Structure–Activity Relationships in NHC–Silver Complexes as Antimicrobial Agents
Abstract
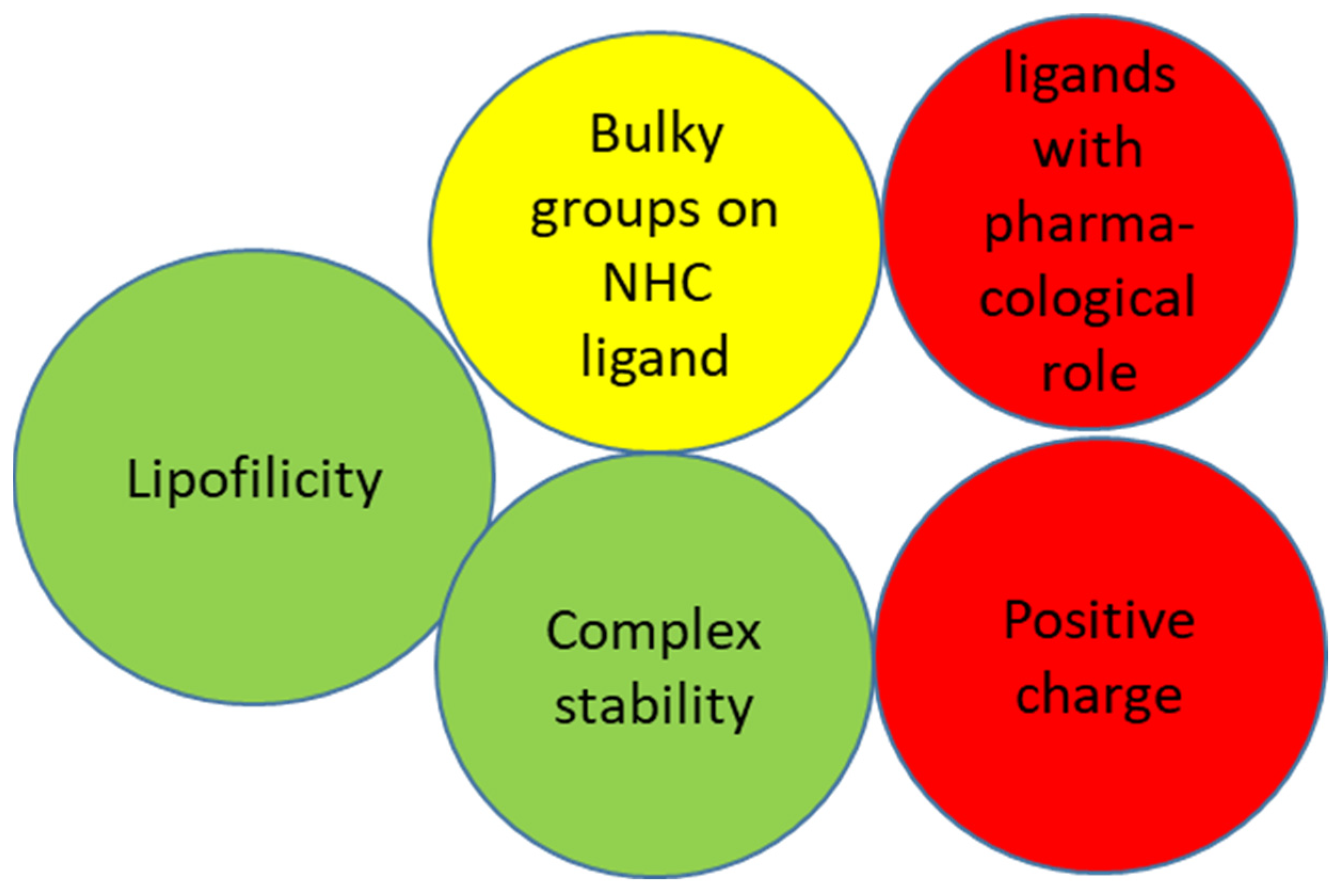
1. Introduction
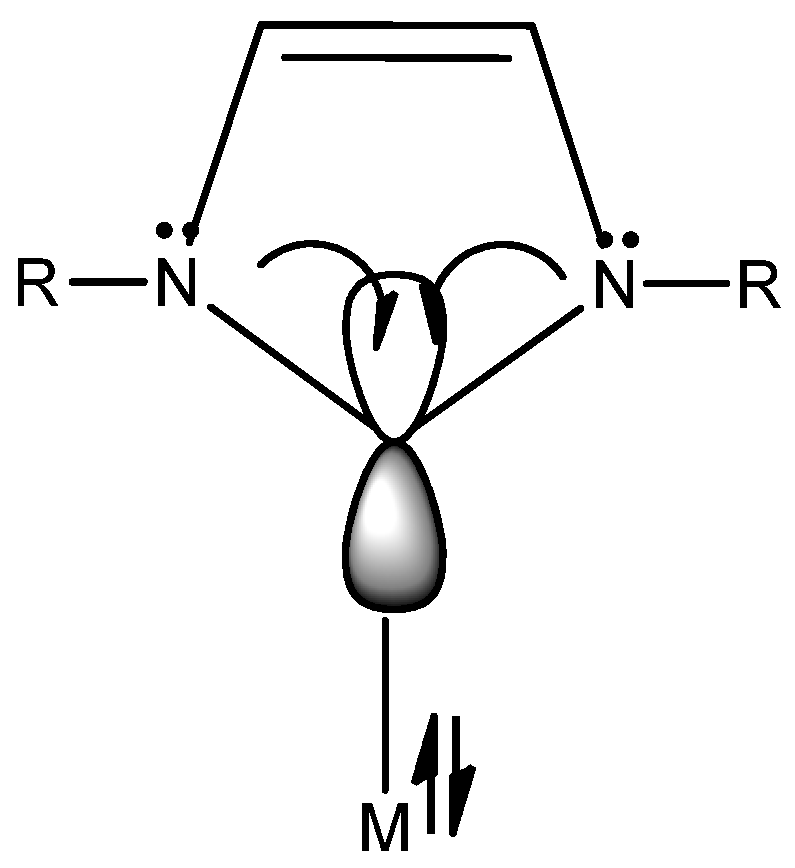
2. N-Heterocyclic Carbenes (NHC) Ligands
3. NHC–Silver Mononuclear Complexes
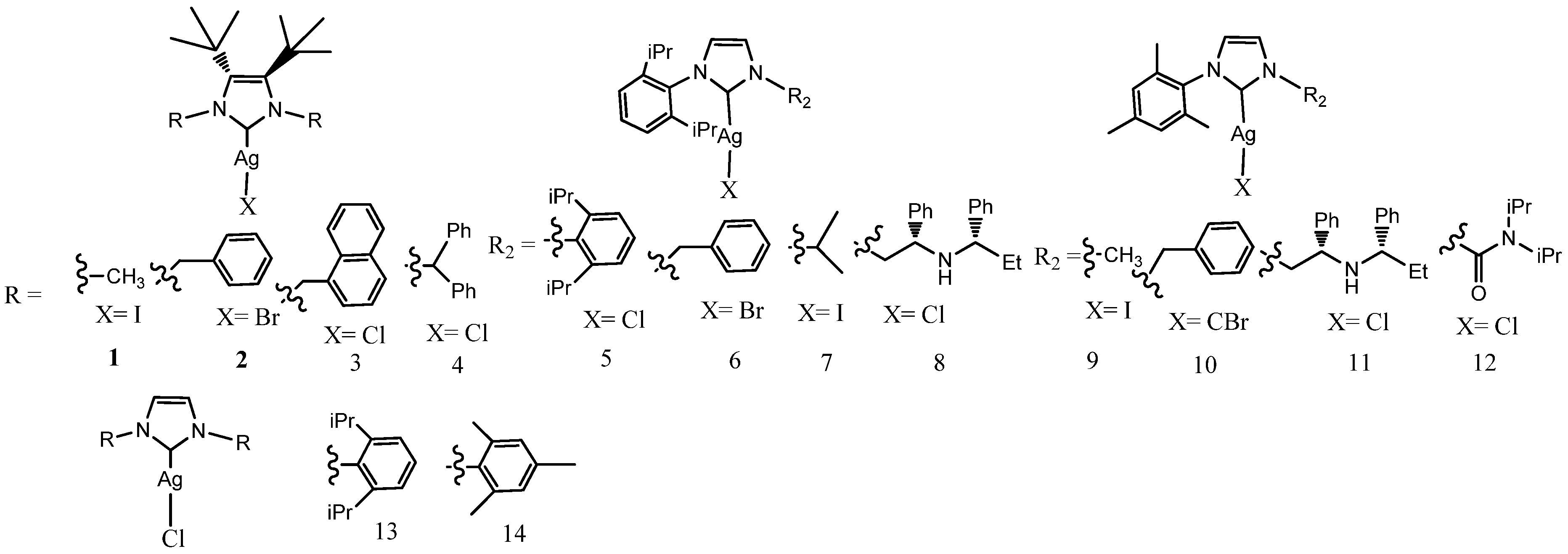
3.1. Benzyl-Substituted NHC–Silver Complexes
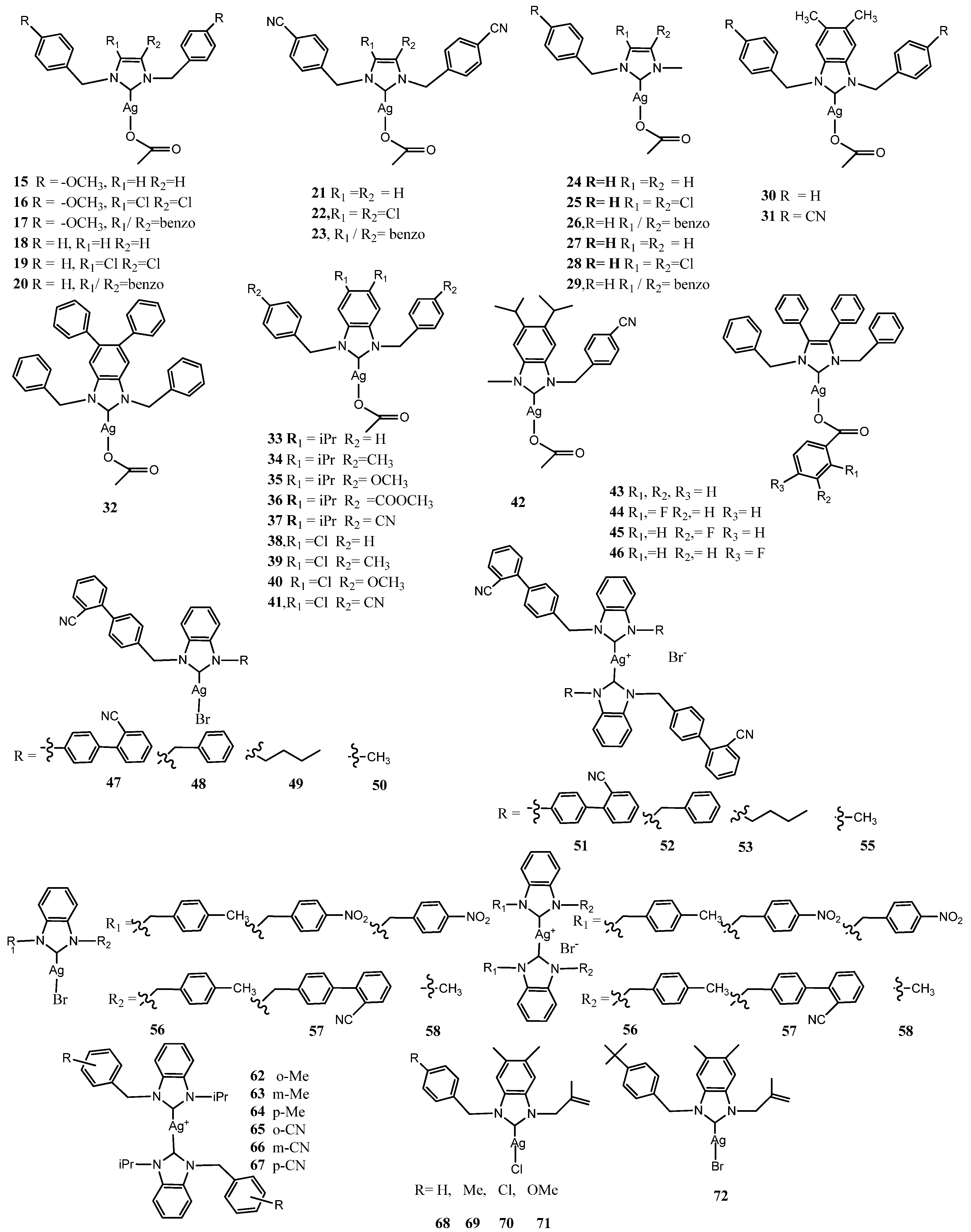
3.2. NHC–Silver Complexes Designed with Steric Hindrance
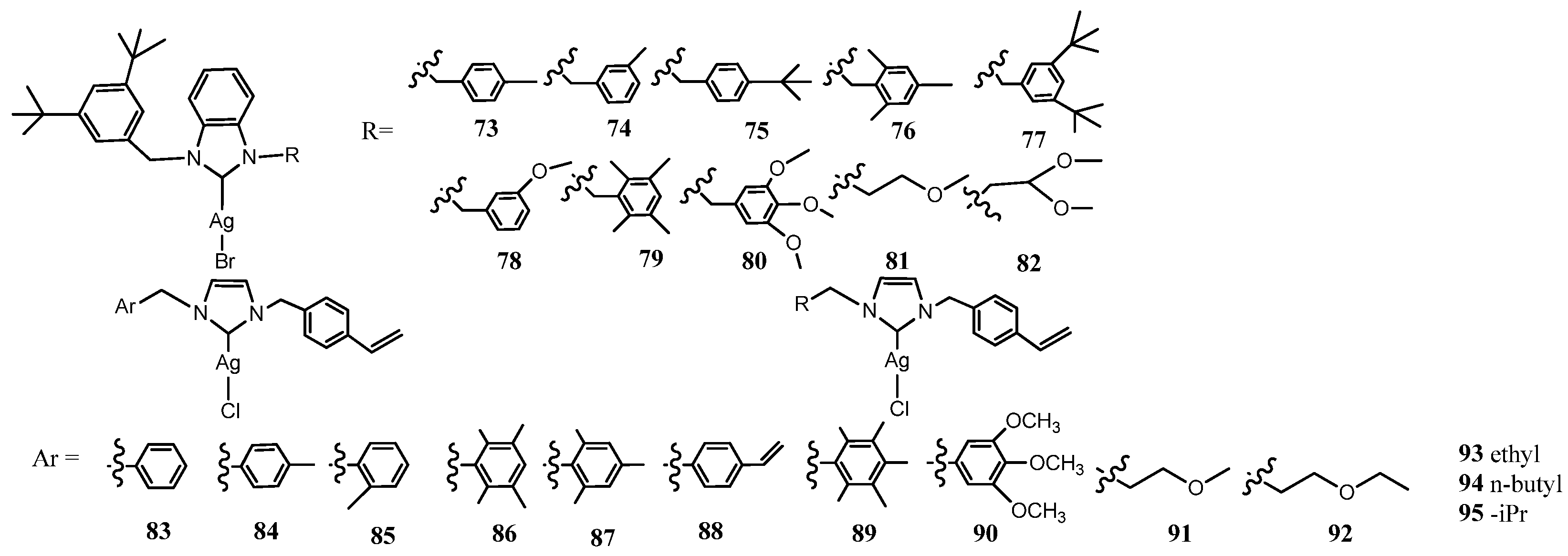
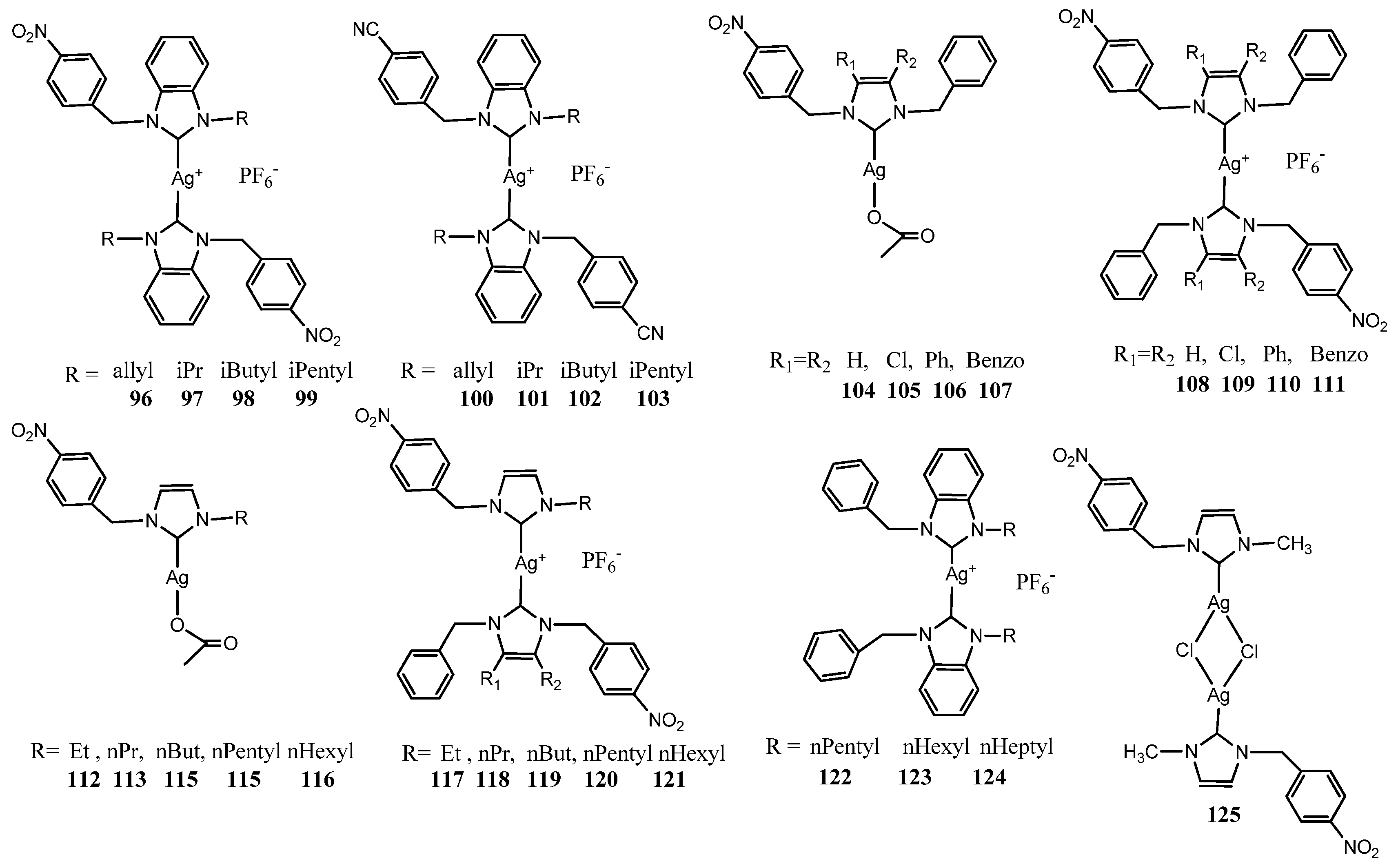
3.3. NHC–Silver Complexes Bearing p–Nitrobenzyl Group
3.4. NHC–Silver Complexes Bearing Naphthalil Group

3.5. NHC–Silver Complexes Bearing Electron Withdrawing Substituents
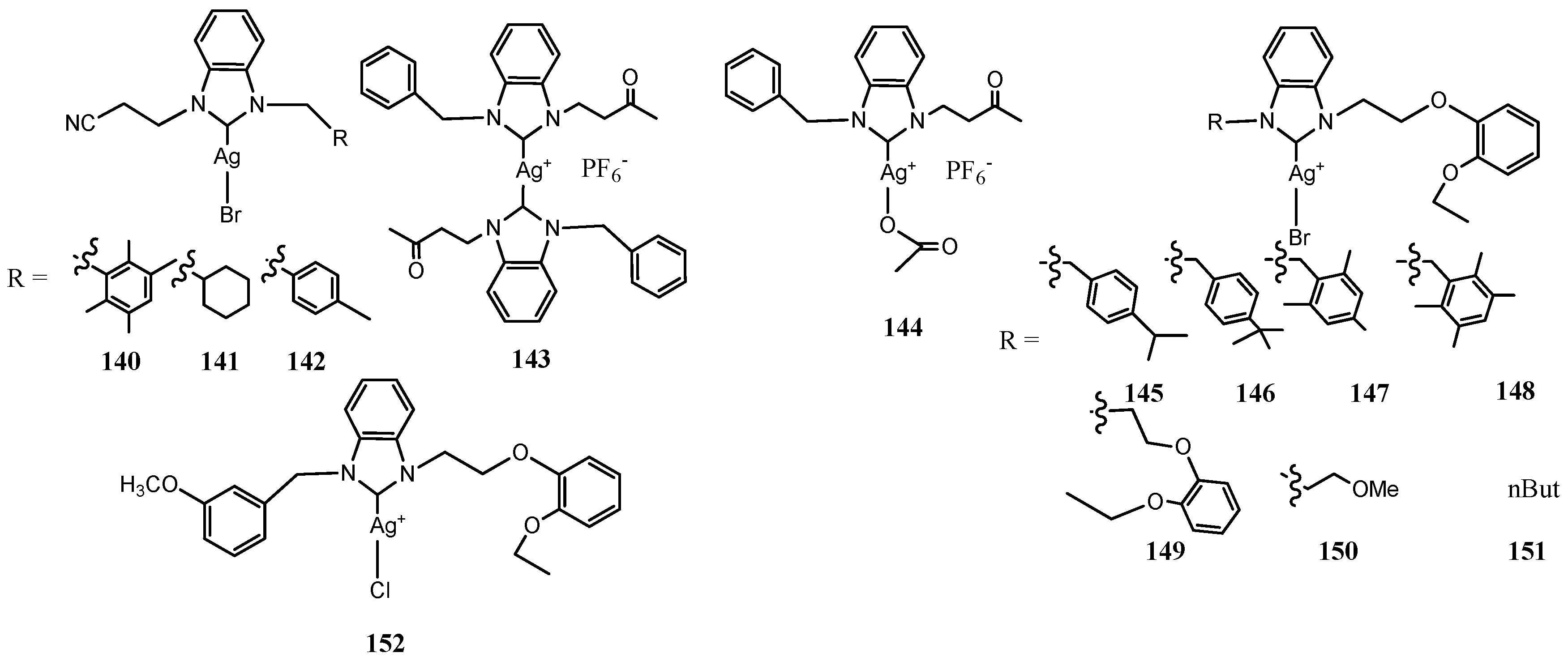
3.6. NHC–Silver Complexes Bearing Aliphatic Substituents
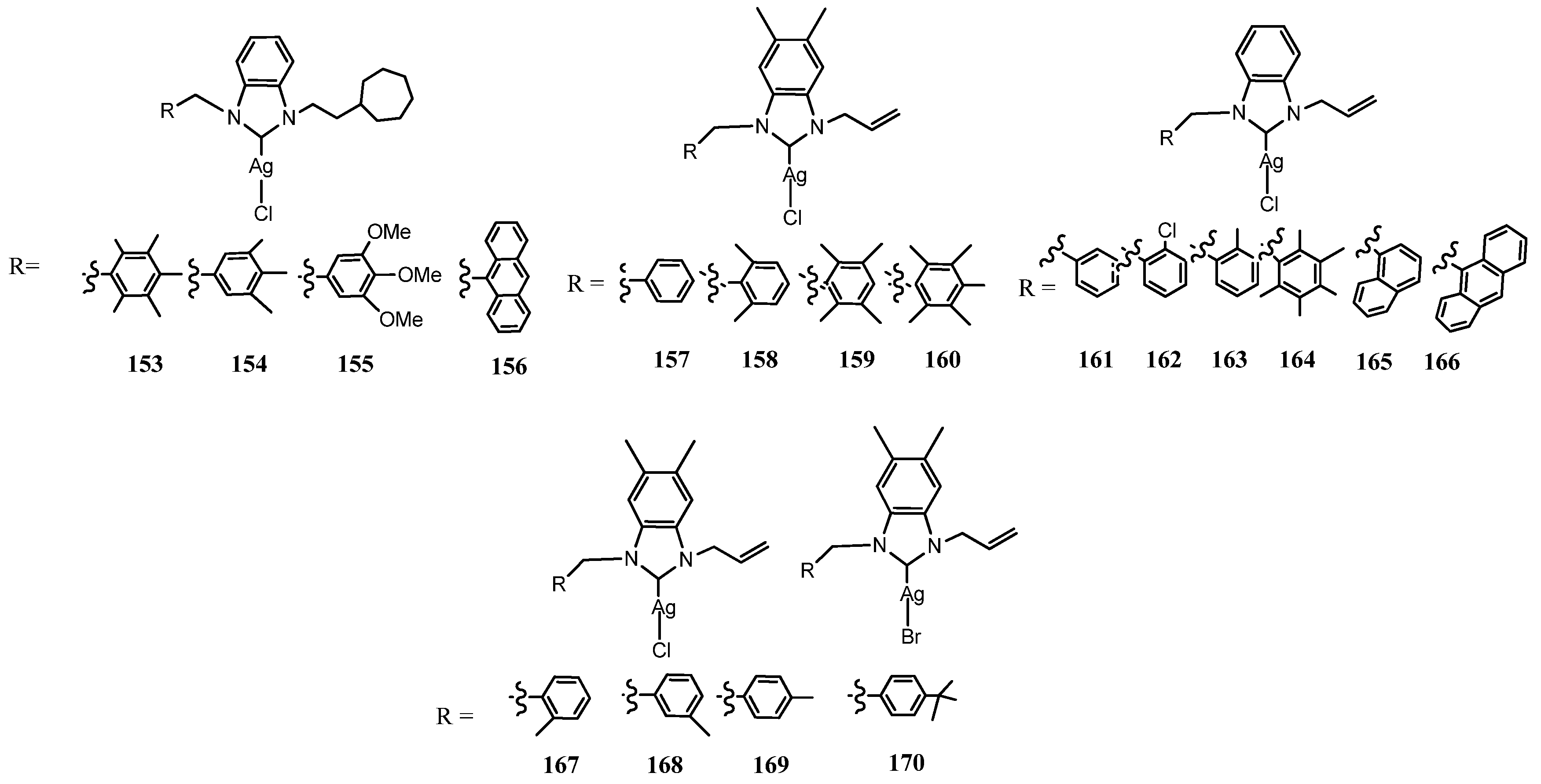
3.7. NHC–Silver Complexes with Nitrogenous Substituents
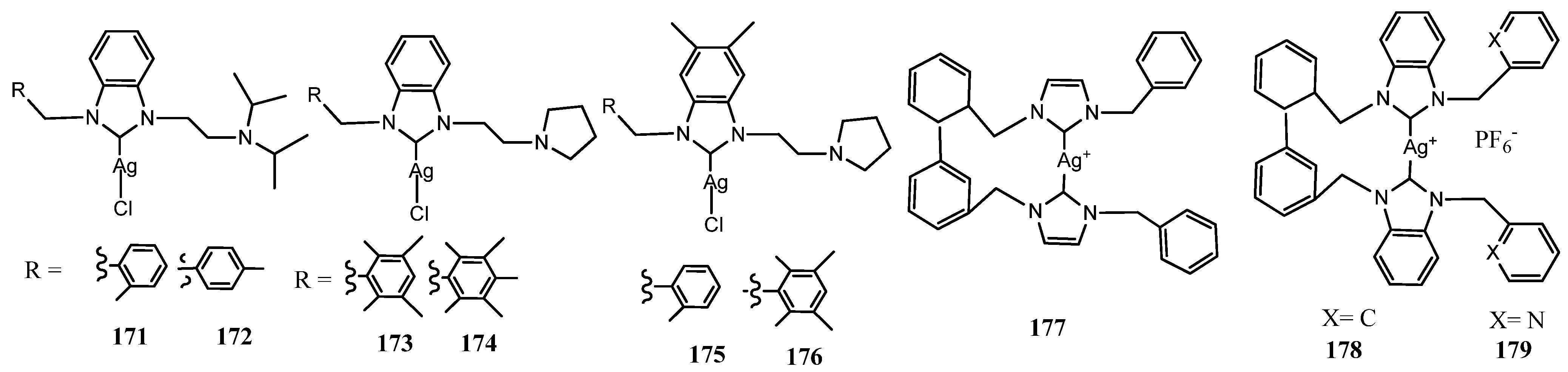
3.8. Unusual NHC–Silver Complexes
| Entry | N° of Tested Strains | Highest Activity Against | Concentration | ZoI or MIC or CA * | Ref. |
|---|---|---|---|---|---|
| 1, 7 | 5 | E. coli | 4 mg mL−1 | MIC | [27] |
| 14 | 5 | S. aureus | 1 mg mL−1 | MIC | [27] |
| 32 | 7 | M. smegmatis | 5 mg mL−1 | MIC | [31] |
| 42 | 2 | S. aureus | 7 mm | CA | [35] |
| 47, 51 | 2 | S. aureus | 6.25 μM | MIC | [37] |
| 57 | 2 | S. aureus | 25 μM | MIC | [38] |
| 62 | 2 | S. aureus | 14 mm at 12 μL | ZoI | [39] |
| 72 | 5 | E. faecalis–C. albicans | 14 μM | MIC | [40] |
| 77 | 4 | All strains | 6.25 μg mL−1 | MIC | [41] |
| 83, 86, 87 | 6 | C. tropicali–C. albicans | 25 μg mL−1 | MIC | [42] |
| 89 | 11 | S. aureus | 19 mm at 50 μL | ZoI | [43] |
| 96 | 2 | S. aureus | 17 mm at 2.85 nM | ZoI | [44] |
| 102 | 2 | S. aureus | 17 mm at 2.85 nM | ZoI | [44] |
| 107 | 6 | S. aureus–E. coli | 8 μgmL−1 | MIC | [45] |
| 110 | 6 | E. coli | 8 μgmL−1 | MIC | [45] |
| 115–116 | 6 | S. aureus–E. coli | 16.0 μgmL−1 | MIC | [46] |
| 120–121 | 6 | S. aureus–E. coli | 16.0 μgmL−1 | MIC | [46] |
| 123–124 | 2 | S. aureus | 12.5 μgmL−1 | MIC | [47] |
| 125 | 4 | S. aureus–P. aeruginosa | 12 mm at 9 μL | ZoI | [48] |
| 126 | 6 | B. subtitis | 16.0 μgmL−1 | MIC | [49] |
| 127 | 6 | S. aureus | 6.25 μgmL−1 | MIC | [51] |
| 128 | 6 | C. albicans–C tropicalis | 6.25 μgmL−1 | MIC | [51] |
| 135 | 8 | E. coli J53 + pMG101 | 8 μgmL−1 | MIC | [52] |
| 137 | 2 | S. aureus | 13.5 mm at 25 μgmL−1 | ZoI | [53] |
| 142 | 9 | S. aureus | 12.5 μgmL−1 | MIC | [54] |
| 143–144 | 2 | S. aureus–E. coli | 31.25 μgmL−1 | MIC | [55] |
| 151 | 6 | E. faecalis–S. aureus | 6.25μgmL−1 | MIC | [56] |
| 153–156 | 6 | C. albicans–C tropicalis | 6.25 μgmL−1 | MIC | [57] |
| 153–156 | 6 | E. faecalis–S. aureus | 6.25 μgmL−1 | MIC | [57] |
| 158, 160 | 4 | E. faecalis–S. aureus–E. coli | 7.8 μgmL−1 | MIC | [58] |
| 161–166 | 6 | E. coli | ≤3.9 μgmL−1 | MIC | [59] |
| 170 | 5 | S. aureus | <1.9 μgmL−1 | MIC | [61] |
| 173 | 6 | L. monocytogenes | 0.24 mgmL−1 | MIC | [62] |
| 177–179 | 3 | P. aeruginosa E322 | 10 ppm | MIC | [63] |
| 182 | 2 | P. aeruginosa | 39 μM | MIC | [64] |
| 188–189 | 2 | E. coli–E.subtitis | 5 μgmL−1 | MIC | [65] |
| 192–194 | 4 | All Strains | <1μM | MIC | [66] |
| 200–201 | 4 | A. baumannii | 2 μg mL−1 | MIC | [67] |
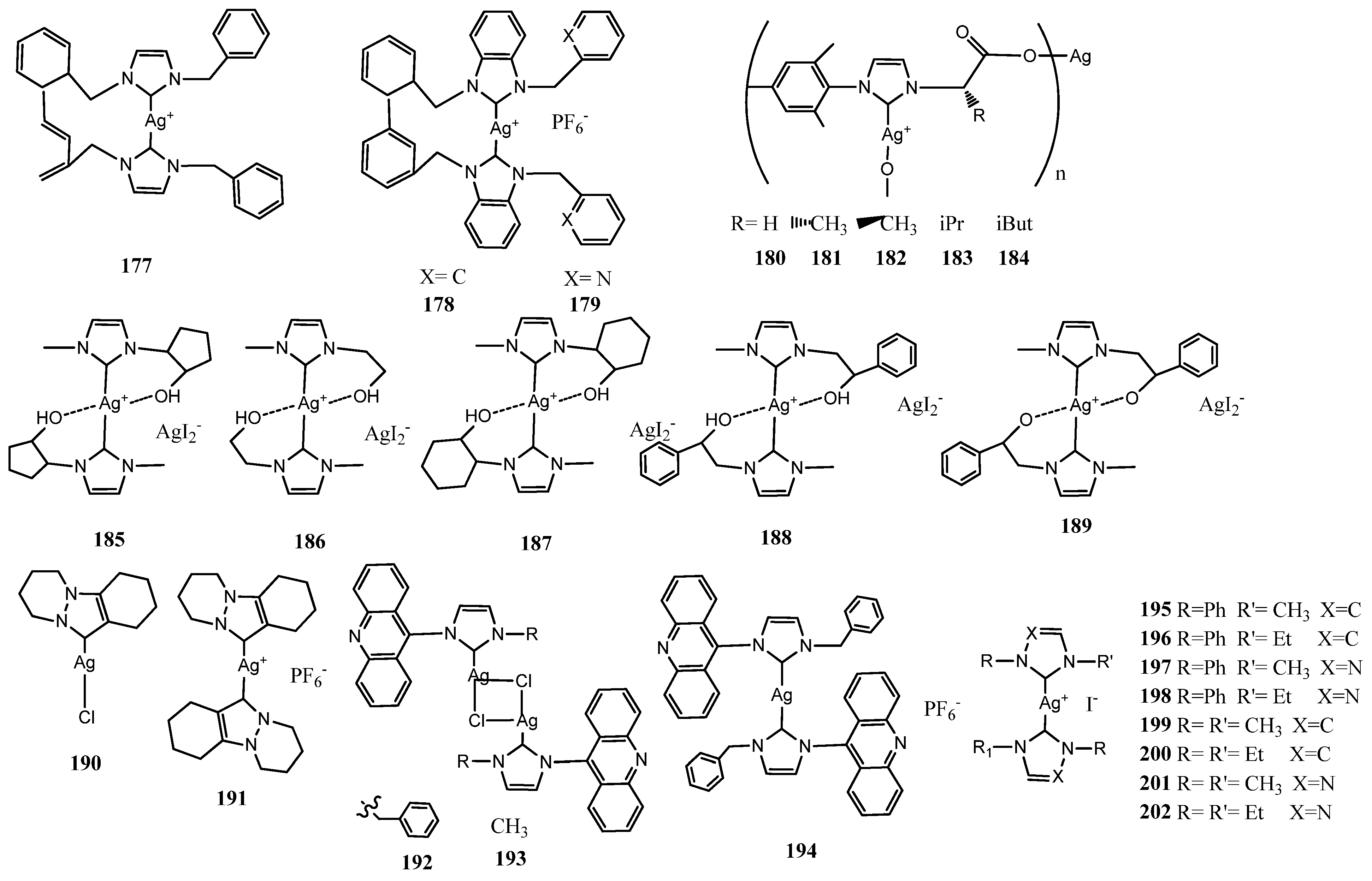
4. NHC–Silver Complex with Ligand in Dual Activity
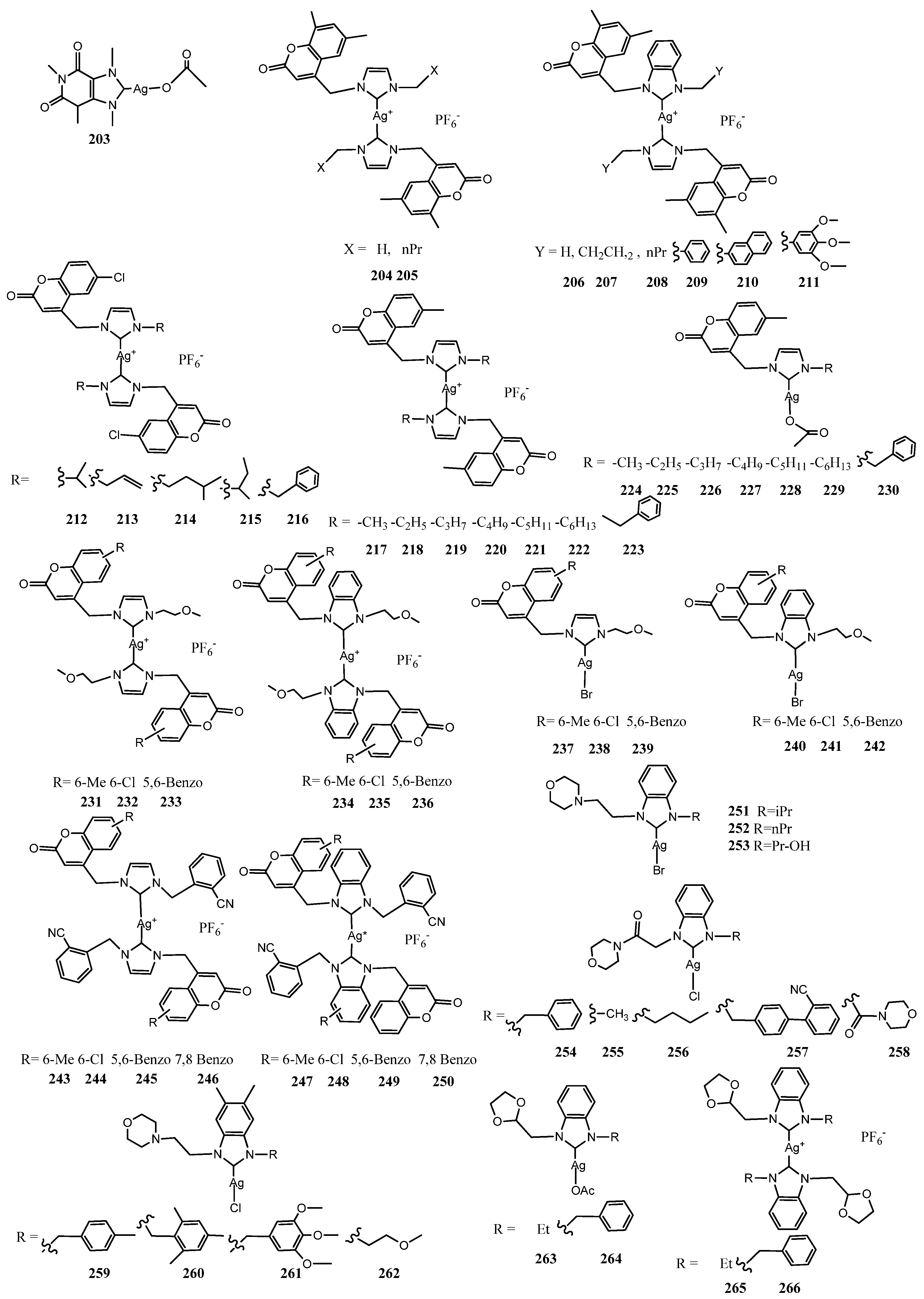
5. Binuclear NHC–Silver Complexes

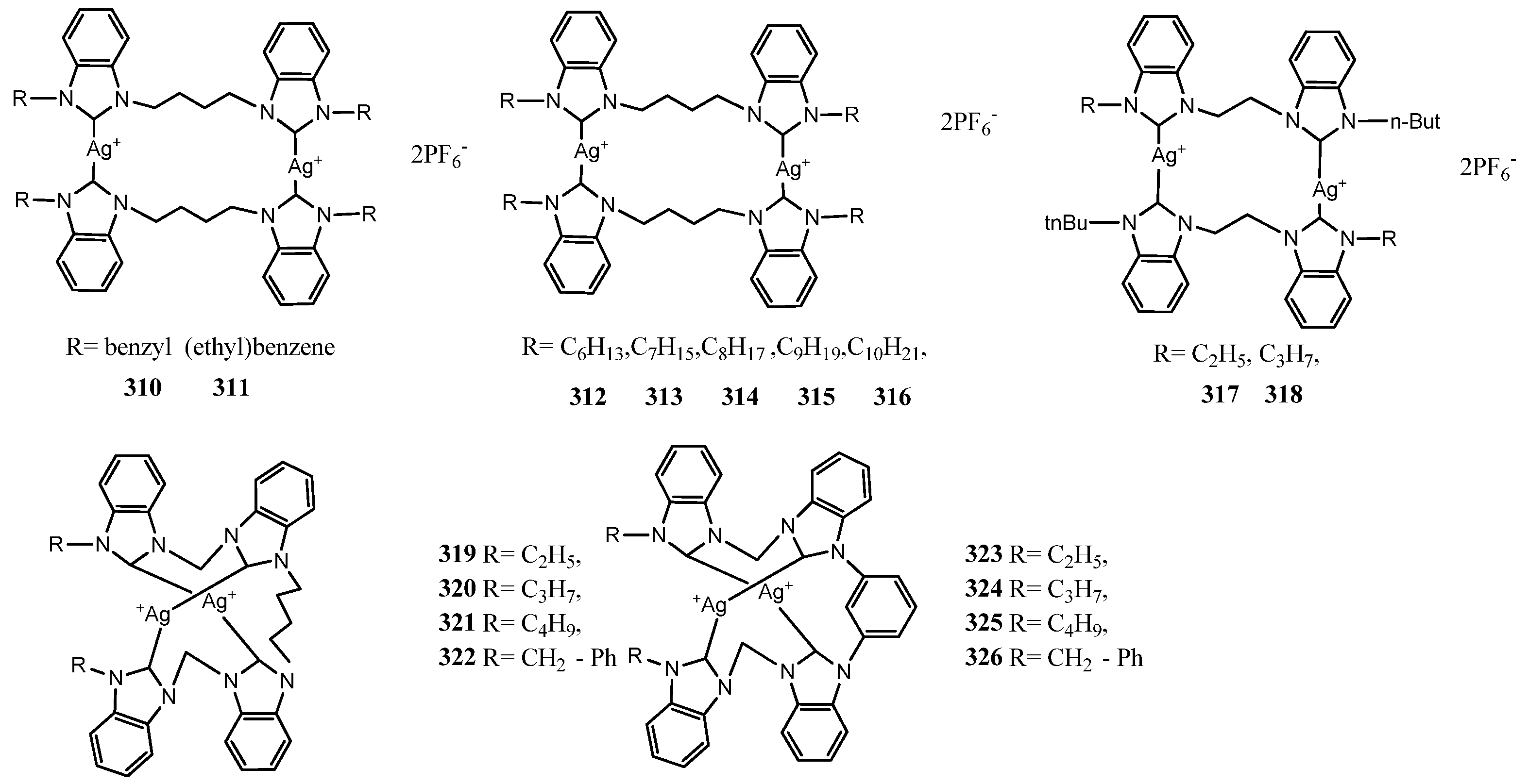
6. Encapsulated Nanosystems Silver–NHC Complexes
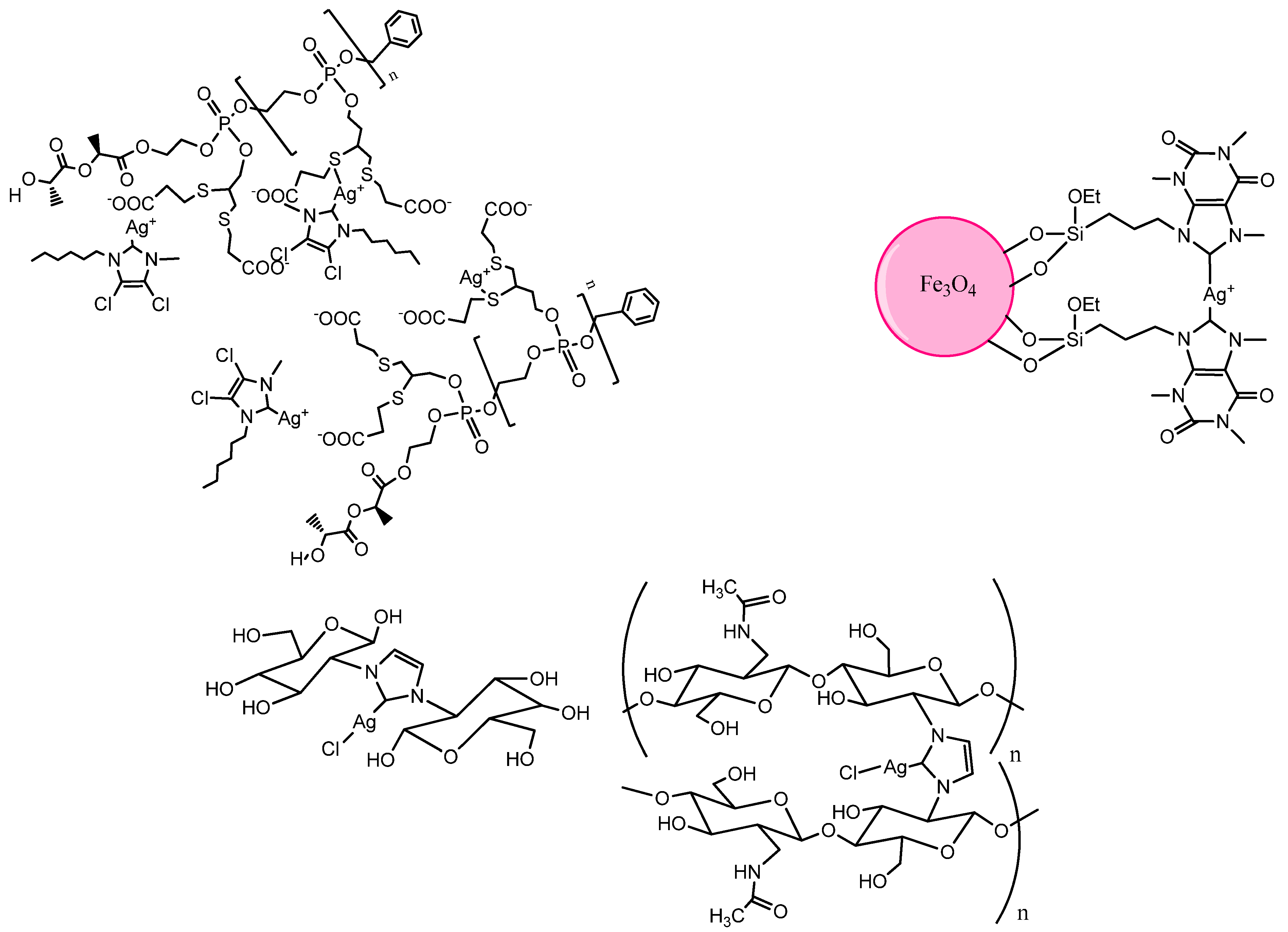
7. Perspective and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Russell, A.D.; Path, F.R.C.; Hugo, W.B. Antimicrobial Activity and Action of Silver. Prog. Med. Chem. 1994, 31, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.; Wood, F. Nanocrystalline silver dressings in wound management: A review. Int. J. Nanomed. 2006, 1, 441–449. [Google Scholar] [CrossRef]
- Von Nägeli, V. Über oligodynamische Erscheinungen in lebenden Zellen. Deut. Schr. Schweiz. Naturforsch. Ges. 1893, 33, 174–182. [Google Scholar]
- Lansdown, A.B.G. A review of the use of silver in wound care: Facts and fallacies. Br. J. Nurs. 2004, 13, S6. [Google Scholar] [CrossRef]
- Fox, C.L., Jr. Silver Sulfadiazine—A New Topical Therapy for Pseudomonas in Burns Therapy of Pseudomonas Infection in Burns. Arch. Surg. 1968, 96, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Silver, S. Bacterial silver resistance: Molecular biology and uses and misuses of silver compounds. FEMS Microbiol. Rev. 2003, 27, 341–353. [Google Scholar] [CrossRef]
- Gupta, A.; Maynes, M.; Silver, S. Effects of Halides on Plasmid-Mediated Silver Resistance in Escherichia coli. Appl. Environ. Microbiol. 1998, 64, 5042–5045. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B.G. Silver I: Its antibacterial properties and mechanism of action. J. Wound Care 2002, 11, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio silver; its interactions with peptides and bacteria, and its use in medicine. Chem. Rev. 2013, 33, 139–148. [Google Scholar] [CrossRef]
- Duran, N.; Durán, M.; Bispo de Jesus, M.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles; a new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Wells, T.N.C.; Scully, P.; Paravicini, G.; Proudfoot, A.E.I.; Payton, M.A. Mechanism of Irreversible Inactivation of Phosphomannose Isomerases by Silver Ions and Flamazine. Biochemistry 1995, 34, 7896–7903. [Google Scholar] [CrossRef] [PubMed]
- Morones Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver enhances antibiotic activity against Gram-negative bacteria. Sci. Trans. Med. 2013, 5, 190ra181. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Wang, H.; Yan, A.; Liu, Z.; Yang, X.; Xu, Z.; Wang, Y.; Wang, R.; Koohi-Moghadam, M.; Hu, L.; Xia, W.; et al. Deciphering molecular mechanism of silver by integrated omic approaches enables enhancing its antimicrobial efficacy in E. coli. PLoS Biol. 2019, 17, e3000292. [Google Scholar] [CrossRef] [PubMed]
- Graham, C. The role of silver in wound healing. Br. J. Nurs. 2005, 14, S22–S28. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, L.; Cianci, M.; Gonzales Vara, A.; Ciurli, S. The structure of urease inactivated by Ag(I): A new paradigm for enzyme inhibition by heavy metals. Dalton Trans 2018, 47, 8240–8247. [Google Scholar] [CrossRef]
- Arduengo, A.J., III; Harlow, R.L.; Kline, M. A Stable crystalline carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- de Frémont, P.; Marion, N.; Nolan, S.P. Carbenes: Synthesis, properties, and organometallic chemistry. Coord. Chem. Rev. 2009, 253, 862–892. [Google Scholar] [CrossRef]
- Bourissou, D.; Guerret, O.; Gabbaϊ, F.P.; Bertrand, G. Stable Carbenes. Chem. Rev. 2000, 100, 39–92. [Google Scholar] [CrossRef]
- Fantasia, S.; Petersen, J.L.; Jacobsen, H.; Cavallo, L.; Nolan, S.P. Electronic Properties of N-Heterocyclic Carbene (NHC) Ligands: Synthetic, Structural, and Spectroscopic Studies of (NHC)Platinum(II) Complexes. Organometallics 2007, 26, 5880–5889. [Google Scholar] [CrossRef]
- Gusev, D.G. Electronic and Steric Parameters of 76 N-Heterocyclic Carbenes in Ni(CO)3(NHC). Organometallics 2009, 28, 6458–6461. [Google Scholar] [CrossRef]
- Satija, G.; Sharma, B.; Madan, A.; Iqubal, A.; Shaquiquzzaman, M.; Akhter, M.; Parvez, S.; Khan, M.A.; Alam, M.M. Benzimidazole based derivatives as anticancer agents: Structure activity relationship analysis for various targets. J. Heter. Chem. 2022, 59, 22–66. [Google Scholar] [CrossRef]
- Song, D.; Ma, S. Recent Development of Benzimidazole-Containing Antibacterial Agents. ChemMedChem 2016, 16, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Melaiye, A.; Simons, R.S.; Milsted, A.; Pingitore, F.; Wesdemiotis, C.; Tessier, C.A.; Youngs, W.J. Formation of water-soluble pincer silver(I)-carbene complexes: A novel antimicrobial agent. J. Med. Chem. 2004, 47, 973–977. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, S.A.; Patil, R.; Keri, R.S.; Budagumpi, S.; Balakrishna, G.R.; Tacke, M. N-heterocyclic carbene metal complexes as bioorganometallic antimicrobial and anticancer drugs. Future Med. Chem. 2015, 7, 1305–1333. [Google Scholar] [CrossRef]
- Kascatan-Nebioglu, A.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. N-Heterocyclic carbene–silver complexes: A new class of antibiotics. Coord. Chem. Rev. 2007, 251, 884–895. [Google Scholar] [CrossRef]
- Roland, S.; Jolivalt, C.; Cresteil, T.; Eloy, L.; Bouhours, P.; Hequet, A.; Mansuy, V.; Vanucci, C.; Paris, J.M. Investigation of a series of Silver-N-heterocyclic carbenes as antibacterial agents: Activity, synergistic effects, and cytotoxicity. Chem. Eur. J. 2011, 17, 1442–1446. [Google Scholar] [CrossRef]
- Patil, S.; Claffey, J.; Deally, A.; Hogan, M.; Gleeson, B.; Miguel, L.; Méndez, M.; Müller-Bunz, H.; Paradisi, F.; Tacke, M. Synthesis, cytotoxicity and antibacterial studies of p-methoxybenzylsubstituted and benzyl-substituted N-heterocyclic carbene–silver complexes. Eur. J. Inorg. Chem. 2010, 7, 1020–1031. [Google Scholar] [CrossRef]
- Patil, S.; Deally, A.; Gleeson, B.; Müller-Bunz, H.; Paradisi, F.; Tacke, M. Synthesis, cytotoxicity and antibacterial studies of symmetrically and non-symmetrically benzyl- or p-Cyanobenzyl-substituted N-heterocyclic Carbene–Silver complexes. Appl. Organomet. Chem. 2010, 24, 781–793. [Google Scholar] [CrossRef]
- Patil, S.; Deally, A.; Gleeson, B.; Müller-Bunz, H.; Paradisi, F.; Tacke, M. Novel benzyl-substituted N-heterocyclic carbene–silver acetate complexes: Synthesis, cytotoxicity and antibacterial studies. Metallomics 2011, 3, 74–88. [Google Scholar] [CrossRef]
- Sharkey, M.A.; O’Gara, J.P.; Gordon, S.V.; Hackenberg, F.; Healy, C.; Paradisi, F.; Patil, S.; Schaible, B.; Tacke, M. Investigations into the Antibacterial Activity of the Silver-Based Antibiotic Drug Candidate SBC3. Antibiotics 2012, 1, 25–28. [Google Scholar] [CrossRef]
- Browne, N.; Hackenberg, F.; Streciwilk, W.; Tacke, M.; Kavanagh, K. Assessment of in vivo antimicrobial activity of the carbene silver(I) acetate derivative SBC3 using Galleria mellonella larvae. Biometals 2014, 27, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Piatek, M.; O’Beirne, C.; Beato, Z.; Tacke, M.; Kavanagh, K. Exposure of Candida parapsilosis to the silver (I) compound SBC3 induces alterations in the proteome and reduced virulence. Metallomics 2022, 14, mfac046. [Google Scholar] [CrossRef] [PubMed]
- Piatek, M.; O’Beirne, C.; Beato, Z.; Tacke, M.; Kavanagh, K. Pseudomonas aeruginosa and Staphylococcus aureus Display Differential Proteomic Responses to the Silver(I) Compound, SBC3. Antibiotics 2023, 12, 348. [Google Scholar] [CrossRef] [PubMed]
- Streciwilk, W.; Cassidy, J.; Hackenberg, F.; Müller-Bunz, H.; Paradisi, F.; Tacke, M. Synthesis, cytotoxic and antibacterial studies of p-benzyl-substituted NHC-silver(I) acetate compounds derived from 4,5-di-pdiisopropylphenyl-or 4,5-di-p-chlorophenyl-1H-imidazole. J. Organomet. Chem. 2014, 749, 88–99. [Google Scholar] [CrossRef]
- O’Beirne, C.; Alhamad, N.F.; Ma, Q.; Müller-Bunz, H.; Kavanagh, K.; Buder, G.; Zhu, X.; Tacke, M. Synthesis, structures and antimicrobial activity of novel NHC* and Ph3P-Ag(I)-benzoate derivatives. Inorg. Chim. Acta 2019, 486, 294–303. [Google Scholar] [CrossRef]
- Aher, S.; Das, A.; Muskawar, P.; Osborne, J.; Bhagat, P. Silver (I) complexes of imidazolium based N-heterocyclic carbenes for antibacterial applications. J. Mol. Liq. 2017, 231, 396–403. [Google Scholar] [CrossRef]
- Aher, S.; Das, A.; Muskawar, P.; Osborne, J.; Bhagat, P. Synthesis, sharacterization and antimicrobial properties of methylbenzyl and nitrobenzyl containing imidazolium-based silver N-heterocyclic carbenes. J. Mol. Liq. 2017, 233, 270–277. [Google Scholar] [CrossRef]
- Haque, R.A.; Haziz, U.F.M.; Abdullah, A.A.; Shaheeda, N.; Razali, M.R. New non-functionalized and nitrile-functionalized benzimidazolium salts and their silver(I) complexes: Synthesis, crystal structures and antibacterial studies. Polyhedron 2016, 109, 208–217. [Google Scholar] [CrossRef]
- Sahin, N.; Üstün, E.; Tutar, U.; Çelik, C.; Gürbüz, N.; Özdemir, I. Antimicrobial activity, inhibition of biofilm formation, and molecular docking study of novel Ag-NHC complexes. J. Organomet. Chem. 2021, 954–955, 122082. [Google Scholar] [CrossRef]
- Kaloğlu, N.; Özdemir, I.; Günal, S.; Özdemir, I. Synthesis and antimicrobial activity of bulky 3,5-di-tert-butyl substituent-containing silver–N-heterocyclic carbene complexes. Appl. Organomet. Chem. 2017, 31, e3803. [Google Scholar] [CrossRef]
- Gök, Y.; SarJ, Y.; Akkoç, S.; Özdemir, E.; Günal, S. Antimicrobial Studies of N-Heterocyclic Carbene Silver Complexes Containing Benzimidazol-2-ylidene Ligand. Int. J. Inorg. Chem. 2014, 2014, 191054. [Google Scholar] [CrossRef]
- Gök, Y.; Akkoc, S.; Erdoğan, H.; Albayrak, S. In Vitro antimicrobial studies of new benzimidazolium salts and silver N-heterocyclic carbene complexes. J. Enzyme Inhib. Med. Chem. 2016, 31, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Subramanya Prasad, T.V.; Shahini, C.R.; Patil, S.A.; Huang, X.; Bugarin, A.; Patil, S.A. Non-symmetrically p-nitrobenzyl- and p-cyanobenzyl-substituted N-heterocyclic carbene-silver(I) complexes: Synthesis, characterization and antibacterial studies. J. Coord. Chem. 2017, 70, 600–614. [Google Scholar] [CrossRef]
- Shahini, C.R.; Achar, G.; Budagumpi, S.; Tacke, M.; Patil, S.A. Non-symmetrically p-nitrobenzyl-substituted N-heterocyclic carbene-silver(I) complexes as metallopharmaceutical agents. Appl. Organomet. Chem. 2017, 31, e3819. [Google Scholar] [CrossRef]
- Shahini, C.R.; Achar, G.; Budagumpi, S.; Tacke, M.; Patil, S.A. Synthesis, structural investigation and antibacterial studies of non-symmetrically p–nitrobenzyl substituted benzimidazole N-heterocyclic carbene-silver(I) complexes. Inorg. Chim. Acta 2017, 466, 432–441. [Google Scholar] [CrossRef]
- Asekunowo, P.O.; Haque, R.A.; Razali, M.R. Sterically modulated silver(I) complexes of N-benzyl-substituted N-heterocyclic carbenes: Synthesis, crystal structures and bioactivity. Transit. Met. Chem. 2015, 40, 79–88. [Google Scholar] [CrossRef]
- Carpio-Granillo, M.; Zuno-Cruz, F.J.; Sanchez-Cabrera, G.; Rojo-Gomez, E.G.; Gonzalez-Abrego, D.O.; Coronel-Olivares, C.; Caviedes, M.F.; Andrade-Lopez, N.; Rosales-Hoz, M.J.; Leyva, M.A. p-Nitrobenzyl-substituted N–heterocyclic carbene in Silver(I) and Gold(I) complexes and their antibacterial activities. Polyhedron 2022, 217, 115726. [Google Scholar] [CrossRef]
- Streciwilk, W.; Terenzi, A.; Lo Nardo, F.; Prochnow, P.; Bandow, J.E.; Keppler, B.K.; Ott, I. Synthesis and biological evaluation of organometallic complexes bearing bis-1,8-naphthalimide ligands. Eur. J. Inorg. Chem. 2018, 26, 3104–3112. [Google Scholar] [CrossRef]
- Lu, J.; Vlamis-Gardikas, A.; Kandasamy, K.; Zhao, R.; Gustafsson, T.N.; Engstrand, L.; Hoffner, S.; Engman, L.; Holmgren, A. Inhibition of bacterial thioredoxin reductase: An antibiotic mechanism targeting bacteria lacking glutathione. FASEB J. 2013, 27, 1394–1403. [Google Scholar] [CrossRef]
- Gök, Y.; Akkoç, S.; Çelikal, Ö.Ö.; Özdemir, I.; Günal, S. In Vitro antimicrobial studies of naphthalen-1-ylmethyl substituted silver N-heterocyclic carbene complexes. Arab. J. Chem. 2019, 12, 2513–2518. [Google Scholar] [CrossRef]
- Wright, B.D.; Shah, P.N.; McDonald, L.J.; Shaeffer, M.L.; Wagers, P.O.; Panzner, M.J.; Smolen, J.; Tagaev, J.; Tessier, C.A.; Cannon, C.L.; et al. Synthesis, characterization, and antimicrobial activity of silver carbene complexes derived from 4,5,6,7-tetrachlorobenzimidazole against antibiotic resistant bacteria. Dalton Trans. 2012, 41, 6500–6506. [Google Scholar] [CrossRef] [PubMed]
- Asekunowo, P.O.; Haque, R.A.; Razali, M.R.; Avicor, S.W.; Wajidi, M.F.F. Synthesis and characterization of nitrile functionalized silver(I)-N-heterocyclic carbene complexes: DNA binding, cleavage studies, antibacterial properties and mosquitocidal activity against the dengue vector, aedes albopictus. Eur. J. Med. Chem. 2018, 150, 601–615. [Google Scholar] [CrossRef]
- Türker, D.; Üstün, E.; Günal, S.; Yıldız, H.; Düşünceli, S.D.; Özdemir, İ. Cyanopropyl functionalized benzimidazolium salts and their silver N-heterocyclic carbene complexes: Synthesis, antimicrobial activity, and theoretical analysis. Arch. Pharm. 2022, 355, e2200041. [Google Scholar] [CrossRef]
- Haque, R.A.; Iqbal, M.A.; Mohamad, F.; Razali, M.R. Antibacterial and DNA cleavage activity of carbonyl functionalized N-heterocyclic carbene-silver(I) and selenium compounds. J. Mol. Struct. 2018, 1155, 362–370. [Google Scholar] [CrossRef]
- Kaloğlu, M.; Kaloğlu, N.; Özdemir, İ.; Günal, S.; Özdemir, İ. Novel benzimidazol-2-ylidene carbene precursors and their silver(I) complexes: Potential antimicrobial agents. Bioorg. Med. Chem. 2016, 24, 3649–3656. [Google Scholar] [CrossRef]
- Karataş, M.O.; Günal, S.; Mansur, A.; Alıcı, B.; Çetinkaya, E. Synthesis and antimicrobial properties of cycloheptyl substituted benzimidazolium salts and their silver(I) carbene complexes. J. Heter. Commun. 2016, 22, 357–361. [Google Scholar] [CrossRef]
- Üstün, E.; Şahin, N.; Çelik, C.; Tutar, U.; Özdemir, N.; Gürbüz, N.; Özdemir, İ. Synthesis, characterization, antimicrobial and antibiofilm activity, and molecular docking analysis of NHC precursors and their Ag-NHC complexes. Dalton Trans. 2021, 50, 15400–15412. [Google Scholar] [CrossRef]
- Tutar, U.; Celik, C.; Sahin, N. Allyl Functionalized Benzimidazolium-Derived Ag(I)-N-Heterocyclic Carbene Complexes: Anti-Biofilm and Antimicrobial Properties. Pharm. Chem. J. 2022, 56, 54–60. [Google Scholar] [CrossRef]
- Achar, G.; Hokrani, P.P.; Brinda, K.N.; Małecki, J.G.; Budagumpi, S. Synthesis, characterization, crystal structure and antibacterial properties of N– and O–functionalized (benz)imidazolium salts and their N–heterocyclic carbene silver(I) complexes. J. Mol. Struct. 2019, 1196, 627–636. [Google Scholar] [CrossRef]
- Çelik, C.; Üstün, E.; Şahin, N.; Tutar, U. Antimicrobial and antibiofilm activities and bovine serum albumin binding properties of benzimidazolium derivative NHC salts and their Ag(I)-NHC complexes. Appl. Organomet. Chem. 2022, 36, e6891. [Google Scholar] [CrossRef]
- Mnasri, A.; Mejri, A.; Al-Hazmy, S.M.; Arfaoui, Y.; Özdemir, I.; Gürbüz, N.; Hamdi, N. Silver–N-heterocyclic carbene complexes-catalyzed multicomponent reactions: Synthesis, spectroscopic characterization, density functional theory calculations, and antibacterial study. Arch. Pharm. 2021, 354, e2100111. [Google Scholar] [CrossRef]
- Muniyappan, N.; Advaya, G.R.; Sujitha, E.; Sabiah, S. Picolyl and benzyl functionalized biphenyl NHC carbenes and their silver complexes: Sigma donating and antimicrobial properties. J. Organomet. Chem. 2021, 954–955, 122075. [Google Scholar] [CrossRef]
- Sánchez, A.; Carrasco, C.J.; Montilla, F.; Álvarez, E.; Galindo, A.; Pérez-Aranda, M.; Pajuelo, E.; Alcudia, A. Antimicrobial Properties of Amino-Acid-Derived N-Heterocyclic Carbene Silver Complexes. Pharmaceutics 2022, 14, 748. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, M.; Saturnino, C.; Cianciulli, E.I.; Varcamonti, M.; Zanfardino, A.; Tommonaro, G.; Longo, P. Silver (I) N-heterocyclic carbene complexes: Synthesis, characterization and antibacterial activity. J. Organomet. Chem. 2013, 725, 46–53. [Google Scholar] [CrossRef]
- Prencipe, F.; Zanfardino, A.; Di Napoli, M.; Rossi, F.; D’Errico, S.; Piccialli, G.; Mangiatordi, G.F.; Saviano, M.; Ronga, L.; Varcamonti, M.; et al. Silver (I) N-Heterocyclic Carbene Complexes: A Winning and Broad Spectrum of Antimicrobial Properties. Int. J. Mol. Sci. 2021, 22, 2497. [Google Scholar] [CrossRef]
- Mather, J.C.; Wyllie, J.A.; Hamilton, A.; Soares da Costa, T.P.; Barnard, P.J. Antibacterial silver and gold complexes of imidazole and 1,2,4-triazole derived N-heterocyclic carbenes. Dalton Trans. 2022, 51, 12056. [Google Scholar] [CrossRef] [PubMed]
- Kascatan-Nebioglu, A.; Melaiye, A.; Hindi, K.; Durmus, S.; Panzner, M.J.; Hogue, L.A.; Mallett, R.J.; Hovis, C.E.; Coughenour, M.; Crosby, S.D.; et al. Synthesis from Caffeine of a Mixed N-Heterocyclic Carbene–Silver Acetate Complex Active against Resistant Respiratory Pathogens. J. Med. Chem. 2006, 49, 6811–6818. [Google Scholar] [CrossRef] [PubMed]
- Karatas, M.O.; Olgundeniz, B.; Günal, S.; Özdemir, İ.; Alıcı, B.; Çetinkaya, E. Synthesis, characterization and antimicrobial activities of novel silver(I) complexes with coumarin substituted N-heterocyclic carbene ligands. Bioorg. Med. Chem. 2016, 24, 643–650. [Google Scholar] [CrossRef]
- Achar, G.; Uppendranath, K.; Ramya, V.C.; Biffis, A.; Keri, R.S.; Budagumpi, S. Synthesis, characterization, crystal structure and biological studies of silver(I) complexes derived from coumarin-tethered N-heterocyclic carbene ligands. Polyhedron 2017, 123, 470–479. [Google Scholar] [CrossRef]
- Achar, G.; Shahini, C.R.; Patil, S.A.; Budagumpi, S. Synthesis, structural characterization, crystal structures and antibacterial potentials of coumarin-tethered N-heterocyclic carbene silver(I) complexes. J. Organomet. Chem. 2017, 833, 28–42. [Google Scholar] [CrossRef]
- Achar, G.; Agarwal, P.; Brinda, K.N.; Małecki, J.G.; Keri, R.S.; Budagumpi, S. Ether and coumarin–functionalized (benz)imidazolium salts and their silver(I)–N–heterocyclic carbene complexes: Synthesis, characterization, crystal structures and antimicrobial studies. J. Organomet. Chem. 2018, 854, 64e75. [Google Scholar] [CrossRef]
- Yıldırım, I.; Aktaş, A.; Celepci, D.B.; Kırbağ, S.; Kutlu, T.; Gök, T.; Aygün, M. Synthesis, characterization, crystal structure, and antimicrobial studies of 2-morpholinoethyl-substituted benzimidazolium salts and their silver(I)-N-heterocyclic carbene complexes. Res. Chem. Intermed. 2017, 43, 6379–6393. [Google Scholar] [CrossRef]
- Aher, S.; Das, A.; Muskawar, P.; Osborne, J.; Bhagat, P. In Vitro antimicrobial evaluation, effects of halide concentration and hemolysis study of silver-N-heterocyclic carbene complexes. Res. Chem. Intermed. 2018, 44, 2099–2110. [Google Scholar] [CrossRef]
- Shahini, C.R.; Achar, G.; Budagumpi, S.; Müller-Bunz, H.; Tacke, M.; Patil, S.A.L. Benzoxazole and dioxolane substituted benzimidazole-based N-heterocyclic carbine-silver(I) complexes: Synthesis, structural characterization and In Vitro antimicrobial activity. J. Organomet. Chem. 2018, 868, 1–13. [Google Scholar] [CrossRef]
- Lasmari, S.; Ikhlef, S.; Boulcina, R.; Mokrani, E.H.; Bensouici, C.; Gürbüz, N.; Dündar, M.; Karcıi, H.; Özdemir, I.; Koçh, A.; et al. New silver N–heterocyclic carbenes complexes: Synthesis, Molecular docking study and biological activities evaluation as cholinesterase inhibitors and antimicrobials. J. Mol. Struct. 2021, 1238, 130399. [Google Scholar] [CrossRef]
- Haque, R.A.; Asekunowo, P.O.; Budagumpi, S. Binuclear silver(I) complexes of p-xylyl/2,6-lutidinyl linked bis-N heterocyclic carbene ligands: Synthesis, crystal structures and biological evaluation. Inorg. Chem. Commun. 2014, 47, 56–59. [Google Scholar] [CrossRef]
- Sakamoto, R.; Morozumi, S.; Yanagawa, Y.; Toyama, M.; Takayama, A.; Kasuga, N.C.; Nomiya, K. Synthesis, characterization, and structure–activity relationship of the antimicrobial activities of dinuclear N-heterocyclic carbene (NHC)-silver(I) complexes. J. Inorg. Biochem. 2016, 163, 110–117. [Google Scholar] [CrossRef]
- Haziz, U.F.M.; Haque, R.A.; Amirul, A.A.; Shaheeda, N.; Razali, M.R. Synthesis, structures and antibacterial studies of non-functionalized and nitrile-functionalized bis-benzimidazolium salts and respective dinuclear silver(I)-N-heterocyclic carbene complexes. Polyhedron 2016, 117, 628–636. [Google Scholar] [CrossRef]
- Haziz, U.F.M.; Haque, R.A.; Amirul, A.A.; Aidda, O.N.; Razali, M.R. New class of non-symmetrical homo-dibenzimidazolium salts and their dinuclear silver(I) di-NHC complexes. J. Organomet. Chem. 2019, 899, 120914. [Google Scholar] [CrossRef]
- Loh, Y.L.; Haziz, U.F.M.; Haque, R.A.; Amirul, A.A.; Aidda, O.N.; Razali, M.R. The effect of short alkane bridges in stability of bisbenzimidazole-2-ylidene silver(I) complexes: Synthesis, crystal structure and antibacterial activity. J. Coord. Chem. 2019, 72, 894–907. [Google Scholar] [CrossRef]
- Habib, A.; Iqbal, M.A.; Bhatti, H.N.; Kamal, A.; Kamal, S. Synthesis of alkyl/aryl linked binuclear silver(I)-N-Heterocyclic carbene complexes and evaluation of their antimicrobial, hemolytic and thrombolytic potential. Inorg. Chem. Commun. 2020, 111, 107670. [Google Scholar] [CrossRef]
- Ashraf, R.; Bhatti, H.N.; Iqbal, M.A.; Jamil, Y. Synthesis of aryl linked complexes, DNA interaction study and biological potentials. Inorg. Chem. Commun. 2020, 119, 108077. [Google Scholar] [CrossRef]
- Nadeem, R.Y.; Yaqoob, M.; Yam, W.; Haque, R.A.; Iqbal, M.A. Synthesis, characterization and biological evaluation of Bis-benzimidazolium salts and their silver(I)-N-heterocyclic carbene complexes. Med. Chem. Res. 2022, 31, 1783–1791. [Google Scholar] [CrossRef]
- Hussaini, S.Y.; Haque, R.A.; Haziz, U.F.M.; Amirul, A.A.; Razali, M.R. Dinuclear silver(I) and gold(I) N-heterocyclic carbene complexes of N-alkyl substituted bis-benzimidazol-2-ylidenes with aliphatic spacer: Synthesis, characterizations and antibacterial studies. J. Mol. Struct. 2021, 1246, 131187. [Google Scholar] [CrossRef]
- Haziz, U.F.M.; Haque, R.A.; Amirul, A.A.; Razali, M.R. Synthesis, Structural Analysis and Antibacterial Studies of Bis and Open Chain Tetra N-Heterocyclic Carbene Dinuclear Silver(I) Complexes. J. Mol. Struct. 2021, 1236, 130301. [Google Scholar] [CrossRef]
- Panzner, M.J.; Hindi, K.M.; Wright, B.D.; Taylor, J.B.; Han, D.S.; Youngs, W.J.; Cannon, C.L. A theobromine derived silver N-heterocyclic carbene: Synthesis, characterization, and antimicrobial efficacy studies on cystic fibrosis relevant pathogens. Dalton Trans. 2009, 35, 7308–7313. [Google Scholar] [CrossRef]
- Maxwell, A. The interaction between coumarin drugs and DNA gyrase. Mol. Microbiol. 1993, 9, 681–686. [Google Scholar] [CrossRef]
- Kaczor, A.; Witek, K.; Podlewska, S.; Sinou, V.; Czekajewska, J.; Żesławska, E.; Doroz-Płonka, A.; Lubelska, A.; Latacz, G.; Nitek, W.; et al. Molecular Insights into an Antibiotic Enhancer Action of New Morpholine-Containing 5-Arylideneimidazolones in the Fight against MDR Bacteria. Int. J. Mol. Sci. 2021, 22, 2062. [Google Scholar] [CrossRef]
- Belhi, Z.; Karci, H.; Dündar, M.; Gürbüz, N.; Özdemir, İ.; Koç, A.; Cheriti, A.; Özdemir, İ. Novel benzimidazolium salts and their silver(I)-N-heterocyclic carbene complexes: Synthesis, characterization and their biological properties. J. Coord. Chem. 2023, 76, 120–133. [Google Scholar] [CrossRef]
- Haque, R.A.; Salman, A.W.; Budagumpi, S.; Abdullah, A.A.; Abdul Majid, A.M.S. Sterically tuned Ag(I)- and Pd(II)- N-heterocyclic carbene complexes of imidazol-2-ylidenes: Synthesis, crystal structures, and in vitro antibacterial and anticancer studies. Metallomics 2013, 5, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Hindi, K.M.; Ditto, A.J.; Panzner, M.J.; Medvetz, D.A.; Han, D.S.; Hovis, C.E.; Hilliard, J.K.; Taylor, J.B.; Yun, Y.H.; Cannon, C.L.; et al. The antimicrobial efficacy of sustained release silver–carbene complex-loaded L-tyrosine polyphosphate nanoparticles: Characterization, in vitro and in vivo studies. Biomaterials 2009, 30, 3771–3779. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, F.; Diaferia, C.; Rossi, F.; Ronga, L.; Tesauro, D. Forward Precision Medicine: Micelles for Active Targeting Driven by Peptides. Molecules 2021, 26, 4049. [Google Scholar] [CrossRef]
- Li, Y.; Hindi, K.; Watts, K.M.; Taylor, J.B.; Zhang, K.; Li, Z.; Hunstad, D.A.; Cannon, C.L.; Youngs, W.J.; Wooley, K.L. Shell crosslinked nanoparticles carrying silver antimicrobials as therapeutics. Chem. Commun. 2010, 46, 121–123. [Google Scholar] [CrossRef]
- Ornelas-Megiatto, C.; Shah, P.N.; Wich, P.R.; Cohen, J.L.; Tagaev, J.A.; Smolen, J.A.; Wright, B.D.; Panzner, M.J.; Youngs, W.J.; Fréchet, J.M.J.; et al. Aerosolized Antimicrobial Agents Based on Degradable Dextran Nanoparticles Loaded with Silver Carbene Complexes. Mol. Pharm. 2012, 9, 3012–3022. [Google Scholar] [CrossRef]
- Lim, Y.H.; Tiemann, K.M.; Heo, G.S.; Wagers, P.O.; Rezenom, Y.H.; Zhang, S.; Zhang, F.; Youngs, W.J.; Hunstad, D.A.; Wooley, K.L. Preparation and In Vitro Antimicrobial Activity of Silver-Bearing Degradable Polymeric Nanoparticles of Polyphosphoester-block-Poly(l-lactide). ACS Nano 2015, 9, 1995–2008. [Google Scholar] [CrossRef]
- Necola, M.R.; Gurovic, M.S.V.; Ruiz Díaz, S.; Silbestri, G.F. Binding silver to chitooligosaccharides through N-heterocyclic carbenes: Synthesis and antimicrobial activity. Carbohydrate Res. 2019, 471, 6–12. [Google Scholar] [CrossRef]
- Gajare, S.P.; Bansode, P.A.; Patil, P.V.; Patil, A.D.; Pore, D.M.; Sonawane, K.D.; Dhanavade, M.J.; Khot, V.M.; Rashinkar, G.S. Anticancer, Antibacterial and Hyperthermia Studies of a Caffeine-Based N-Heterocyclic Carbene Silver Complex Anchored on Magnetic Nanoparticles. Chem. Select 2021, 6, 1958–2019. [Google Scholar] [CrossRef]
- Tsyba, I.; Bun-kit Mui, B.; Bau, R.; Noguchi, R.; Nomiya, K. Synthesis and Structure of a Water-Soluble Hexanuclear Silver(I) Nicotinate Cluster Comprised of a “Cyclohexane-Chair”-Type of Framework, Showing Effective Antibacterial and Antifungal Activities: Use of “Sparse Matrix” Techniques for Growing Crystals of Water-Soluble. Inorganic Complexes. Inorg. Chem. 2003, 42, 8028–8032. [Google Scholar] [CrossRef]
- Stenger-Smith, J.; Chakraborty, I.; Sameera, W.M.C.; Mascharak, P.K. Antimicrobial silver (I) complexes derived from aryl-benzothiazoles as turn-on sensors: Syntheses, properties and density functional studies. Inorg. Chim. Acta 2018, 471, 326–335. [Google Scholar] [CrossRef]

| Entry | N° Strains | Highest Activity Against | Concentration | ZoI or MIC or % of G * | Ref. |
|---|---|---|---|---|---|
| 203 | 36 | E. coli–Burkholderia | 1 μg mL−1 | MIC | [68] |
| 210 | 6 | E. faecalis–S. aureus | 25 μgmL−1 | MIC | [69] |
| 210 | 6 | C. albicans–C tropicalis | 25 μgmL−1 | MIC | [69] |
| 212–216 | 6 | P. aeruginosa | 8 μgmL−1 | MIC | [70] |
| 212 | 6 | P. aeruginosa | 24 mm at 12 μL | ZoI | [70] |
| 217, 220 | 6 | P. aeruginosa | 8 μgmL−1 | MIC | [71] |
| 231–236 | 4 | S. aureus–E. coli | 16 μgmL−1 | MIC | [72] |
| 243–245 | 2 | E. coli | 8 μgmL−1 | MIC | [60] |
| 253 | 3 | S. aureus–E. coli | 5.85 μgmL−1 | MIC | [73] |
| 255 | 2 | S. aureus | 6.25 µmolL−1 | MIC | [74] |
| 263–264 | 7 | All Strains | >95% | % of G | [75] |
| 271, 275 | 5 | E. coli | 6.25 μgmL−1 | MIC | [76] |
| 271 | 5 | P. aeruginosa | 6.25 μgmL−1 | MIC | [76] |
| 278–279 | 2 | E. coli–S. aureus | 27.0 mm at 100μL mL−1 | ZoI | [77] |
| 284 | 2 | E. coli–S. aureus | 12.5 μgmL−1 | MIC | [53] |
| 285 | 8 | S. aureus–B. subtilis | 15.7 μgmL−1 | MIC | [78] |
| 288–293 | 2 | E. coli and S. aureus | 8 ± 1 mm at 9 μL | ZoI | [79] |
| 295–296 | 2 | E. coli and S. aureus | 13 ± 0.6 mm at 6 μL | ZoI | [80] |
| 302 | 2 | E. coli–S. aureus | 12.5 µM | MIC | [81] |
| 305 | 4 | M. brunensis | 15 ± 0.9 μgmL−1 | MIC | [82] |
| 308 | 3 | S. aureus | 2.09 µM | MIC | [83] |
| 310 | 2 | S. aureus | 0.382 μgmL−1 | MIC | [84] |
| 316 | 2 | S. aureus | 20 ± 1 mm at 12 μL | ZoI | [85] |
| 317 | 2 | E. coli | 1.56 μM | MIC | [86] |
| 322 | 2 | S. aureus | 20 ± 1 mm at 12 μL | ZoI | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronga, L.; Varcamonti, M.; Tesauro, D. Structure–Activity Relationships in NHC–Silver Complexes as Antimicrobial Agents. Molecules 2023, 28, 4435. https://doi.org/10.3390/molecules28114435
Ronga L, Varcamonti M, Tesauro D. Structure–Activity Relationships in NHC–Silver Complexes as Antimicrobial Agents. Molecules. 2023; 28(11):4435. https://doi.org/10.3390/molecules28114435
Chicago/Turabian StyleRonga, Luisa, Mario Varcamonti, and Diego Tesauro. 2023. "Structure–Activity Relationships in NHC–Silver Complexes as Antimicrobial Agents" Molecules 28, no. 11: 4435. https://doi.org/10.3390/molecules28114435
APA StyleRonga, L., Varcamonti, M., & Tesauro, D. (2023). Structure–Activity Relationships in NHC–Silver Complexes as Antimicrobial Agents. Molecules, 28(11), 4435. https://doi.org/10.3390/molecules28114435



.jpg)




