Substituents Regulate the Cyclization of Conjugated Alkynes to Accurately Construct Cyclo-(E)-[3]dendralenes
Abstract
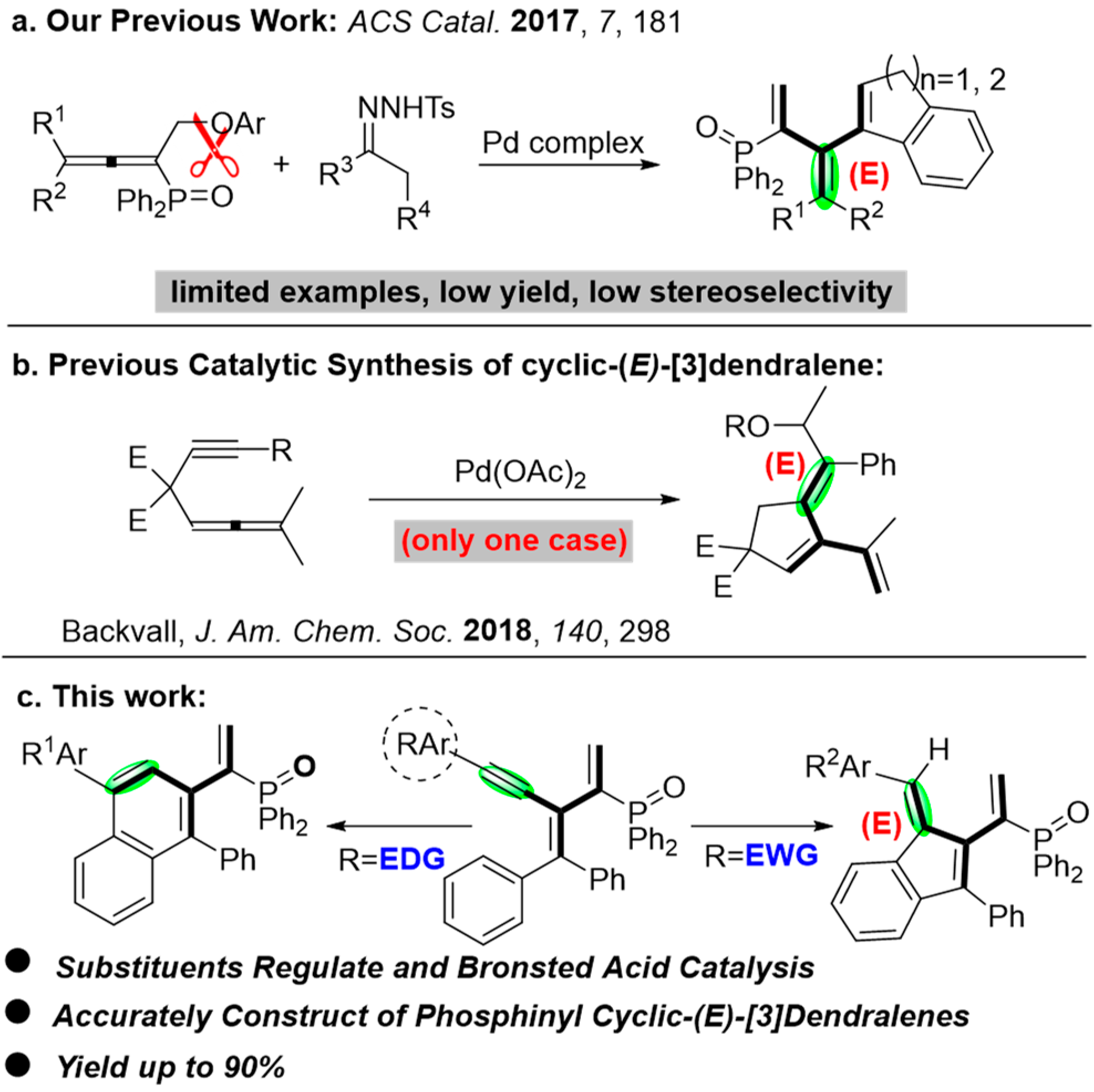
:1. Introduction
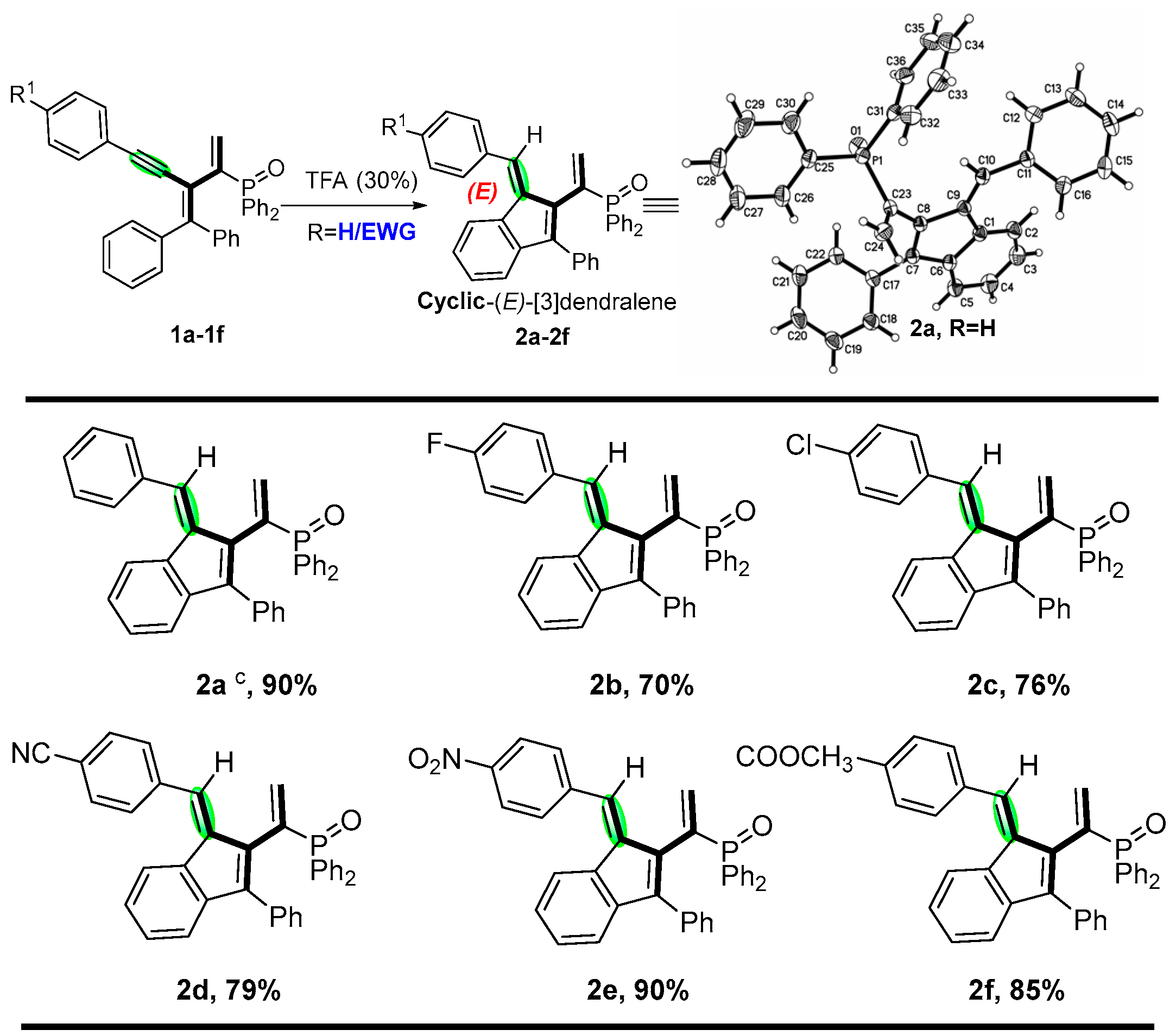
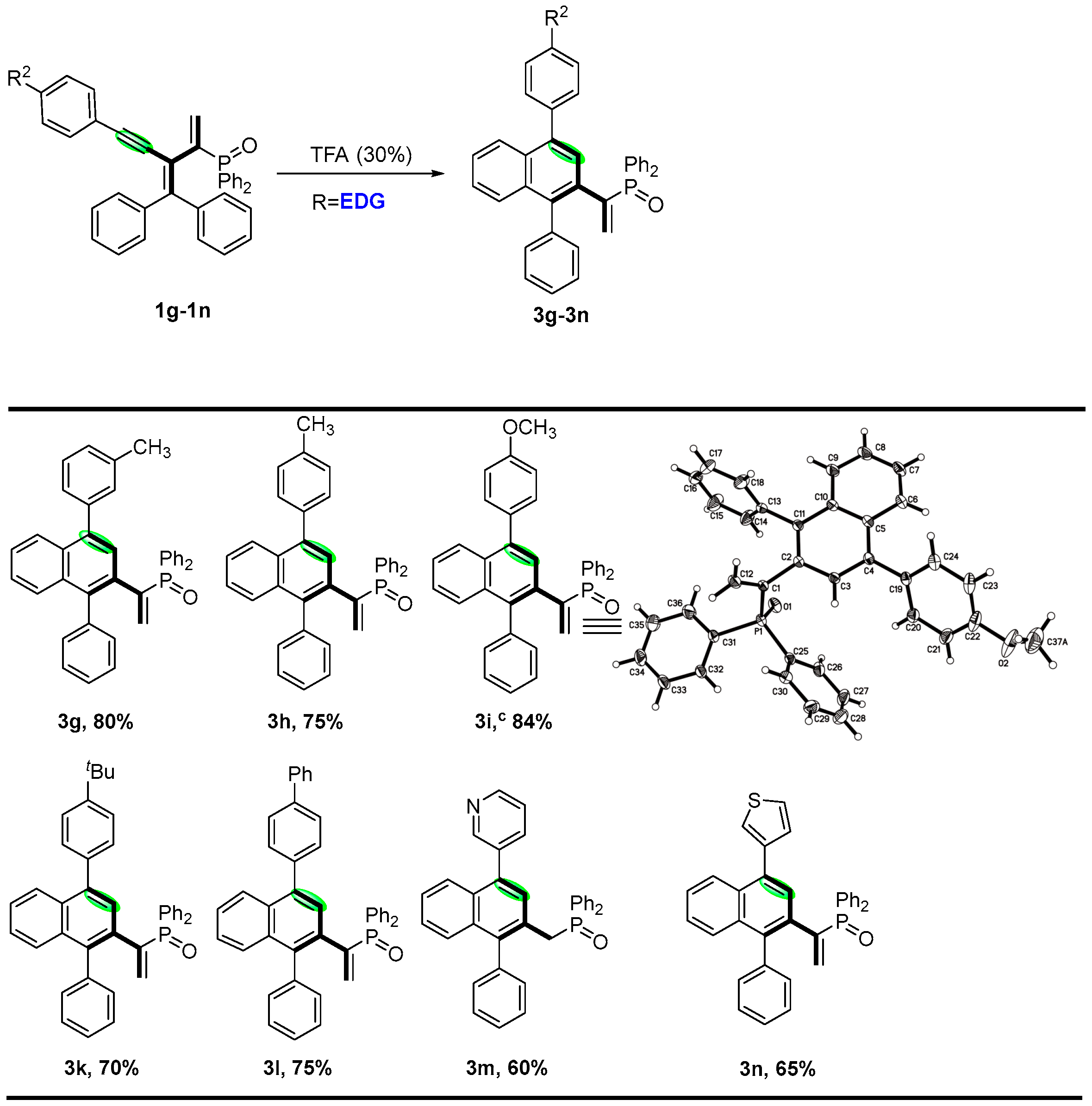
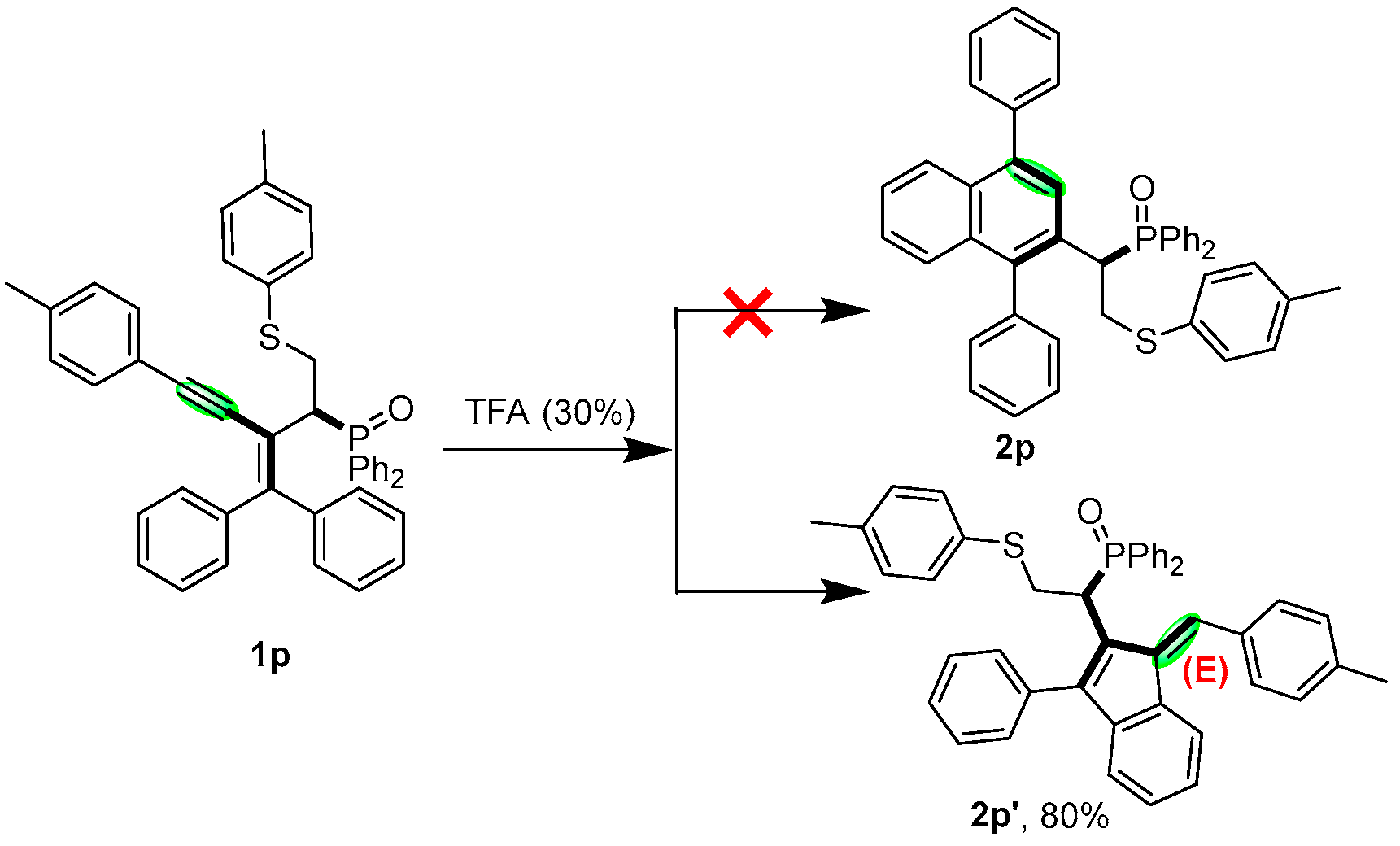
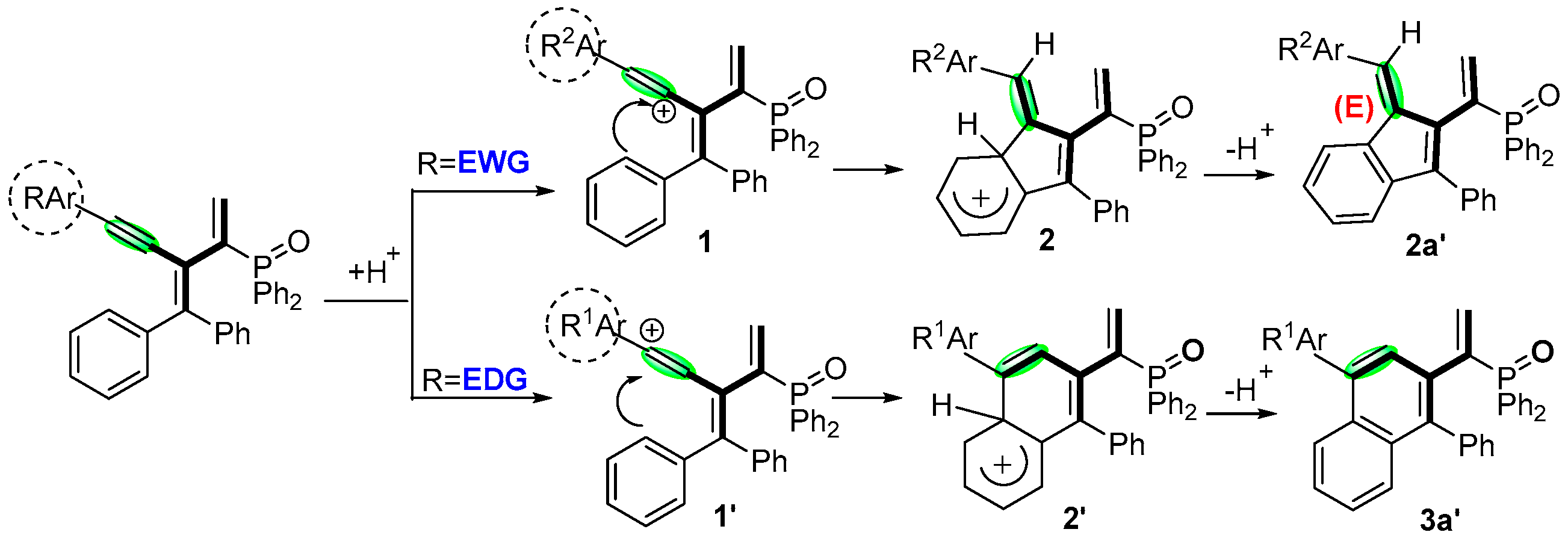
2. Results and Discussion
3. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sherburn, M.S. Preparation and Synthetic Value of π-Bond-Rich Branched Hydrocarbons. Acc. Chem. Res. 2015, 48, 1961. [Google Scholar] [CrossRef]
- Mackay, E.G.; Sherburn, M.S. Demystifying the dendralenes. Pure Appl. Chem. 2013, 85, 1227. [Google Scholar] [CrossRef]
- Hopf, H. Forgotten hydrocarbons prepared. Nature 2009, 460, 183. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.D.; Bojase, G.; Paddon-Row, M.N.; Sherburn, M.S. Practical Synthesis of the Dendralene Family Reveals Alternation in Behavior. Angew. Chem. Int. Ed. 2009, 48, 4836. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Amamoto, S.; Kishi, H.; Takeshita, H.; Miya, M.; Shiomi, T. Anionic Polymerization of 2-Phenyl[3]dendralene and 2-(4-Methoxyphenyl)[3]dendralene. Macromolecules 2013, 46, 7282. [Google Scholar] [CrossRef]
- Takamura, Y.; Takenaka, K.; Toda, T.; Takeshita, H.; Miya, M.; Shiomi, T. Anionic Polymerization of 2-Hexyl [3]dendralene. Macromol. Chem. Phys. 2018, 219, 1700046. [Google Scholar] [CrossRef]
- Ravinder, P.; Subramanian, V. Theor. Density functional theory studies on the Diels–Alder reaction of [3]dendralene with C60: An attractive approach for functionalization of fullerene. Chem. Acc. 2012, 131, 1128. [Google Scholar] [CrossRef]
- Paddon-Row, M.N.; Sherburn, M.S. On the origin of the alternating Diels–Alder reactivity in [n]dendralenes. Chem. Commun. 2012, 48, 832. [Google Scholar] [CrossRef]
- Saglam, M.F.; Fallon, T.; Paddon-Row, M.N.; Sherburn, M.S. Discovery and Computational Rationalization of Diminishing Alternation in [n]Dendralenes. J. Am. Chem. Soc. 2016, 138, 1022. [Google Scholar] [CrossRef]
- Hosoya, H. Mathematical Features of the Genealogy of Acyclic Conjugated Polyenes. Bull. Chem. Soc. Jpn. 2019, 92, 205. [Google Scholar] [CrossRef]
- Breiten, B.; Wu, Y.-L.; Jarowski, P.D.; Gisselbrecht, J.-P.; Boudon, C.; Griesser, M.; Onitsch, C.; Gescheidt, G.; Schweizer, W.B.; Langer, N.; et al. Donor-substituted octacyano [4]dendralenes: A new class of cyano-rich non-planar organic acceptors. Chem. Sci. 2011, 2, 88. [Google Scholar]
- Shoji, T.; Miura, K.; Araki, T.; Maruyama, A.; Ohta, A.; Sekiguchi, R.; Ito, S.; Okujima, T. Synthesis of 2-Methyl-1-azulenyl Tetracyanobutadienes and Dicyanoquinodimethanes: Substituent Effect of 2-Methyl Moiety on the Azulene Ring toward the Optical and Electrochemical Properties. J. Org. Chem. 2018, 83, 6690. [Google Scholar] [CrossRef]
- Michinobu, T.; Diederich, F. The [2 + 2] Cycloaddition-Retroelectrocyclization (CA-RE) Click Reaction: Facile Access to Molecular and Polymeric Push-Pull Chromophores. Angew. Chem. Int. Ed. 2018, 57, 3552. [Google Scholar] [CrossRef]
- Pronin, S.V.; Shenvi, R.A. Synthesis of a Potent Antimalarial Amphilectene. J. Am. Chem. Soc. 2012, 134, 19604. [Google Scholar] [PubMed]
- Volla, C.M.R.; Bäckvall, J.-E. Palladium-Catalyzed Aerobic Domino Oxidative Carbocyclization-Alkynylation of Allenynes. Angew. Chem. Int. Ed. 2013, 52, 14209. [Google Scholar]
- Fallon, T.; Willis, A.C.; Paddon-Row, M.N.; Sherburn, M.S. Furanodendralenes. J. Org. Chem. 2014, 79, 3185. [Google Scholar] [PubMed]
- Bartholomeyzik, T.; Mazuela, J.; Pendrill, R.; Deng, Y.; Bäckvall, J.-E. Palladium-Catalyzed Oxidative Arylating Carbocyclization of Allenynes: Control of Selectivity and Role of H2O. Angew. Chem. Int. Ed. 2014, 53, 8696. [Google Scholar] [CrossRef]
- Thies, N.; Haak, E. Ruthenium-Catalyzed Synthesis of 2,3-Cyclo[3]dendralenes and Complex Polycycles from Propargyl Alcohols. Angew. Chem. Int. Ed. 2015, 54, 4097. [Google Scholar] [CrossRef]
- Tan, S.M.; Willis, A.C.; Paddon-Row, M.N.; Sherburn, M.S. Multicomponent Diene-Transmissive Diels–Alder Sequences Featuring Aminodendralenes. Angew. Chem. Int. Ed. 2016, 55, 3081. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, K.; Shibata, Y.; Tanaka, K. Rhodium-Catalyzed Cross-Cyclotrimerization and Dimerization of Allenes with Alkynes. Angew. Chem. Int. Ed. 2016, 55, 6753. [Google Scholar] [CrossRef]
- Lippincott, D.J.; Linstadt, R.T.H.; Maser, M.R.; Lipshutz, B.H. Synthesis of Functionalized [3], [4], [5] and [6]Dendralenes through Palladium-Catalyzed Cross-Couplings of Substituted Allenoates. Angew. Chem. Int. Ed. 2017, 56, 847. [Google Scholar] [CrossRef]
- Qiu, Y.; Posevins, D.; Bäckvall, J.-E. Selective Palladium-Catalyzed Allenic C−H Bond Oxidation for the Synthesis of [3]Dendralenes. Angew. Chem. Int. Ed. 2017, 56, 13112. [Google Scholar] [CrossRef]
- Rivera-Chao, E.; Fañanás-Mastral, M. Synthesis of Stereodefined Borylated Dendralenes through Copper-Catalyzed Allylboration of Alkynes. Angew. Chem. Int. Ed. 2018, 57, 9945. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, P.; Deng, Y.; Shao, Z. Regioselectivity Switch in Palladium-Catalyzed Allenylic Cycloadditions of Allenic Esters: [4 + 1] or [4 + 3] Cycloaddition/Cross-Coupling. Angew. Chem. Int. Ed. 2019, 131, 4758. [Google Scholar] [CrossRef]
- Li, H.; Gontla, R.; Flegel, J.; Merten, C.; Ziegler, S.; Antonchick, A.P.; Waldmann, H. Enantioselective Formal C(sp3)−H Bond Activation in the Synthesis of Bioactive Spiropyrazolone Derivatives. Angew. Chem. Int. Ed. 2019, 58, 307. [Google Scholar] [CrossRef]
- Li, S.C.; Hou, B.; Wang, J.B. Palladium-Catalyzed Oxidative Coupling of the Allenic C–H Bond with α-Diazo Esters: Synthesis of [3]Dendralenes. J. Org. Chem. 2021, 86, 5371–5379. [Google Scholar] [CrossRef] [PubMed]
- Hopf, H.; Sherburn, M.S. Dendralenes Branch Out: Cross-Conjugated Oligoenes Allow the Rapid Generation of Molecular Complexity. Angew. Chem. Int. Ed. 2012, 51, 2298. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, W.C.; Zhang, C.Y.; Wu, L. Recent Progress in the Synthesis of Dendralenes: A Decade Update. Chin. J. Org. Chem. 2021, 41, 1081. [Google Scholar] [CrossRef]
- Mao, M.; Zhang, L.; Chen, Y.-Z.; Zhu, J.; Wu, L. Palladium-Catalyzed Coupling of Allenylphosphine Oxides with N-Tosylhydrazones toward Phosphinyl [3]Dendralenes. ACS Catal. 2017, 7, 181. [Google Scholar] [CrossRef]
- Bartholomeyzik, T.; Pendrill, R.; Lihammar, R. Kinetics and Mechanism of the Palladium-Catalyzed Oxidative Arylating Carbocyclization of Allenynes. J. Am. Chem. Soc. 2018, 140, 298. [Google Scholar] [CrossRef]
- Wei, K.; Luo, K.; Liu, F.; Wu, L.; Wu, L.-Z. Visible-Light-Driven Selective Alkenyl C–P Bond Cleavage of Allenylphosphine Oxides. Org. Lett. 2019, 21, 1994. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mao, M.; Ji, H.-J.; Xu, J.-Y.; Wu, L. Palladium-Catalyzed Cleavage of α-Allenylic Aryl Ether toward Pyrazolemethylene-Substituted Phosphinyl Allenes and Their Transformations via Alkenyl C–P(O) Cleavage. Org. Lett. 2017, 19, 1946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, J.; Ma, J.; Wu, L.; Zhang, W.-H. Visible-Light-Driven α-Allenylic C–O Bond Cleavage and Alkenyl C–S Formation: Metal-Free and Oxidant-Free Thiolation of Allenyl Phosphine Oxides. Org. Lett. 2017, 19, 6308. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.T.; Wu, J.J.; Zhang, C.Y.; Mao, M.; Ji, Y.G.; Wu, L.; Zhang, W.H. Cascade Alkynylation and Highly Selective Hydrogenation Catalyzed by Binaphthyl-Palladium Nanoparticles Accessing Phosphinyl (Z)-[3]Dendralenes. Org. Lett. 2019, 21, 6383. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Zhang, L.; Lu, A.-M.; Yang, F.; Wu, L. α-Allenyl Ethers as Starting Materials for Palladium Catalyzed Suzuki–Miyaura Couplings of Allenylphosphine Oxides with Arylboronic Acids. J. Org. Chem. 2015, 80, 673. [Google Scholar] [CrossRef]
- Liu, P.; Deng, C.-L.; Lei, X.; Lin, G. Tandem Amination/Cycloisomerization of Aryl Propargylic Alcohols with 2-Aminopyridines as an Expedient Route to Imidazo [1,2-a]pyridines. Eur. J. Org. Chem. 2011, 2011, 7308–7316. [Google Scholar] [CrossRef]
- Xia, Y.T.; Xie, X.Y.; Cui, S.H.; Ji, Y.G.; Wu, L. Secondary phosphine oxides stabilized Au/Pd nanoalloys: Metal components-controlled regioselective hydrogenation toward phosphinyl (Z)-[3]dendralenes. Chem. Commun. 2019, 55, 11699. [Google Scholar] [CrossRef]
- Franco, M.; Azzena, U.; Melloni, G. A novel synthesis of methylene-1H-indenes (benzofulvenes) by cyclisation of phenyl-substituted but-1-en-3-ynes. J. Chem. Soc. Perkin Trans. 1993, 1, 2957. [Google Scholar]
- Luo, X.-L.; Chen, X.-W.; Chen, L.; Zhang, K.; Chen, Y.-B. Xanthate-mediated synthesis of (E)-alkenes by semi-hydrogenation of alkynes using water as the hydrogen donor. Chem. Commun. 2019, 55, 2170. [Google Scholar] [CrossRef]

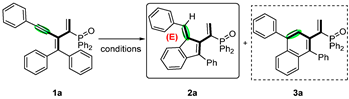
| Entry | Catalyst | Solvent | Yield (2a/3a, %) b |
|---|---|---|---|
| 1 | AgSbF6 | DCM | 90/trace |
| 2 | AgSbF6 | DMF | 0/0 |
| 3 | AgSbF6 | 1,4-dioxane | 78/<5 |
| 4 | AgSbF6 | toluene | 63/<5 |
| 5 | AgSbF6 | CH3CN | 0/0 |
| 6 | AgCl | DCM | trace/0 |
| 7 | AgOAc | DCM | trace/0 |
| 8 | H3PO4 | DCM | 40/0 |
| 9 | TsOH | DCM | 61/0 |
| 10 | TFA | DCM | 90/0 |
| 11 | Pivalic acid | DCM | 0/0 |
| 12 c | TFA | DCM | trace/0 |
| 13 d | TFA | TFA | 87/<5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.-T.; Li, Y.-X.; Bi, T.-T.; Lian, W.; Wang, X.; Yan, M.; Guo, T. Substituents Regulate the Cyclization of Conjugated Alkynes to Accurately Construct Cyclo-(E)-[3]dendralenes. Molecules 2023, 28, 4382. https://doi.org/10.3390/molecules28114382
Xia Y-T, Li Y-X, Bi T-T, Lian W, Wang X, Yan M, Guo T. Substituents Regulate the Cyclization of Conjugated Alkynes to Accurately Construct Cyclo-(E)-[3]dendralenes. Molecules. 2023; 28(11):4382. https://doi.org/10.3390/molecules28114382
Chicago/Turabian StyleXia, Yun-Tao, Ya-Xin Li, Tong-Tong Bi, Wei Lian, Xia Wang, Meng Yan, and Tao Guo. 2023. "Substituents Regulate the Cyclization of Conjugated Alkynes to Accurately Construct Cyclo-(E)-[3]dendralenes" Molecules 28, no. 11: 4382. https://doi.org/10.3390/molecules28114382
APA StyleXia, Y.-T., Li, Y.-X., Bi, T.-T., Lian, W., Wang, X., Yan, M., & Guo, T. (2023). Substituents Regulate the Cyclization of Conjugated Alkynes to Accurately Construct Cyclo-(E)-[3]dendralenes. Molecules, 28(11), 4382. https://doi.org/10.3390/molecules28114382






