Cattail-Grass-Derived Porous Carbon as High-Capacity Anode Material for Li-Ion Batteries
Abstract
1. Introduction
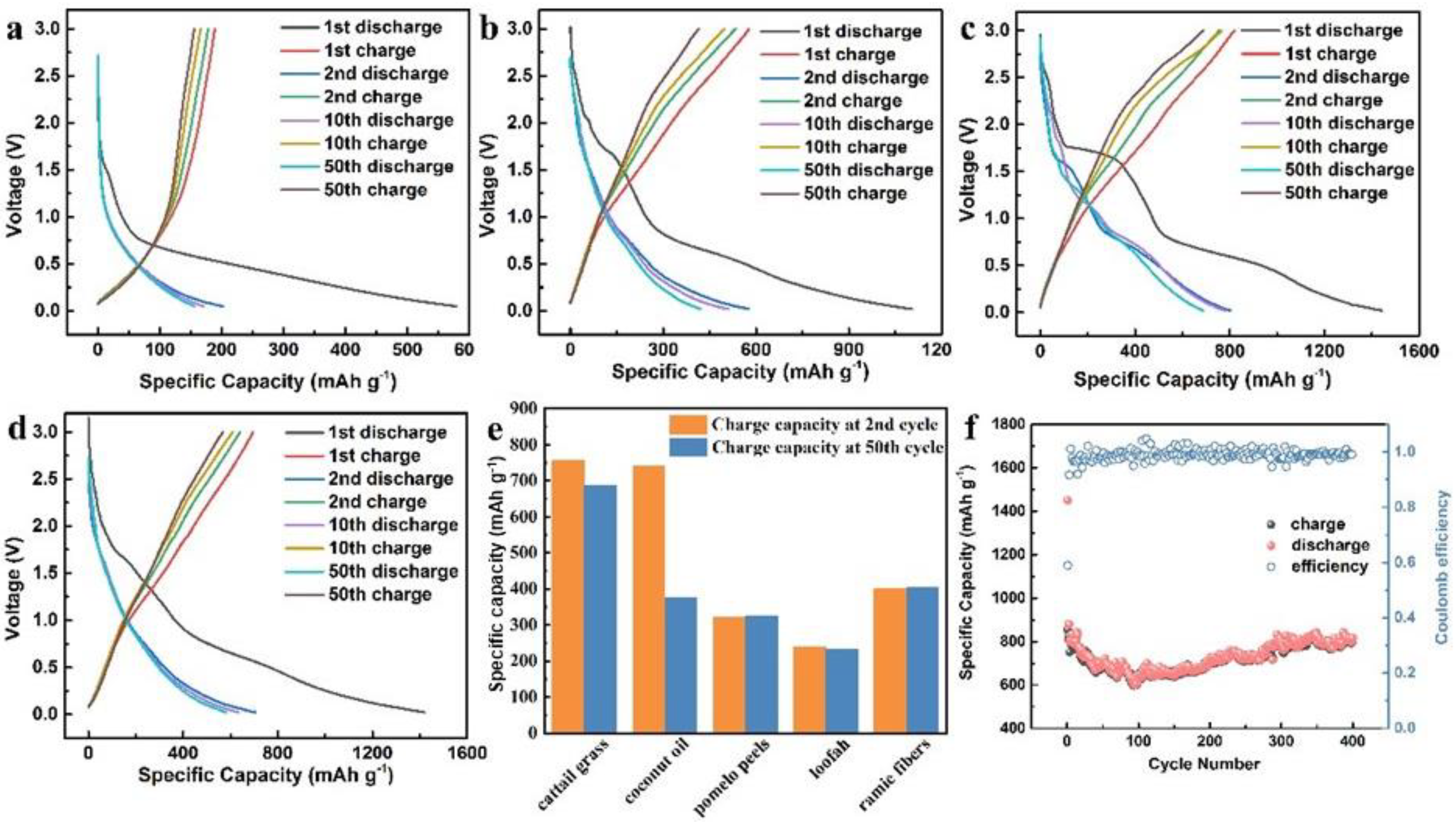
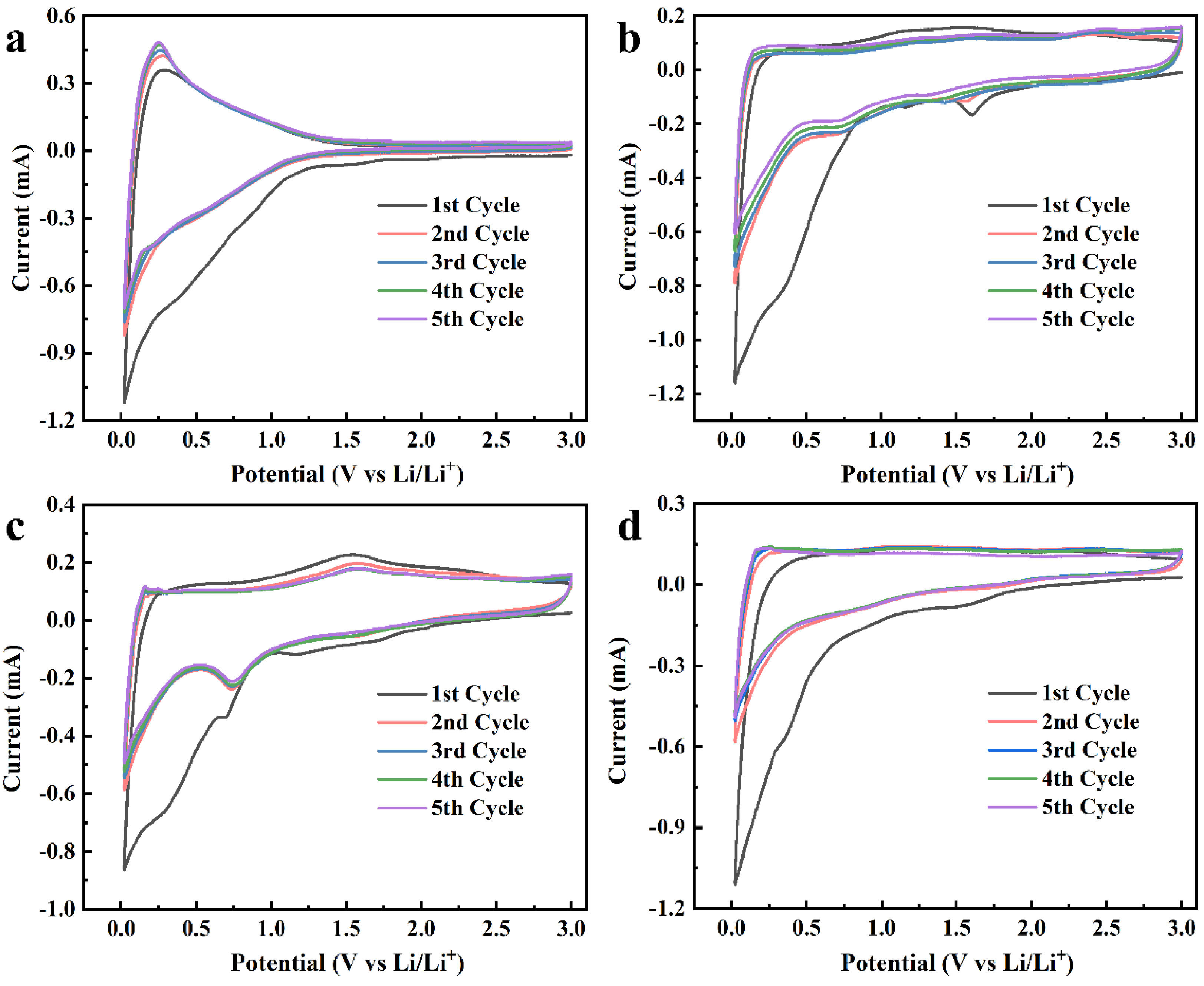
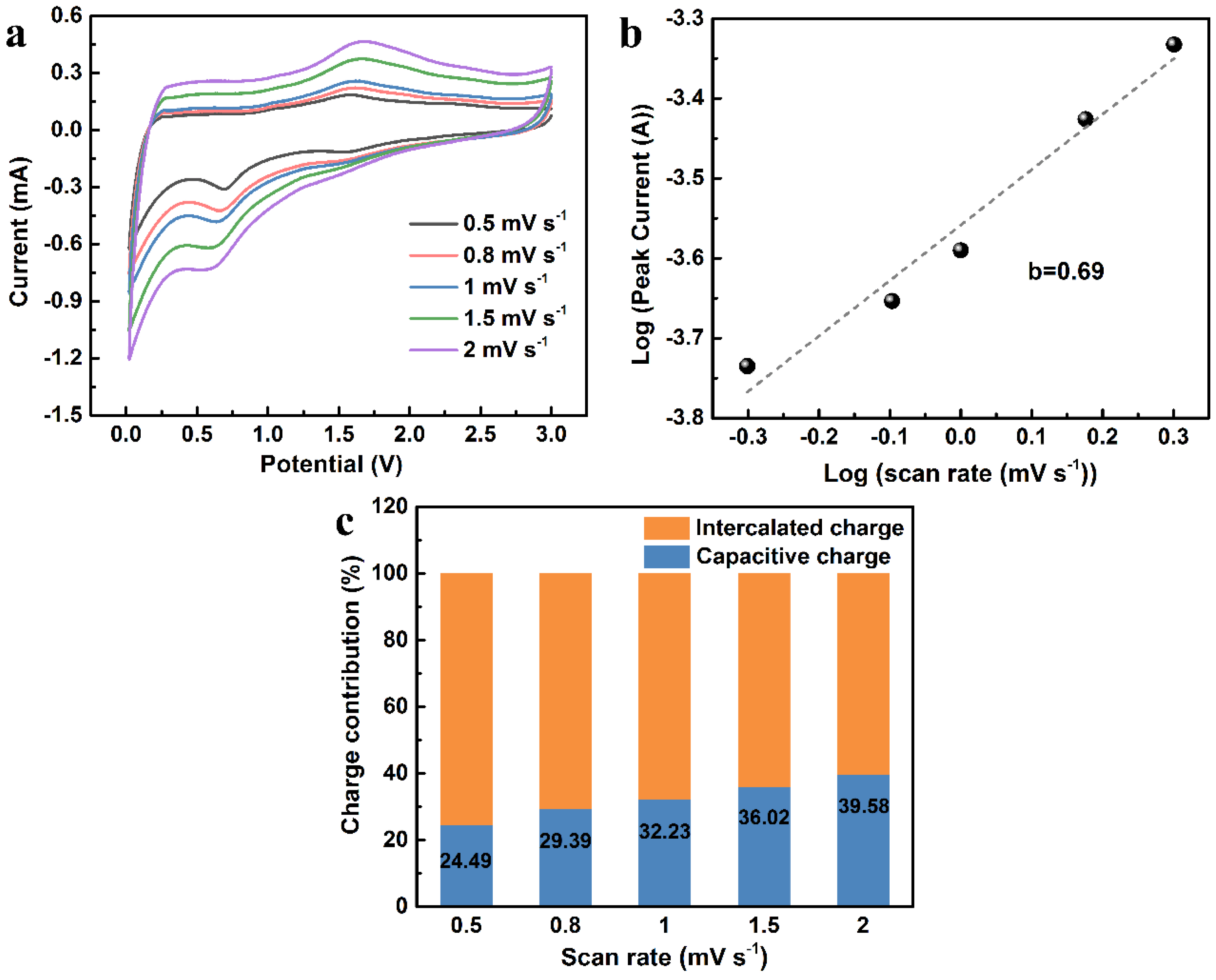
2. Results and Discussion
3. Experimental and Methods
3.1. Material Preparation
3.2. Material Characterizations
3.3. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, Q.; Gao, C.L.; Zhang, W.X.; Luo, S.H.; Zhou, M.; Liu, Y.G.; Liu, R.H.; Zhang, Y.H.; Wang, Z.Y.; Hao, A.M. Biomorphic carbon derived from corn husk as a promising anode materials for potassium ion battery. Electrochim. Acta 2019, 324, 7. [Google Scholar] [CrossRef]
- Sun, X.L.; Wang, X.H.; Feng, N.; Qiao, L.; Li, X.W.; He, D.Y. A new carbonaceous material derived from biomass source peels as an improved anode for lithium ion batteries. J. Anal. Appl. Pyrolysis 2013, 100, 181–185. [Google Scholar] [CrossRef]
- Raj, K.A.; Panda, M.R.; Dutta, D.P.; Mitra, S. Bio-derived mesoporous disordered carbon: An excellent anode in sodium-ion battery and full-cell lab prototype. Carbon 2019, 143, 402–412. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, Z.H.; Yin, S.Y.; Guo, Z.P.; Wang, S.Q.; Feng, C.Q. Biomass carbon micro/nano-structures derived from ramie fibers and corncobs as anode materials for lithium-ion and sodium-ion batteries. Appl. Surf. Sci. 2016, 379, 73–82. [Google Scholar] [CrossRef]
- Rasheed, T.; Naveed, A.; Nabeel, F.; Ahmed, M.S.; Ali, J.; Khan, S.U.D. Bio-mass derived ultrahigh-energy storage porous graphitic carbon for advanced anode material in lithium battery. Mater. Chem. Phys. 2020, 242, 5. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Yang, D.F.; Narayan, R.; Raju, K.; Kumar, N.A.; Zhao, X.S. Biomass derived carbon nanoparticle as anodes for high performance sodium and lithium ion batteries. Nano Energy 2016, 26, 346–352. [Google Scholar] [CrossRef]
- Zheng, F.C.; Liu, D.; Xia, G.L.; Yang, Y.; Liu, T.; Wu, M.Z.; Chen, Q.W. Biomass waste inspired nitrogen-doped porous carbon materials as high-performance anode for lithium-ion batteries. J. Alloys Compd. 2017, 693, 1197–1204. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Muruganantham, R.; Tai, S.H.; Chang, B.K.; Wu, S.C.; Chueh, Y.L.; Liu, W.R. Coffee grounds-derived carbon as high performance anode materials for energy storage applications. J. Taiwan Inst. Chem. Eng. 2019, 97, 178–188. [Google Scholar] [CrossRef]
- Yu, K.F.; Li, J.; Qi, H.; Liang, C. High-capacity activated carbon anode material for lithium-ion batteries prepared from rice husk by a facile method. Diam. Relat. Mat. 2018, 86, 139–145. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Q.; Chen, W.L.; Wan, M.; Li, X.C.; Wang, L.L.; Xue, L.H.; Zhang, W.X. High capacity hard carbon derived from lotus stem as anode for sodium ion batteries. J. Power Sources 2018, 378, 331–337. [Google Scholar] [CrossRef]
- Ma, G.Y.; Huang, K.S.; Zhuang, Q.C.; Ju, Z.C. Superior cycle stability of nitrogen-doped graphene nanosheets for Na-ion batteries. Mater. Lett. 2016, 174, 221–225. [Google Scholar] [CrossRef]
- Luo, X.F.; Yang, C.H.; Peng, Y.Y.; Pu, N.W.; Ger, M.D.; Hsieh, C.T.; Chang, J.K. Graphene nanosheets, carbon nanotubes, graphite, and activated carbon as anode materials for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 10320–10326. [Google Scholar] [CrossRef]
- Murali, G.; Harish, S.; Ponnusamy, S.; Ragupathi, J.; Therese, H.A.; Navaneethan, M.; Muthamizhchelvan, C. Hierarchically porous structured carbon derived from peanut shell as an enhanced high rate anode for lithium ion batteries. Appl. Surf. Sci. 2019, 492, 464–472. [Google Scholar] [CrossRef]
- Ghannam, M.M.; Heiba, Z.K.; Sanad, M.M.S.; Mohamed, M.B. Functional properties of ZnMn2O4/MWCNT/graphene nanocomposite as anode material for Li-ion batteries. Appl. Phys. A 2020, 126, 332. [Google Scholar] [CrossRef]
- Hamidouche, F.; Sanad, M.M.S.; Ghebache, Z.; Boudieb, N. Effect of polymerization conditions on the physicochemical and electrochemical properties of SnO2/polypyrrole composites for supercapacitor applications. J. Mol. Struct. 2022, 1251, 131964. [Google Scholar] [CrossRef]
- Sanad, M.M.S.; El-Sadek, M.H. Porous niobium carbide as promising anode for high performance lithium-ions batteries via cost-effective processing. Diam. Relat. Mat. 2021, 121, 108722. [Google Scholar] [CrossRef]
- Khan, M.; Ahmad, N.; Lu, K.W.; Sun, Z.H.; Wei, C.H.; Zheng, X.J.; Yang, R.Z. Nitrogen-doped carbon derived from onion waste as anode material for high performance sodium-ion battery. Solid State Ion. 2020, 346, 7. [Google Scholar] [CrossRef]
- Wan, H.R.; Hu, X.F. Nitrogen doped biomass-derived porous carbon as anode materials of lithium ion batteries. Solid State Ion. 2019, 341, 8. [Google Scholar] [CrossRef]
- Sankar, S.; Saravanan, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Lee, S.; Kim, D.Y. Spherical activated-carbon nanoparticles derived from biomass green tea wastes for anode material of lithium-ion battery. Mater. Lett. 2019, 240, 189–192. [Google Scholar] [CrossRef]
- Wang, L.L.; Li, Y.T.; Yang, K.L.; Lu, W.Q.; Yu, J.G.; Gao, J.; Liao, G.; Qu, Y.N.; Wang, X.F.; Li, X.F.; et al. Hierarchical Porous Carbon Microspheres Derived from Biomass-Corncob as Ultra-High Performance Supercapacitor Electrode. Int. J. Electrochem. Sci. 2017, 12, 5604–5617. [Google Scholar] [CrossRef]
- Yu, X.L.; Lu, J.M.; Zhan, C.Z.; Lv, R.T.; Liang, Q.H.; Huang, Z.H.; Shen, W.C.; Kang, F.Y. Synthesis of activated carbon nanospheres with hierarchical porous structure for high volumetric performance supercapacitors. Electrochim. Acta 2015, 182, 908–916. [Google Scholar] [CrossRef]
- Liu, S.L.; Wang, Y.N.; Lu, K.T. Preparation and pore characterization of activated carbon from Ma bamboo (Dendrocalamus latiflorus) by H3PO4 chemical activation. J. Porous Mat. 2014, 21, 459–466. [Google Scholar] [CrossRef]
- Yu, K.F.; Wang, J.J.; Wang, X.F.; Liang, J.C.; Liang, C. Sustainable application of biomass by-products: Corn straw-derived porous carbon nanospheres using as anode materials for lithium ion batteries. Mater. Chem. Phys. 2020, 243, 8. [Google Scholar] [CrossRef]
- Acevedo, S.; Giraldo, L.; Moreno-Pirajan, J.C. Enthalpies of immersion in benzene, cyclohexane and water of granular activated carbons prepared by chemical activation with solutions of MgCl2 and CaCl2. J. Therm. Anal. Calorim. 2015, 121, 1279–1285. [Google Scholar] [CrossRef]
- Liu, B.; Gu, J.; Zhou, J.B. High surface area rice husk-based activated carbon prepared by chemical activation with ZnCl2-CuCl2 composite activator. Environ. Prog. Sustain. Energy 2016, 35, 133–140. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, E.J.; Wang, J.; Liu, Z.M.; Ge, J.M.; Yu, X.Z.; Yang, H.G.; Lu, B.A. Potato derived biomass porous carbon as anode for potassium ion batteries. Electrochim. Acta 2019, 293, 364–370. [Google Scholar] [CrossRef]
- Gao, C.L.; Wang, Q.; Luo, S.H.; Wang, Z.Y.; Zhang, Y.H.; Liu, Y.G.; Hao, A.M.; Guo, R. High performance potassium-ion battery anode based on biomorphic N-doped carbon derived from walnut septum. J. Power Sources 2019, 415, 165–171. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Lv, W.M.; Mu, C.P.; Zhao, J.; Wang, B.C. Activated hard carbon from orange peel for lithium/sodium ion battery anode with long cycle life. J. Alloys Compd. 2017, 701, 870–874. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, Y.G.; Wan, J.Y.; Henderson, D.; Zhang, X.G.; Hu, L.B. High Temperature Carbonized Grass as a High Performance Sodium Ion Battery Anode. ACS Appl. Mater. Interfaces 2017, 9, 391–397. [Google Scholar] [CrossRef]
- Lotfabad, E.M.; Ding, J.; Cui, K.; Kohandehghan, A.; Kalisvaart, W.P.; Hazelton, M.; Mitlin, D. High-Density Sodium and Lithium Ion Battery Anodes from Banana Peels. ACS Nano 2014, 8, 7115–7129. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Y.; Chen, K.; Zhang, N.; Zhao, X.Q.; Zhao, F.H.; Dou, Z.F.; He, X.M.; Wang, L. Biomass-derived Activated Carbon for Rechargeable Lithium-Sulfur Batteries. BioResources 2015, 10, 155–168. [Google Scholar] [CrossRef]
- Chen, Q.; Pu, W.H.; Hou, H.J.; Hu, J.P.; Liu, B.C.; Li, J.F.; Cheng, K.; Huang, L.; Yuan, X.Q.; Yang, C.Z.; et al. Activated microporous-mesoporous carbon derived from chestnut shell as a sustainable anode material for high performance microbial fuel cells. Bioresour. Technol. 2018, 249, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.F.; Shao, G.Y.; Guo, C.Q.; Lu, Y.X.; Dong, X.C.; Zhong, Y.W.; Liu, A.H. Long cycle life, low self-discharge carbon anode for Li-ion batteries with pores and dual-doping. J. Alloys Compd. 2019, 802, 620–627. [Google Scholar] [CrossRef]
- Arie, A.A.; Kristianto, H.; Demir, E.; Cakan, R.D. Activated porous carbons derived from the Indonesian snake fruit peel as anode materials for sodium ion batteries. Mater. Chem. Phys. 2018, 217, 254–261. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Ge, X.; Lu, M.; Xue, X.; Wang, Z.; Yue, N.; Zhang, J.; Lang, X.; Jiang, Q.; et al. Etching-courtesy NH4+ pre-intercalation enables highly-efficient Li+ storage of MXenes via the renaissance of interlayer redox. J. Energy Chem. 2022, 72, 26–32. [Google Scholar] [CrossRef]
- Yang, H.; Qin, T.; Zhou, X.; Feng, Y.; Wang, Z.; Ge, X.; Yue, N.; Li, D.; Zhang, W.; Zheng, W. Boosting the kinetics of PF6− into graphitic layers for the optimal cathode of dual-ion batteries: The rehearsal of pre-intercalating Li+. J. Energy Chem. 2022, 71, 392–399. [Google Scholar] [CrossRef]
- Dou, Y.L.; Liu, X.; Yu, K.F.; Wang, X.F.; Liu, W.P.; Liang, J.C.; Liang, C. Biomass porous carbon derived from jute fiber as anode materials for lithium-ion batteries. Diam. Relat. Mat. 2019, 98, 9. [Google Scholar] [CrossRef]
- Li, Z.L.; Zhang, K.; Li, M.Y.; Liu, C.L.; Dong, W.S. Pore size-controlled synthesis of 3D hierarchical porous carbon materials for lithium-ion batteries. J. Porous Mat. 2018, 25, 1047–1056. [Google Scholar] [CrossRef]
- Wu, Z.R.; Wang, L.P.; Huang, J.; Zou, J.; Chen, S.L.; Cheng, H.; Jiang, C.; Gao, P.; Niu, X.B. Loofah-derived carbon as an anode material for potassium ion and lithium ion batteries. Electrochim. Acta 2019, 306, 446–453. [Google Scholar] [CrossRef]
- Tang, Y.H.; Chen, J.J.; Wang, X.; Wang, X.X.; Zhao, Y.; Mao, Z.Y.; Wang, D.J. Fabrication of highly N-Doped graphene-like carbon templated from g-C3N4 nanosheets as promising Li-ions battery anode. Electrochim. Acta 2019, 324, 8. [Google Scholar] [CrossRef]
- Yu, C.Y.; Hou, H.Y.; Liu, X.X.; Yao, Y.; Liao, Q.S.; Dai, Z.P.; Li, D.D. Old-loofah-derived hard carbon for long cyclicity anode in sodium ion battery. Int. J. Hydrogen Energy 2018, 43, 3253–3260. [Google Scholar] [CrossRef]
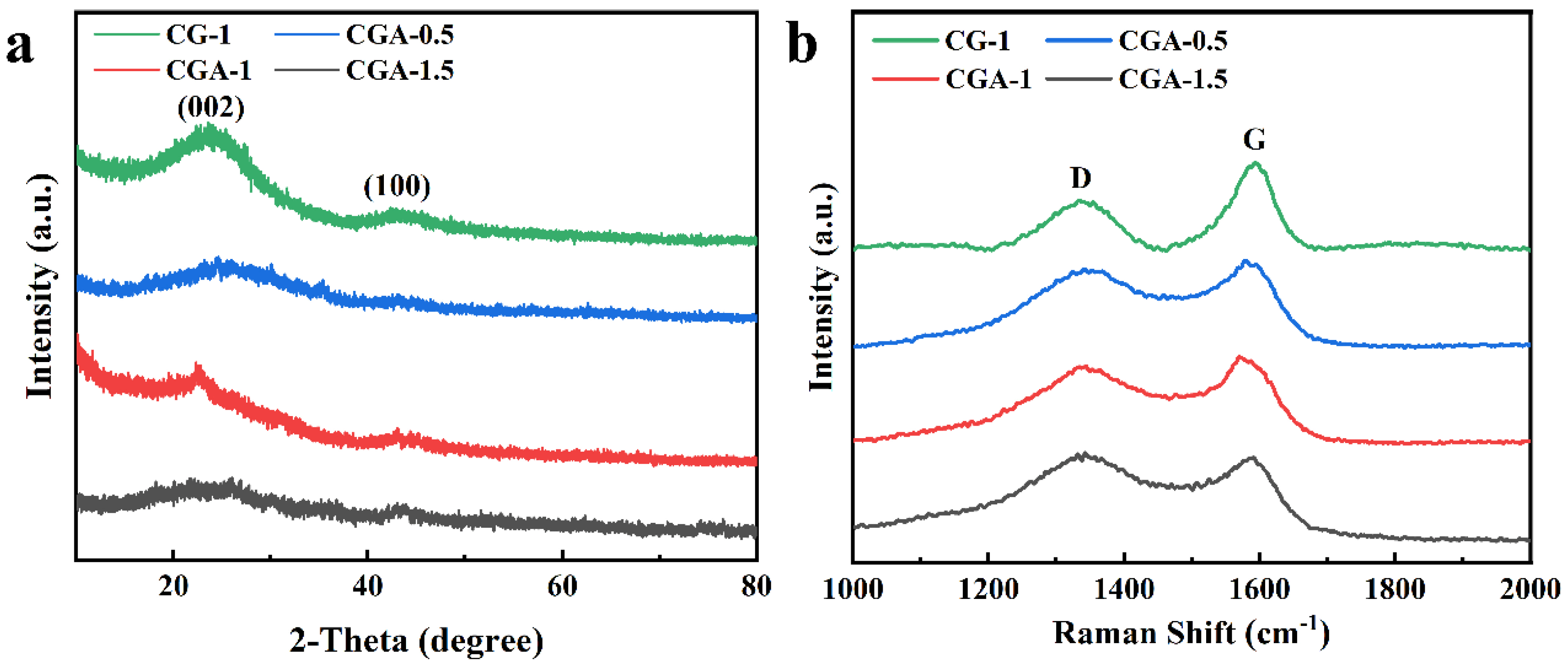




| Sample | d002 (nm) | L (nm) | ID/IG | SBET (m2 g−1) | Vmicro (cm3 g−1) | Vmeso and macro (cm3 g−1) |
|---|---|---|---|---|---|---|
| CG-1 | 0.374 | 1.058 | 0.577 | 1.175 | 0.000 | 0.010 |
| CGA-0.5 | 0.391 | 0.665 | 0.866 | 886.472 | 0.414 | 0.110 |
| CGA-1 | 0.392 | 0.671 | 0.889 | 714.808 | 0.322 | 0.152 |
| CGA-1.5 | 0.394 | 0.696 | 1.056 | 755.076 | 0.359 | 0.190 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Song, L.; Huo, D.; Yang, Y.; Zhang, N.; Liang, J. Cattail-Grass-Derived Porous Carbon as High-Capacity Anode Material for Li-Ion Batteries. Molecules 2023, 28, 4427. https://doi.org/10.3390/molecules28114427
Li H, Song L, Huo D, Yang Y, Zhang N, Liang J. Cattail-Grass-Derived Porous Carbon as High-Capacity Anode Material for Li-Ion Batteries. Molecules. 2023; 28(11):4427. https://doi.org/10.3390/molecules28114427
Chicago/Turabian StyleLi, Hui, Lingyue Song, Dongxing Huo, Yu Yang, Ning Zhang, and Jinglong Liang. 2023. "Cattail-Grass-Derived Porous Carbon as High-Capacity Anode Material for Li-Ion Batteries" Molecules 28, no. 11: 4427. https://doi.org/10.3390/molecules28114427
APA StyleLi, H., Song, L., Huo, D., Yang, Y., Zhang, N., & Liang, J. (2023). Cattail-Grass-Derived Porous Carbon as High-Capacity Anode Material for Li-Ion Batteries. Molecules, 28(11), 4427. https://doi.org/10.3390/molecules28114427





