CrossRef]
- Brito, T.B.N.; Lima, L.R.S.; Santos, M.C.B.; Moreira, R.F.A.; Cameron, L.C.; Fai, A.E.C.; Ferreira, M.S.L. Antimicrobial, Antioxidant, Volatile and Phenolic Profiles of Cabbage-Stalk and Pineapple-Crown Flour Revealed by GC-MS and UPLC-MSE. Food Chem. 2021, 339, 127882. [Google Scholar] [CrossRef]
- Juśkiewicz, J.; Wróblewska, M.; Jarosławska, J.; Baliński, P.; Matusevičius, P.; Zduńczyk, P.; Biedrzycka, E.; Zduńczyk, Z. Effects of Inulin Supplemented to Cellulose-Free or Cellulose-Rich Diets on Caecal Environment and Biochemical Blood Parameters in Rats. J. Anim. Feed Sci. 2009, 18, 709–722. [Google Scholar] [CrossRef]
- Juśkiewicz, J.; Zduńczyk, Z.; Żary-Sikorska, E.; Król, B.; Milala, J.; Jurgoński, A. Effect of the Dietary Polyphenolic Fraction of Chicory Root, Peel, Seed and Leaf Extracts on Caecal Fermentation and Blood Parameters in Rats Fed Diets Containing Prebiotic Fructans. Brit. J. Nutr. 2010, 105, 710–720. [Google Scholar] [CrossRef]
- Juśkiewicz, J.; Ašmanskaitė, L.; Zduńczyk, Z.; Matusevičius, P.; Wróblewska, M.; Žilinskienė, A. Metabolic Response of the Gastrointestinal Tract and Serum Parameters of Rabbits to Diets Containing Chicory Flour Rich in Inulin. J. Anim. Physiol. Anim. N. 2007, 92, 113–120. [Google Scholar] [CrossRef]
- Fotschki, B.; Jurgonski, A.; Fotschki, J.; Majewski, M.; Ognik, K.; Juśkiewicz, J. Dietary Chicory Inulin-Rich Meal Exerts Greater Healing Effects than Fructooligosaccharide Preparation in Rats with Trinitrobenzenesulfonic Acid-Induced Necrotic Colitis. Pol. J. Food Nutr. Sci. 2019, 69, 147–155. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, W.; Ma, Z.; Liu, Y.; Mu, J.; Wang, J.; Stipkovits, L.; Wu, G.; Sun, J.; Hui, X. Enzymatic-Modified Dietary Fibre Fraction Extracted from Potato Residue Regulates the Gut Microbiotas and Production of Short-Chain Fatty Acids of C57BL/6 Mice. J. Funct. Foods 2021, 84, 104606. [Google Scholar] [CrossRef]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential Oils and Their Application in Food Safety. Front. Sustain. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Feng, W.; Liu, J.; Cheng, H.; Zhang, D.; Tan, Y.; Peng, C. Dietary Compounds in Modulation of Gut Microbiota-Derived Metabolites. Front Nutr. 2022, 9, 939571. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Hou, X.; Li, S.; Luo, Q.; Shen, G.; Wu, H.; Li, M.; Liu, X.; Chen, A.; Ye, M.; Zhang, Z. Discovery and Identification of Antimicrobial Peptides in Sichuan Pepper (Zanthoxylum bungeanum Maxim) Seeds by Peptidomics and Bioinformatics. Appl. Microbiol. Biotechnol. 2019, 103, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.L.; Yeow, C.C.; Teo, K.C.; Gnanaraj, C.; Chang, Y.P. Valorizing Cabbage (Brassica oleracea L. Var. Capitata) and Capsicum (Capsicum annuum L.) Wastes: In Vitro Health-Promoting Activities. J. Food Sci. Technol. 2019, 56, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.E.; Gibson, G.R.; de Pascual-Teresa, S. Metabolism of Anthocyanins by Human Gut Microflora and Their Influence on Gut Bacterial Growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef]
- Quecan, B.X.V.; Santos, J.T.C.; Rivera, M.L.C.; Hassimotto, N.M.A.; Almeida, F.A.; Pinto, U.M. Effect of Quercetin Rich Onion Extracts on Bacterial Quorum Sensing. Front. Microbiol. 2019, 10, 867. [Google Scholar] [CrossRef]
- Zamora-Mendoza, L.; Guamba, E.; Miño, K.; Romero, M.P.; Levoyer, A.; Alvarez-Barreto, J.F.; Machado, A.; Alexis, F. Antimicrobial Properties of Plant Fibers. Molecules 2022, 27, 7999. [Google Scholar] [CrossRef]
- Alvarado, A.; Pacheco-Delahaye, E.; Hevia, P. Value of a tomato byproduct as a source of dietary fiber in rats. Plant Foods Hum. Nutr. 2001, 56, 335–348. [Google Scholar] [CrossRef]
- Szabo, K.; Diaconeasa, Z.; Cătoi, A.-F.; Vodnar, D.C. Screening of Ten Tomato Varieties Processing Waste for Bioactive Components and Their Related Antioxidant and Antimicrobial Activities. Antioxidants 2019, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Taveira, M.; Silva, L.R.; Vale-Silva, L.A.; Pinto, E.; Valentão, P.; Ferreres, F.; Guedes de Pinho, P.; Andrade, P.B. Lycopersicon Esculentum Seeds: An Industrial Byproduct as an Antimicrobial Agent. J. Agric. Food Chem. 2010, 58, 9529–9536. [Google Scholar] [CrossRef] [PubMed]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ornelas-Paz, J.D.J.; López-Mata, M.A.; Del-Toro-Sánchez, C.L.; Ayala-Zavala, J.F.; Márquez-Ríos, E. Total Phenolic, Flavonoid, Tomatine, and Tomatidine Contents and Antioxidant and Antimicrobial Activities of Extracts of Tomato Plant. Int. J. Anal. Chem. 2015, 2015, 284071. [Google Scholar] [CrossRef]
- Ricci, A.; Bernini, V.; Maoloni, A.; Cirlini, M.; Galaverna, G.; Neviani, E.; Lazzi, C. Vegetable By-Product Lacto-Fermentation as a New Source of Antimicrobial Compounds. Microorganisms 2019, 7, 607. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Upadhyay, N.; Singh, A.K.; Meena, G.S.; Arora, S. Organic Solvent-Free Extraction of Carotenoids from Carrot Bio-Waste and Its Physico-Chemical Properties. J. Food Sci. Technol. 2019, 56, 4678–4687. [Google Scholar] [CrossRef]
- Kang, H.; Kim, H. Astaxanthin and β-Carotene in Helicobacter Pylori-Induced Gastric Inflammation: A Mini-Review on Action Mechanisms. J. Cancer Prev. 2017, 22, 57–61. [Google Scholar] [CrossRef]
- Capita, R.; Alonso-Calleja, C. Antibiotic-Resistant Bacteria: A Challenge for the Food Industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef]
- Sanz-Puig, M.; Pina-Pérez, M.C.; Criado, M.N.; Rodrigo, D.; Martínez-López, A. Antimicrobial Potential of Cauliflower, Broccoli, and Okara Byproducts Against Foodborne Bacteria. Foodborne Pathog. Dis. 2015, 12, 39–46. [Google Scholar] [CrossRef]
- Gebrechristos, H.Y.; Ma, X.; Xiao, F.; He, Y.; Zheng, S.; Oyungerel, G.; Chen, W. Potato Peel Extracts as an Antimicrobial and Potential Antioxidant in Active Edible Film. Food Sci. Nutr. 2020, 8, 6338–6345. [Google Scholar] [CrossRef]
- Perry, C.C.; Weatherly, M.; Beale, T.; Randriamahefa, A. Atomic force microscopy study of the antimicrobial activity of aqueous garlic versus ampicillin against Escherichia coli and Staphylococcus aureus. J. Sci. Food Agric. 2009, 89, 958–964. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial Activity of Phenolic Acids against Commensal, Probiotic and Pathogenic Bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Diet-Derived Phenols in Plasma and Tissues and Their Implications for Health. Planta Med. 2004, 70, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Krause, L.; Somerset, S. Associations between Micronutrient Intakes and Gut Microbiota in a Group of Adults with Cystic Fibrosis. Clin. Nutr. 2017, 36, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Fehlbaum, S.; Seifert, N.; Richard, N.; Bruins, M.J.; Sybesma, W.; Rehman, A.; Steinert, R.E. Effects of Colon-Targeted Vitamins on the Composition and Metabolic Activity of the Human Gut Microbiome—A Pilot Study. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the Gut Microbiome and Gastrointestinal Health in Humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Somrani, M.; Inglés, M.-C.; Debbabi, H.; Abidi, F.; Palop, A. Garlic, Onion, and Cinnamon Essential Oil Anti-Biofilms’ Effect against Listeria Monocytogenes. Foods 2020, 9, 567. [Google Scholar] [CrossRef]
- Benkeblia, N. Antimicrobial Activity of Essential Oil Extracts of Various Onions (Allium cepa) and Garlic (Allium sativum). LWT—Food Sci. Technol. 2004, 37, 263–268. [Google Scholar] [CrossRef]
- De-Montijo-Prieto, S.; Razola-Díaz, M.D.C.; Gómez-Caravaca, A.M.; Guerra-Hernandez, E.J.; Jiménez-Valera, M.; Garcia-Villanova, B.; Ruiz-Bravo, A.; Verardo, V. Essential Oils from Fruit and Vegetables, Aromatic Herbs, and Spices: Composition, Antioxidant, and Antimicrobial Activities. Biology 2021, 10, 1091. [Google Scholar] [CrossRef]
- Palaniswamy, U.R.; Bible, B.B.; McAvoy, R.J. Oxalic Acid Concentrations in Purslane (Portulaca oleraceae L.) Is Altered by the Stage of Harvest and the Nitrate to Ammonium Ratios in Hydroponics. Sci. Hortic. 2004, 102, 267–275. [Google Scholar] [CrossRef]
- Mitchell, T.; Kumar, P.; Reddy, T.; Wood, K.D.; Knight, J.; Assimos, D.G.; Holmes, R.P. Dietary oxalate and kidney stone formation. Am. J. Physiol. Renal. Physiol. 2019, 316, F409–F413. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, K.; Saini, V.; Mitchell, T. Oxalate Alters Cellular Bioenergetics, Redox Homeostasis, Antibacterial Response, and Immune Response in Macrophages. Front. Immunol. 2021, 12, 694865. [Google Scholar] [CrossRef] [PubMed]
- Obi, R.K.; Nwanebu, F.C.; Ndubuisi, U.U.; Orji, N.M. Antibacterial qualities and phytochemical screening of the oils of Curcubita pepo and Brassica nigra. J. Med. Plants Res. 2009, 3, 429–432. [Google Scholar]
- Odilia, M.R.; Putri, D.T.Z.A.; Rosetyadewi, A.W.; Wijayanti, A.D.; Budiyanto, A.; Jadi, A.R.; Pratama, A.M. Identification of Antinutritional, Antioxidant, and Antimicrobial Activity of Plants That Cause Livestock Poisoning in Bojonegoro Regency, Indonesia. Vet. World 2022, 15, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary Fibre in Foods: A Review. J. Food Sci. Technol. 2011, 49, 255–266. [Google Scholar] [CrossRef]
- Yusuf, K.; Saha, S.; Umar, S. Health Benefits of Dietary Fiber for the Management of Inflammatory Bowel Disease. Biomedicines 2022, 10, 1242. [Google Scholar] [CrossRef]
- Biswas, V.; Praveen, A.; Marisetti, A.L.; Sharma, A.; Kumar, V.; Sahu, S.K.; Tewari, D. A Mechanistic Overview on Impact of Dietary Fibres on Gut Microbiota and Its Association with Colon Cancer. Dietetics 2022, 1, 182–202. [Google Scholar] [CrossRef]
- Tuohy, K.M.; Conterno, L.; Gasperotti, M.; Viola, R. Up-Regulating the Human Intestinal Microbiome Using Whole Plant Foods, Polyphenols, and/or Fiber. J. Agric. Food Chem. 2012, 60, 8776–8782. [Google Scholar] [CrossRef]
- Cuervo, A.; Valdés, L.; Salazar, N.; de los Reyes-Gavilán, C.G.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. Pilot Study of Diet and Microbiota: Interactive Associations of Fibers and Polyphenols with Human Intestinal Bacteria. J. Agric. Food Chem. 2014, 62, 5330–5336. [Google Scholar] [CrossRef]
- Nyam, K.L.; Lau, M.; Tan, C.P. Fibre from pumpkin (Cucurbita pepo L.) seeds and rinds: Physico-chemical properties, antioxidant capacity and application as bakery product ingredients. Malays J. Nutr. 2013, 19, 99–109. [Google Scholar]
- Barczynska, R.; Jurgoński, A.; Slizewska, K.; Juśkiewicz, J.; Kapusniak, J. Effects of Potato Dextrin on the Composition and Metabolism of the Gut Microbiota in Rats Fed Standard and High-Fat Diets. J. Funct. Foods 2017, 34, 398–407. [Google Scholar] [CrossRef]
- Ma, S.; Ren, B.; Diao, Z.; Chen, Y.; Qiao, Q.; Liu, X. Physicochemical Properties and Intestinal Protective Effect of Ultra-Micro Ground Insoluble Dietary Fibre from Carrot Pomace. Food Funct. 2016, 7, 3902–3909. [Google Scholar] [CrossRef] [PubMed]
- Gutöhrlein, F.; Morales-Medina, R.; Boje, A.-L.; Drusch, S.; Schalow, S. Modulating the Hydration Properties of Pea Hull Fibre by Its Composition as Affected by Mechanical Processing and Various Extraction Procedures. Food Hydrocoll. 2020, 107, 105958. [Google Scholar] [CrossRef]
- Hodson, R. Inflammatory Bowel Disease. Nature 2016, 540, S97. [Google Scholar] [CrossRef]
- Cross, R.K.; Lapshin, O.; Finkelstein, J. Patient Subjective Assessment of Drug Side Effects in Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2008, 42, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, K.; Feuerstein, J.D. New Developments in Ulcerative Colitis: Latest Evidence on Management, Treatment, and Maintenance. Drugs Context 2019, 8, 212572. [Google Scholar] [CrossRef]
- Kikut, J.; Konecka, N.; Ziętek, M.; Kulpa, D.; Szczuko, M. Diet Supporting Therapy for Inflammatory Bowel Diseases. Eur. J. Nutr. 2021, 60, 2275–2291. [Google Scholar] [CrossRef]
- Farzaei, M.; Rahimi, R.; Abdollahi, M. The Role of Dietary Polyphenols in the Management of Inflammatory Bowel Disease. Curr. Pharm. Biotechnol. 2015, 16, 196–210. [Google Scholar] [CrossRef]
- da Fonseca Machado, A.P.; Geraldi, M.V.; do Nascimento, R.D.P.; Moya, A.M.T.M.; Vezza, T.; Diez-Echave, P.; Gálvez, J.J.; Cazarin, C.B.B.; Júnior, M.R.M. Polyphenols from Food By-Products: An Alternative or Complementary Therapy to IBD Conventional Treatments. Food Res. Int. 2021, 140, 110018. [Google Scholar] [CrossRef]
- Mateus, V.; Estarreja, J.; Silva, I.; Gonçalves, F.; Teixeira-Lemos, E.; Pinto, R. Effect of Aqueous Extract of Phenolic Compounds Obtained from Red Wine in Experimental Model of Colitis in Mice. Curr. Issues Mol. Biol. 2022, 44, 2745–2758. [Google Scholar] [CrossRef]
- Paesa, M.; Nogueira, D.P.; Velderrain-Rodríguez, G.; Esparza, I.; Jiménez-Moreno, N.; Mendoza, G.; Osada, J.; Martin-Belloso, O.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Valorization of Onion Waste by Obtaining Extracts Rich in Phenolic Compounds and Feasibility of Its Therapeutic Use on Colon Cancer. Antioxidants 2022, 11, 733. [Google Scholar] [CrossRef]
- Loo, Y.T.; Howell, K.; Suleria, H.; Zhang, P.; Gu, C.; Ng, K. Sugarcane Polyphenol and Fiber to Affect Production of Short-Chain Fatty Acids and Microbiota Composition Using in Vitro Digestion and Pig Faecal Fermentation Model. Food Chem. 2022, 385, 132665. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Swanson, K.S.; Fahey, G.C.; Garleb, K.A. Perspective: Physiologic Importance of Short-Chain Fatty Acids from Nondigestible Carbohydrate Fermentation. Adv. Nutr. 2019, 10, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, M.-Y.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic T Reg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Gonçalves, P.; Araújo, J.R.; Di Santo, J.P. A Cross-Talk Between Microbiota-Derived Short-Chain Fatty Acids and the Host Mucosal Immune System Regulates Intestinal Homeostasis and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 558–572. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J.; Olejarz, K.; Zieliński, H.; Piskuła, M.K. Metabolites of Dietary Quercetin: Profile, Isolation, Identification, and Antioxidant Capacity. J. Funct. Foods 2014, 11, 121–129. [Google Scholar] [CrossRef]
- Lyu, Y.-L.; Zhou, H.-F.; Yang, J.; Wang, F.-X.; Sun, F.; Li, J.-Y. Biological Activities Underlying the Therapeutic Effect of Quercetin on Inflammatory Bowel Disease. Mediat. Inflamm. 2022, 2022, 5665778. [Google Scholar] [CrossRef]
- Ju, S.; Ge, Y.; Li, P.; Tian, X.; Wang, H.; Zheng, X.; Ju, S. Dietary Quercetin Ameliorates Experimental Colitis in Mouse by Remodeling the Function of Colonic Macrophages via a Heme Oxygenase-1-Dependent Pathway. Cell Cycle 2018, 17, 53–63. [Google Scholar] [CrossRef]
- Nascimento, R. de P. do; Moya, A.M.T.M.; Machado, A.P. da F.; Geraldi, M.V.; Diez-Echave, P.; Vezza, T.; Galvez, J.; Cazarin, C.B.B.; Maróstica Junior, M.R. Review on the Potential Application of Non-Phenolic Compounds from Native Latin American Food Byproducts in Inflammatory Bowel Diseases. Food Res. Int. 2021, 139, 109796. [Google Scholar] [CrossRef]
- Sabater, C.; Molina-Tijeras, J.A.; Vezza, T.; Corzo, N.; Montilla, A.; Utrilla, P. Intestinal Anti-Inflammatory Effects of Artichoke Pectin and Modified Pectin Fractions in the Dextran Sulfate Sodium Model of Mice Colitis. Artificial Neural Network Modelling of Inflammatory Markers. Food Funct. 2019, 10, 7793–7805. [Google Scholar] [CrossRef]
- Ghishan, F.K.; Kiela, P.R. Vitamins and Minerals in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.; Pala, M.I. Thiamine and Fatigue in Inflammatory Bowel Diseases: An Open-Label Pilot Study. J. Altern. Complement. Med. 2013, 19, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Chernukha, I.; Fedulova, L.; Vasilevskaya, E.; Kulikovskii, A.; Kupaeva, N.; Kotenkova, E. Antioxidant Effect of Ethanolic Onion (Allium Cepa) Husk Extract in Ageing Rats. Saudi J. Biol. Sci. 2021, 28, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Seida, A.A.; El Tanbouly, N.D.; Islam, W.T.; Eid, H.H.; El Maraghy, S.A.; El Senousy, A.S. Bioassay-Guided Fractionation of a Hepatoprotective and Antioxidant Extract of Pea by-Product. Nat. Prod. Res. 2014, 29, 1578–1583. [Google Scholar] [CrossRef]
- Nakajima, S.; Iijima, H.; Egawa, S.; Shinzaki, S.; Kondo, J.; Inoue, T.; Hayashi, Y.; Ying, J.; Mukai, A.; Akasaka, T.; et al. Association of Vitamin K Deficiency with Bone Metabolism and Clinical Disease Activity in Inflammatory Bowel Disease. Nutrition 2011, 27, 1023–1028. [Google Scholar] [CrossRef]
- Shiraishi, E.; Iijima, H.; Shinzaki, S.; Nakajima, S.; Inoue, T.; Hiyama, S.; Kawai, S.; Araki, M.; Yamaguchi, T.; Hayashi, Y.; et al. Vitamin K Deficiency Leads to Exacerbation of Murine Dextran Sulfate Sodium-Induced Colitis. J. Gastroenterol. 2015, 51, 346–356. [Google Scholar] [CrossRef]
- Bolton-Smith, C.; Price, R.J.; Fenton, S.T.; Harrington, D.J.; Shearer, M.J. Compilation of a provisional UK database for the phylloquinone (vitamin K1) content of foods. Br. J. Nutr. 2000, 83, 389–399. [Google Scholar]
- Vagianos, K.; Bector, S.; McConnell, J.; Bernstein, C.N. Nutrition Assessment of Patients With Inflammatory Bowel Disease. J. Parenter. Enter. Nutr. 2007, 31, 311–319. [Google Scholar] [CrossRef]
- Rempel, J.; Grover, K.; El-Matary, W. Micronutrient Deficiencies and Anemia in Children with Inflammatory Bowel Disease. Nutrients 2021, 13, 236. [Google Scholar] [CrossRef]
- Lim, K.; Riddell, L.; Nowson, C.; Booth, A.; Szymlek-Gay, E. Iron and Zinc Nutrition in the Economically-Developed World: A Review. Nutrients 2013, 5, 3184–3211. [Google Scholar] [CrossRef] [PubMed]
- Atsushi, O.; Ikumi, K.; Kyoko, T. Establishment of a Cultivation Method for Leaf Lettuce (Lactuca sativa Var. Crispa) and Komatsuna (Brassica rapa Var. Perviridis) with High Zinc Content for Patients with Zinc Deficiency and Evaluation of Its Effectiveness. J. Sci. Food Agric. 2020, 101, 3202–3207. [Google Scholar] [CrossRef] [PubMed]
- Akram, W.; Garud, N.; Joshi, R. Role of Inulin as Prebiotics on Inflammatory Bowel Disease. Drug Discov. Ther. 2019, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Valcheva, R.; Koleva, P.; Martínez, I.; Walter, J.; Gänzle, M.G.; Dieleman, L.A. Inulin-Type Fructans Improve Active Ulcerative Colitis Associated with Microbiota Changes and Increased Short-Chain Fatty Acids Levels. Gut Microbes 2018, 10, 334–357. [Google Scholar] [CrossRef] [PubMed]
- Pagano, I.; Piccinelli, A.L.; Celano, R.; Campone, L.; Gazzerro, P.; De Falco, E.; Rastrelli, L. Chemical Profile and Cellular Antioxidant Activity of Artichoke By-Products. Food Funct. 2016, 7, 4841–4850. [Google Scholar] [CrossRef] [PubMed]
- Jurgoński, A.; Milala, J.; Juśkiewicz, J.; Zduńczyk, Z.; Król, B. Composition of chicory root, peel, seed and leaf ethanol extracts and biological properties of their non-inulin fraction. Food Technol. Biotechnol. 2011, 49, 40–47. [Google Scholar]
- Juśkiewicz, J.; Glazka, I.; Krol, B.; Zdunczyk, Z. Effect of Chicory Products with Different Inulin Content on Rat Caecum Physiology. J. Anim. Physiol. Anim. Nutr. 2006, 90, 200–207. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jin, Y.-H. Intestinal Bacterial β-Glucuronidase Activity of Patients with Colon Cancer. Arch. Pharm. Res. 2001, 24, 564–567. [Google Scholar] [CrossRef]
- Grzelak-Błaszczyk, K.; Milala, J.; Kołodziejczyk, K.; Sójka, M.; Czarnecki, A.; Kosmala, M.; Klewicki, R.; Fotschki, B.; Jurgoński, A.; Juśkiewicz, J. Protocatechuic Acid and Quercetin Glucosides in Onions Attenuate Changes Induced by High Fat Diet in Rats. Food Funct. 2020, 11, 3585–3597. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Rasool, S.; Geetha, T.; Babu, J.R. Effects and Underlying Mechanisms of Bioactive Compounds on Type 2 Diabetes Mellitus and Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2019, 2019, 8165707. [Google Scholar] [CrossRef]
- Bachmann, M.C.; Bellalta, S.; Basoalto, R.; Gómez-Valenzuela, F.; Jalil, Y.; Lépez, M.; Matamoros, A.; von Bernhardi, R. The Challenge by Multiple Environmental and Biological Factors Induce Inflammation in Aging: Their Role in the Promotion of Chronic Disease. Front. Immunol. 2020, 11, 570083. [Google Scholar] [CrossRef] [PubMed]
- Kocot, A.M.; Wróblewska, B. Fermented products and bioactive food compounds as a tool to activate autophagy and promote the maintenance of the intestinal barrier function. Trends Food Sci. Technol. 2021, 118, 905–919. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Comparative study on antiproliferation properties and cellular antioxidant activities of commonly consumed food legumes against nine human cancer cell lines. Food Chem. 2012, 134, 1287–1296. [Google Scholar] [CrossRef]
- Markiewicz, L.H.; Ogrodowczyk, A.M.; Wiczkowski, W.; Wróblewska, B. Phytate and Butyrate Differently Influence the Proliferation, Apoptosis and Survival Pathways in Human Cancer and Healthy Colonocytes. Nutrients 2021, 13, 1887. [Google Scholar] [CrossRef]
- Drabińska, N. The Evaluation of Amino Acid Profiles in Gluten-Free Mini Sponge Cakes Fortified with Broccoli By-Product. Separations 2022, 9, 81. [Google Scholar] [CrossRef]
- Drabińska, N.; Nogueira, M.; Ciska, E.; Jeleń, H. Effect of Drying and Broccoli Leaves Incorporation on the Nutritional Quality of Durum Wheat Pasta. Pol. J. Food Nutr. Sci. 2022, 72, 273–285. [Google Scholar] [CrossRef]
- Endo, Y.; Hanada, K.; Miyake, M.; Ogawara, K.; Higaki, K. Mechanisms of Cytoprotective Effect of Amino Acids on Local Toxicity Caused by Sodium Laurate, A Drug Absorption Enhancer, in Intestinal Epithelium. J. Pharm. Sci. 2002, 91, 730–743. [Google Scholar] [CrossRef]
- Dai, Z.-L.; Li, X.-L.; Xi, P.-B.; Zhang, J.; Wu, G.; Zhu, W.-Y. Metabolism of Select Amino Acids in Bacteria from the Pig Small Intestine. Amino Acids 2012, 42, 1597–1608. [Google Scholar] [CrossRef]
- Ma, N.; Ma, X. Dietary Amino Acids and the Gut-Microbiome-Immune Axis: Physiological Metabolism and Therapeutic Prospects. Compr. Rev. Food Sci. 2019, 18, 221–242. [Google Scholar] [CrossRef]
- Nour, V.; Panaite, T.D.; Ropota, M.; Turcu, R.; Trandafir, I.; Corbu, A.R. Nutritional and bioactive compounds in dried tomato processing waste. CYTA—J. Food 2018, 16, 222–229. [Google Scholar] [CrossRef]
- Cha, J.H.; Kim, W.K.; Ha, A.W.; Kim, M.H.; Chang, M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pract. 2017, 11, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Hazewindus, M.; Haenen, G.R.M.M.; Weseler, A.R.; Bast, A. The anti-inflammatory effect of lycopene complements the antioxidant action of ascorbic acid and α-tocopherol. Food Chem. 2012, 132, 954–958. [Google Scholar] [CrossRef]
- Abbasi-Parizad, P.; De Nisi, P.; Scaglia, B.; Scarafoni, A.; Pilu, S.; Adani, F. Recovery of phenolic compounds from agro-industrial by-products: Evaluating antiradical activities and immunomodulatory properties. Food Bioprod. Process. 2021, 127, 338–348. [Google Scholar] [CrossRef]
- Pawankar, R.; Canonica, G.W.; Holgate, S.T.; Lockey, R.F. ; World Allergy Organization. World Allergy Organization (WAO) White Book on Allergy; WAO: Milwaukee, WI, USA, 2011. [Google Scholar]
- Cao, J.; Chen, W.; Zhang, Y.; Zhang, Y.; Zhao, X. Content of Selected Flavonoids in 100 Edible Vegetables and Fruits. Food Sci. Technol. Res. 2010, 16, 395–402. [Google Scholar] [CrossRef]
- Hirano, T.; Higa, S.; Arimitsu, J.; Naka, T.; Ogata, A.; Shima, Y.; Fujimoto, M.; Yamadori, T.; Ohkawara, T.; Kuwabara, Y.; et al. Luteolin, a Flavonoid, Inhibits AP-1 Activation by Basophils. Biochem. Biophys. Res. Commun. 2006, 340, 1–7. [Google Scholar] [CrossRef]
- Hirano, T.; Arimitsu, J.; Higa, S.; Naka, T.; Ogata, A.; Shima, Y.; Fujimoto, M.; Yamadori, T.; Ohkawara, T.; Kuwabara, Y.; et al. Luteolin, a Flavonoid, Inhibits CD40 Ligand Expression by Activated Human Basophils. Int. Arch. Allergy Immunol. 2006, 140, 150–156. [Google Scholar] [CrossRef]
- Jang, T.Y.; Jung, A.-Y.; Kyung, T.-S.; Kim, D.-Y.; Hwang, J.-H.; Kim, Y.H. Anti-Allergic Effect of Luteolin in Mice with Allergic Asthma and Rhinitis. Cent. Eur. J. Immunol. 2017, 1, 24–29. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Wang, S.; Guo, Q.; Li, Z.; Liu, H.; Wang, C. Structural characterisation and immunomodulatory activity of polysaccharides from white asparagus skin. Carbohydr. Polym. 2020, 227, 115314. [Google Scholar] [CrossRef]
- Wang, N.; Jia, G.; Wang, X.; Liu, Y.; Li, Z.; Bao, H.; Guo, Q.; Wang, C.; Xiao, D. Fractionation, structural characteristics and immunomodulatory activity of polysaccharide fractions from asparagus (Asparagus officinalis L.) skin. Carbohydr. Polym. 2021, 256, 117514. [Google Scholar] [CrossRef]
- Nie, C.; Zhu, P.; Ma, S.; Wang, M.; Hu, Y. Purification, characterization and immunomodulatory activity of polysaccharides from stem lettuce. Carbohydr. Polym. 2018, 188, 236–242. [Google Scholar] [CrossRef]
- Šeregelj, V.; Pezo, L.; Šovljanski, O.; Lević, S.; Nedović, V.; Markov, S.; Tomić, A.; Čanadanović-Brunet, J.; Vulić, J.; Šaponjac, V.T.; et al. New concept of fortified yogurt formulation with encapsulated carrot waste extract. LWT 2021, 138, 110732. [Google Scholar] [CrossRef]
- Cañete, A.; Cano, E.; Muñoz-Chápuli, R.; Carmona, R. Role of vitamin a/retinoic acid in regulation of embryonic and adult hematopoiesis. Nutrients 2017, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Aguirre Calvo, T.R.; Perullini, M.; Santagapita, P.R. Encapsulation of betacyanins and polyphenols extracted from leaves and stems of beetroot in Ca(II)-alginate beads: A structural study. J. Food Eng. 2018, 235, 32–40. [Google Scholar] [CrossRef]
- Vulić, J.; Čanadanović-Brunet, J.; Ćetković, G.; Tumbas, V.; Djilas, S.; Četojević-Simin, D.; Čanadanović, V. Antioxidant and cell growth activities of beet root pomace extracts. J. Funct. Foods 2012, 4, 670–678. [Google Scholar] [CrossRef]
- Vulić, J.J.; Ćebović, T.N.; Čanadanović, V.M.; Ćetković, G.S.; Djilas, S.M.; Čanadanović-Brunet, J.M.; Velićanski, A.S.; Cvetković, D.D.; Tumbas, V.T. Antiradical, antimicrobial and cytotoxic activities of commercial beetroot pomace. Food Funct. 2013, 4, 713–721. [Google Scholar] [CrossRef]
- Vulić, J.J.; Ćebović, T.N.; Čanadanović-Brunet, J.M.; Ćetković, G.S.; Čanadanović, V.M.; Djilas, S.M.; Tumbas Šaponjac, V.T. In vivo and in vitro antioxidant effects of beetroot pomace extracts. J. Funct. Foods 2014, 6, 168–175. [Google Scholar] [CrossRef]
- Nopparatmaitree, M.; Nava, M.; Chumsangchotisakun, V.; Saenphoom, P.; Chotnipat, S.; Kitpipit, W. Effect of trimmed asparagus by-products supplementation in broiler diets on performance, nutrients digestibility, gut ecology, and functional meat production. Vet. World 2022, 15, 147–161. [Google Scholar] [CrossRef]
- Monsang, S.J.; Acharya, A.; Gon Choudhury, T.; Kamilya, D. Dietary Asparagus racemosus ethanolic root extract modulates immune-biochemical response, immune gene expression and provides protection against Aeromonas hydrophila in Labeo rohita fingerlings. Aquac. Res. 2022, 53, 4795–4804. [Google Scholar] [CrossRef]
- El-Gindy, Y.M.; Hafsa, S.H.A.; Dosoky, W.M. Effects of potato peel extract on semen quality, sex hormones and immunity of rabbit bucks under intensive breeding system. Andrologia 2020, 52, e13869. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Olejnik, A.; Wieczorek, M.N.; Zembrzuska, J.; Kowalska, K.; Lewandowicz, J.; Lewandowicz, G. Bioactive Substances of Potato Juice Reveal Synergy in Cytotoxic Activity against Cancer Cells of Digestive System Studied In Vitro. Nutrients 2022, 15, 114. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Błażejak, S.; Molenda, M.; Reczek, L. Biosynthesis of β(1,3)/(1,6)-glucans of cell wall of the yeast Candida utilis ATCC 9950 strains in the culture media supplemented with deproteinated potato juice water and glycerol. Eur. Food Res. Technol. 2015, 240, 1023–1034. [Google Scholar] [CrossRef]
- Chen, J.; Seviour, R. Medicinal importance of fungal β-(1→3), (1→6)-glucans. Mycol. Res. 2007, 111, 635–652. [Google Scholar] [CrossRef]
- WHO. Towards Stronger Food Safety Systems and Global Cooperation; WHO: Geneva, Switzerland, 2022; ISBN 9789240057685. [Google Scholar]
- Ramadass, B.; Dokladny, K.; Moseley, P.L.; Patel, Y.R.; Lin, H.C. Sucrose Co-Administration Reduces the Toxic Effect of Lectin on Gut Permeability and Intestinal Bacterial Colonization. Dig. Dis. Sci. 2010, 55, 2778–2784. [Google Scholar] [CrossRef] [PubMed]
- Felker, P.; Bunch, R.; Leung, A.M. Concentrations of Thiocyanate and Goitrin in Human Plasma, Their Precursor Concentrations in Brassica Vegetables, and Associated Potential Risk for Hypothyroidism. Nutr. Rev. 2016, 74, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.; Egli, I. Iron Bioavailability and Dietary Reference Values. AJCN 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- López-Moreno, M.; Garcés-Rimón, M.; Miguel, M. Antinutrients: Lectins, Goitrogens, Phytates and Oxalates, Friends or Foe? JFF 2022, 89, 104938. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Nan, M.; Xue, H.; Bi, Y. Contamination, Detection and Control of Mycotoxins in Fruits and Vegetables. Toxins 2022, 14, 309. [Google Scholar] [CrossRef]
- Costa, J.; Santos, C.; Soares, C.; Rodríguez, R.; Lima, N.; Santos, C. Occurrence of Aflatoxins and Ochratoxin A during Merkén Pepper Powder Production in Chile. Foods 2022, 11, 3843. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Malik, S.; Asi, M.R.; Selamat, J.; Malik, N. Natural occurrence of patulin in different fruits, juices and smoothies and evaluation of dietary intake in Punjab, Pakistan. Food Control 2018, 84, 370–374. [Google Scholar] [CrossRef]
- Delgado, J.A.; Schwarz, P.B.; Gillespie, J.; Rivera-Varas, V.V.; Secor, G.A. Trichothecene Mycotoxins Associated with Potato Dry Rot Caused by Fusarium Graminearum. Phytopathology 2010, 100, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Walravens, J.; Mikula, H.; Rychlik, M.; Asam, S.; Devos, T.; Njumbe Ediage, E.; Diana Di Mavungu, J.; Jacxsens, L.; Van Landschoot, A.; Vanhaecke, L.; et al. Validated UPLC-MS/MS Methods to Quantitate Free and Conjugated Alternaria Toxins in Commercially Available Tomato Products and Fruit and Vegetable Juices in Belgium. J. Agric. Food Chem. 2016, 64, 5101–5109. [Google Scholar] [CrossRef] [PubMed]
- Das, F.; Oliveira, C.; Edite, M. Fungal Metabolites. Fungal Metab. 2016, 1–428. [Google Scholar] [CrossRef]
- Wasilewska, E.; Zlotkowska, D.; Wroblewska, B. Yogurt starter cultures of Streptococcus thermophilus and Lactobacillus bulgaricus ameliorate symptoms and modulate the immune response in a mouse model of dextran sulfate sodium-induced colitis. J. Dairy Sci. 2019, 102, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153. [Google Scholar] [CrossRef] [PubMed]
- Ogrodowczyk, A.M.; Drabińska, N. Crossroad of tradition and innovation—The application of lactic acid fermentation to increase the nutritional and health-promoting potential of plant-based food products—A review. Pol. J. Food Nutr. Sci. 2021, 71, 107–134. [Google Scholar] [CrossRef]
- Chibuike, O.A.; Agwaranze, D.I.; Aliba, N.V.; Chukwuma, K.A.; Blessing, N.C. Fermentation by Lactic Acid Bacteria Consortium and its Effect on Anti-nutritional Factors in Maize Flour. J. Biol. Sci. 2018, 19, 17–23. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef]
- Babalola, R.O.; Giwa, O.E. Effect of fermentation on nutritional and anti-nutritional properties of fermenting Soy beans and the antagonistic effect of the fermenting organism on selected pathogens. Int. Res. J. Microbiol. 2012, 3, 2141–5463. [Google Scholar]
- Adeyemo, S.M.; Onilude, A.A. Enzymatic Reduction of Anti-nutritional Factors in Fermenting Soybeans by Lactobacillus plantarum Isolates from Fermenting Cereals. Niger. Food J. 2013, 31, 84–90. [Google Scholar] [CrossRef]
- Eraslan, G.; Kanbur, M.; Aslan, Ö.; Karabacak, M. The Antioxidant Effects of Pumpkin Seed Oil on Subacute Aflatoxin Poisoning in Mice. Environ. Toxicol. 2011, 165, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Roldán, E.; Sánchez-Moreno, C.; de Ancos, B.; Cano, M.P. Characterisation of onion (Allium cepa L.) by-products as food ingredients with antioxidant and antibrowning properties. Food Chem. 2008, 108, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Ser, S.L.; Cumming, J.R.; Ku, K.M. Comparative phytonutrient analysis of broccoli by-products: The potentials for broccoli by-product utilization. Molecules 2018, 23, 900. [Google Scholar] [CrossRef]
- Masood, S.; Rehman, A.U.; Ihsan, M.A.; Shahzad, K.; Sabir, M.; Alam, S.; Ahmed, W.; Shah, Z.H.; Alghabari, F.; Mehmood, A.; et al. Antioxidant potential and α-glucosidase inhibitory activity of onion (Allium cepa L.) Peel and bulb extracts. Braz. J. Biol. 2023, 83, 00264. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant, anti-inflammatory and DNA scission inhibitory activities of phenolic compounds in selected onion and potato varieties. J. Funct. Foods 2013, 5, 930–939. [Google Scholar] [CrossRef]
- Liao, S.; Liao, L.; Huang, P.; Wang, Y.; Zhu, S.; Wang, X.; Lv, T.; Li, Y.; Fan, Z.; Liu, T.; et al. Effects of Different Levels of Garlic Straw Powder on Growth Performance, Meat Quality, Antioxidant and Intestinal Mucosal Morphology of Yellow-Feathered Broilers. Front. Physiol. 2022, 13, 902995. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.S.; Keum, Y.S.; Sharma, K. Utilization of quercetin and quercetin glycosides from onion (Allium cepa L.) solid waste as an antioxidant, urease and xanthine oxidase inhibitors. Food Chem. 2017, 235, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Salami, A.; Asefi, N.; Kenari, R.E.; Gharekhani, M. Addition of pumpkin peel extract obtained by supercritical fluid and subcritical water as an effective strategy to retard canola oil oxidation. J. Food Meas. Charact. 2020, 14, 2433–2442. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Al-Yousef, H.M.; Al-Qahtani, J.H.; Al-Said, M.S. A hepatonephro-protective phenolic-rich extract from red onion (Allium cepa L.) peels. Pak. J. Pharm. Sci. 2017, 30, 1971–1979. [Google Scholar]
- Multescu, M.; Marinas, I.C.; Susman, I.E.; Belc, N. Byproducts (Flour, Meals, and Groats) from the Vegetable Oil Industry as a Potential Source of Antioxidants. Foods 2022, 11, 253. [Google Scholar] [CrossRef]
- Kalaivani, A.; Sathibabu Uddandrao, V.V.; Brahmanaidu, P.; Saravanan, G.; Nivedha, P.R.; Tamilmani, P.; Swapna, K.; Vadivukkarasi, S. Anti obese potential of Cucurbita maxima seeds oil: Effect on lipid profile and histoarchitecture in high fat diet induced obese rats. Nat. Prod. Res. 2018, 32, 2950–2953. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Santoscoy, R.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Effect of Flavonoids and Saponins Extracted from Black Bean (Phaseolus vulgaris L.) Seed Coats as Cholesterol Micelle Disruptors. Plant Foods Hum. Nutr. 2013, 68, 416–423. [Google Scholar] [CrossRef] [PubMed]
| Side Streams of Vegetable Processing | Bioactive Compounds | Methods | Activity/Observations | Mechanism | Reference |
|---|---|---|---|---|---|
| Onion (Allium cepa): | |||||
| Polyphenols, including flavonoids | A: Extracts (ethyl acetate, n-butanol ethanol, methanol, and water) using the maceration method. B: Antimicrobial activity-disc diffusion method; MIC; MBC and MFC. | Antifungal and antimicrobial properties. Properties were tested against G+ (S. aureus) and G− (E. coli and Salmonella Typhimurium) bacteria and fungi (Aspergillus niger, Candida albicans). The extracts were more active against S. aureus as compared to E. coli and Salmonella Typhimurium. All tested microorganisms were sensitive to studied extracts. | D | [14] |
| A: Extract (ethanol–water). B: Antibacterial susceptibility—Kirby Bauer disk diffusion method and MIC determination. | Antibacterial activity against B. subtilis, E. coli, S. aureus, Salmonella, Pseudomonas aeruginosa. | D | [15] | |
| Fructans, starch, FOS and flavonoids including quercetin | A: Two derived fractions: extract (water/ethanol soluble) rich in FOS (7%) and onion dry residue (3%). B: Rats fed (4 weeks) with an onion by-product powder (10%) and two derived fractions, extract rich in FOS (7%) and onion dry residue (3%) in the diet. Control rats fed with a control diet. SCFA—capillary electrophoresis with indirect UV detection. | The onion by-products as well as the soluble and insoluble fractions had prebiotic effects as evidenced by decreased pH, increased butyrate production, and altered gut microbiota (BGL and GUS) enzyme activities in the caecal contents. | D, ID | [16] |
| A: Extract (using subcritical water extraction—SWE). B: Bacterial counting (log CFU/mL)-plating method. Methanol as a control Extract (ethanol–water). Antibacterial susceptibility—Kirby Bauer disk diffusion method and MIC determination. | Over 0.6 mg/mL of SWE (110 °C) extract exerted a bactericidal effect against B. cereus KCCM 40935 (G+, spore-forming, rod-typed, heat-resistant pathogenic) and 1.2 mg/mL of SWE (160 °C) extract exerted a bacteriostatic effect during culturing. | D | [17] | |
| A: Extract (using subcritical water—SWE). B: Bacterial counting (log CFU/mL)-plating method. Quercetin as a control. | Extract (using subcritical water—SWE). Bacterial counting (log CFU/mL)-plating method. Quercetin as a control. | D | [18] | ||
| Carrot (Daucus carota): | |||||
| Anthocyanins, phenolic acids, carotenes, fibre | A: The fresh material was immediately ground (root) and then dried (root and pomace) in a vacuum dryer at 45 °C for 12 h, grounded and passed through a 0.5-mm mesh sieve to obtain a fine dietary component. B: In vivo experiment with rats. Experimental groups included animals obtained diet with 10% dried preparations; SCFA-GC; bacterial enzymes measured by the rate of release of p-nitrophenol or o-nitrophenol from the respective nitrophenyl glucosides. | In comparison to the control group (standard diet), a significant increase in bacterial caecal α- and β-glucosidase, α- and β-galactosidase, and β-glucuronidase activity was noted in all four carrot groups. In comparison to the control group, a significant increase in all caecal SCFA concentrations followed all four carrot dietary treatments. | D, ID | [19] |
| A: Extract (water). B: MIC determination. | Carrot pomace extracts exhibited inhibitory activity against two methicillin-resistant S. aureus G+ strains (MRSA 1 and 3) and Enterococci 44 (HLAR-VRE). The effectiveness of phenolic compounds was only against G+ bacteria. There was no effect on G− ones: Pseudomonas 30, Klebsiella, E. coli ESBL 365, E. coli 2280). | D | [20] | |
| Soybean (Glycine max): | |||||
| Lactic acid (2.67 g/L); acetic acid (1.87 g/L); total viable count of bacteria (3 × 109 CFU/mL): Lactobacillus, Acetobacter, Burkholderiaceae, Actinobacteria, other. | A: Fresh tofu whey wastewater was collected from three township tofu processing factories and was naturally fermented for 5 days at 25 °C. B: Microbiota analysis—PCR based on 16S rDNA. Chickens were infected with Salmonella enteritidis. The chickens obtained drinking water containing tofu whey wastewater for 7 days. | Reduction in the colonisation and excretion of Salmonella enteritidis in chickens. Tofu whey wastewater supplementation significantly upregulated the relative abundance of Lactobacillus and Burkholderia in control and Salmonella enteritidis-infected chickens. | D | [21] |
| Basic characteristic was identified (proteins, carbohydrates, lipids dietary fibre, isoflavone). | A: Different dosages of okara (7.5% and 15%) B: In vivo experiment with mice (26 weeks of feeding; n = 11–15 per group). Cecal microbial analysis was conducted using the terminal restriction fragment length polymorphism (T-RFLP) method and was subjected to 16S rDNA. Control—mice with a standard diet. | The relative abundance of Clostridiales, Bacteriodales, and Ciriobacteriales was significantly increased by 15% okara diet supplementation compared to control mice. Lactobacillus, Erysipelotrichaceae, Parasutterella, were significantly decreased in the 15% okara group compared to the control group. | D | [22] |
| Broccoli (Brassica oleracea): | |||||
| Glucosinolates, polyphenols (sinapic acid and chlorogenic acid derivatives), dietary fibre, carbohydrates. | A: Extract (ethanol and water). The samples were digested by a simulated gastrointestinal model. B: SCFA were fermented in in vitro human faecal fermentation model and analysed by GCL chromatographic analysis. | SCFA production increased during the fermentation of both extracts by the microbiota. Insoluble fibre fraction extracted from fresh broccoli stalks exhibited a greater prebiotic effect than freeze-dried broccoli stalks, leading to a higher content of total SCFAs with significant differences in the production of acetate, butyrate and other minor SCFAs (isobutyrate, isovalerate, valerate, isocaproate, caproate and heptanoate). | D | [23] |
| Crude protein, fat, uronic acid (pectin), neutral sugars (glucose, xylose, arabinose, fructose, rhamnose, manose, galactose). | A: Total dietary fibre extraction—alcohol insoluble residues method. Modifications: supercritical fluid and enzyme treatments. B: The growth capacity—plating on MRS broth. Evaluation by comparing the percentage of growth in each extract with the positive control (glucose). SCFAs produced in the presence of fibre extracts were determined by a gas chromatograph with a split/split-less injector and a flame ionization detector. | The effect of soluble DF from different parts of the broccoli plant and with different modifications (supercritical fluid and enzyme treatments) on the growth capacity of LAB (L. sakei, L. brevis, L. plantarum, L. casei and Enterococcus faecium). L. sakei, Enterococcus faecium and L. casei showed significant differences in growth rates in the presence of the different extracts. DF extracts from leaf and stem samples showed the highest growth values, while DF extracts from the inflorescences showed the lowest values. In contrast, no significant differences were observed in the growth of L. plantarum andL. brevis in the presence of the different extracts. Treatment with enzymes improved especially the growth of LAB and the production of all the SCFA (acetic, propionic, butyric, isovaleric, isobutyric, isocaproic, caproic, valeric). | D | [24] |
| Sugar beet (Beta vulgaris): | |||||
| Pectin oligosaccharides, monosaccharides representative for the pectin, i.e., galacturonic acid as acidic sugar, and arabinose, galactose, rhamnose | A: Enzymatic (cellulase) and nitric acid extracted, hydrolysed and fractionated. B: The growth of inoculated cells—impedance microbiology (time to detection coincides with the reaching of a cell concentration of about 106–107 cells/mL). Probiotic effect–compared to growth of the species in not supplemented MRS and TSB broths. | Pectin oligosaccharide compounds promoted the growth of LAB. Not all the fractions worked with the same efficiency stimulating LAB, and that pectin oligosaccharides containing a low degree of polymerization arabinans, and little or no free galacturonic acid (and possibly no nitrates), obtained by enzymatic extraction, were the most efficient. No fraction was able to stimulate pathogenic E. coli strains (K88 and K89). | D | [25] |
| Cabbage: | |||||
| Fiber | A: Hydrolyzates; Alcohol-Soluble Fiber (ASF). Two commercial enzymes, Shearzyme Plus and Viscozyme L, were used in the study. B: Growth of intestinal microbiota (L. plantarum ATCC 8014, L. casei ATCC 393, L. delbrueckii subsp. bulgaricus ATCC 11842, and L. acidophilus ATCC 832) were investigated using broth microdilution method and plating on MRS. | Alcohol-soluble dietary fibres were found to promote the growth of LAB. ASF had a significantly greater effect on the growth of all LABs except for L. casei than the control. Especially, L. plantarum and L. delbrueckii grew best in the ASF produced by Shearzyme. | D | [26] |
| Sulfur compounds, such as dimethyl trisulfide, and terpenic compounds, such as phytol and its derivatives, furfural | A: Extracts (aqueous, methanolic, ethanolic) and essential oil. B: Diffusion antimicrobial susceptibility–plating on Mueller–Hinton agar. DIZ (in millimetres) formed around the discs containing the extracts. Negative control-sterilized disc. Positive control—amoxicillin and potassium clavulanate. | The cabbage stalk flour essential oil appeared to be active as an antimicrobial agent against Salmonella sp. (G−), B. cereus (G+), S. aureus (G+), and E. coli (G−). The methanolic extract was active against E. coli and the aqueous extract against S. aureus, and E. coli. Listeria monocytogenes (G+) were not sensitive to all treatments with cabbage stalk flour extracts and essential oil. | D | [27] |
| Chicory (Cichorium intybus) | |||||
| Polyphenolics | A: Drying, 75% ethanol extraction B: In vivo experiment with rats; Diet supplemented with (a) 10% of root extract (PL); (b) 6.5% of peel extract (PM); (c) 8% of peel extract and 0.8% of seed extract (PH); (d) 2.5% of leaf extract with 0.106% of total phenolics (PMc); (e) control—without phenolics. SCFA-GC; faecal bacterial enzymes—the rate of release of p-nitrophenol or o-nitrophenol from the respective nitrophenyl glucosides. Bacterial enzymes were measured by the rate of p- or o-nitrophenol, according to the Juśkiewicz et al. [28] method. | Diet supplementation with the preparations examined did not result in any significant differences in β-glucuronidase activity on day 7, while on day 14, its activity in the PMc group was significantly higher than in the PM and PH diets. The β-glucosidase on day 21 was significantly lower in the PM and PMc vs. C. After 3 and 4 weeks, β-glucuronidase activity was highest in the C and differed significantly from the PM and PH groups. After 4 weeks, β-glucosidase activity in the C and PL groups was significantly higher than in the other groups. | D | [29] |
| Fibre, phenoliccompounds | A: Flour was obtained by drying comminuted roots and ground. B: A 36-day experiment carried out on 54-day-old rabbits fed a diet with the chicory flour at 0, 25 and 50 g/kg. Control—commercial and antibiotic-free diet. | Supplementation of a diet with a chicory flour preparation (both levels) resulted in the lowering of the bacterial enzyme activity in the caecum and colon. | D | [30] |
| Fibre, phenoliccompounds, such as caffeoylquinic acids (CQAs) more specifically mono- and di-CQAs isomers | A: Meal from chicory roots obtained from industrial processing and commercial preparation of FOS produced via the enzymatic hydrolysis of chicory inulin. B: In vivo experiment—Wistar rats with a model of TNBS-induced colitis. Control—diets with dietary cellulose. | Both chicory preparations significantly reduced the pH value of colonic digesta and favourably lowered the caecal activity of bacterial glucuronidase as well as the caecal concentration of putrefactive SCFA in comparison to the control TNBS rats. | D, ID | [31] |
| Potato (Solanum tuberosum): | |||||
| Fibre | A: Freeze-dried, lyophilized, crushed, sieved; ethanol extraction, enzymatic modification of insoluble DF by cellulase and xylanase hydrolysis B: C57BL/6 mice intragastrically fed with 20 mL/(kg d) of low (0.25 mg/(g d), medium (0.50 mg/(g d)), and high dose (1.00 mg/(g d)) of unmodified, or modified potato residue DF. Control-fed with water. SCFA-GC. | Potato residue DF regulated the SCFA production. Unmodified and enzymatic-modified DF extracted from potato residue could promote the production of acetic, n-butyric, isobutyric, valeric, and isovaleric acids while inhibiting the production of propionic acid. DF significantly improved the number and diversity of intestinal microbiota of mice, in particular, the increased ratio of Bacteroidetes to Firmicutes. Cellulase/xylanase improved regulating effects of dietary fibre on gut microbiota. | D | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotschki, J.; Ogrodowczyk, A.M.; Wróblewska, B.; Juśkiewicz, J. Side Streams of Vegetable Processing and Its Bioactive Compounds Support Microbiota, Intestine Milieu, and Immune System. Molecules 2023, 28, 4340. https://doi.org/10.3390/molecules28114340
Fotschki J, Ogrodowczyk AM, Wróblewska B, Juśkiewicz J. Side Streams of Vegetable Processing and Its Bioactive Compounds Support Microbiota, Intestine Milieu, and Immune System. Molecules. 2023; 28(11):4340. https://doi.org/10.3390/molecules28114340
Chicago/Turabian StyleFotschki, Joanna, Anna M. Ogrodowczyk, Barbara Wróblewska, and Jerzy Juśkiewicz. 2023. "Side Streams of Vegetable Processing and Its Bioactive Compounds Support Microbiota, Intestine Milieu, and Immune System" Molecules 28, no. 11: 4340. https://doi.org/10.3390/molecules28114340
APA StyleFotschki, J., Ogrodowczyk, A. M., Wróblewska, B., & Juśkiewicz, J. (2023). Side Streams of Vegetable Processing and Its Bioactive Compounds Support Microbiota, Intestine Milieu, and Immune System. Molecules, 28(11), 4340. https://doi.org/10.3390/molecules28114340