Progress in the Detection of Erythropoietin in Blood, Urine, and Tissue
Abstract
:1. Introduction
2. Detection of Epo Protein in Blood and Urine
2.1. Development of Detection of Epo Protein
2.2. Isoelectric Focusing (IEF)-PAGE
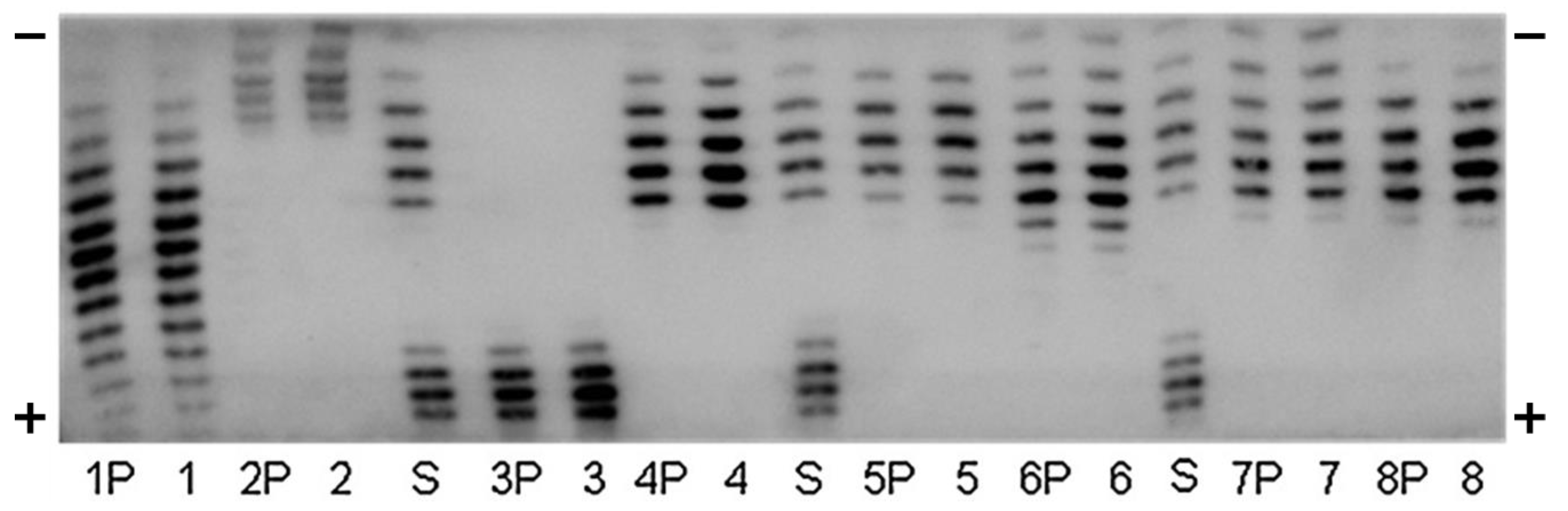
2.3. Sodium N-Lauroylsarcosinate (SAR)-PAGE
2.4. Capillary Electromigration Methods
2.5. Liquid Chromatography/Mass Spectrometry (LC/MS) Analysis
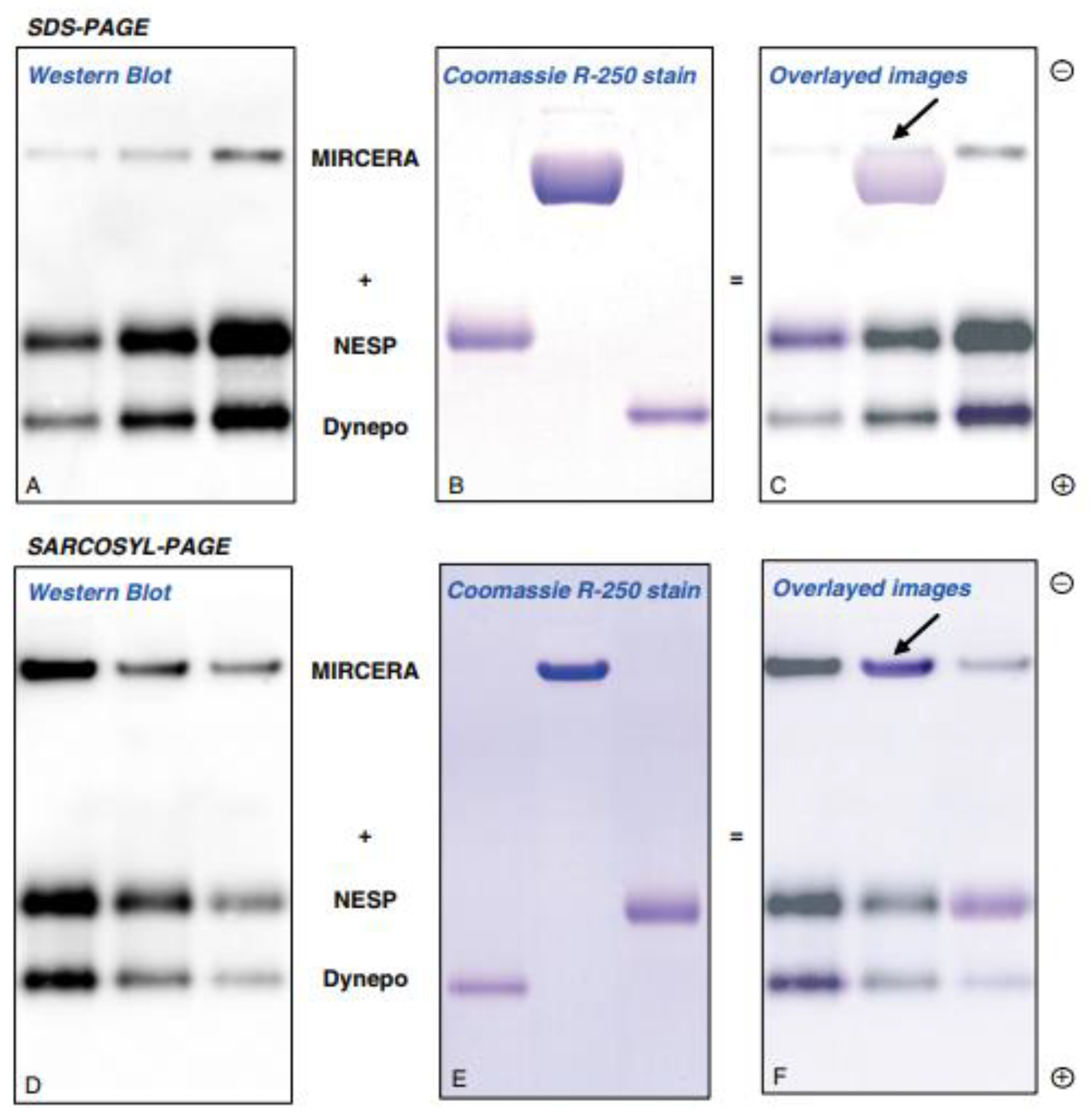
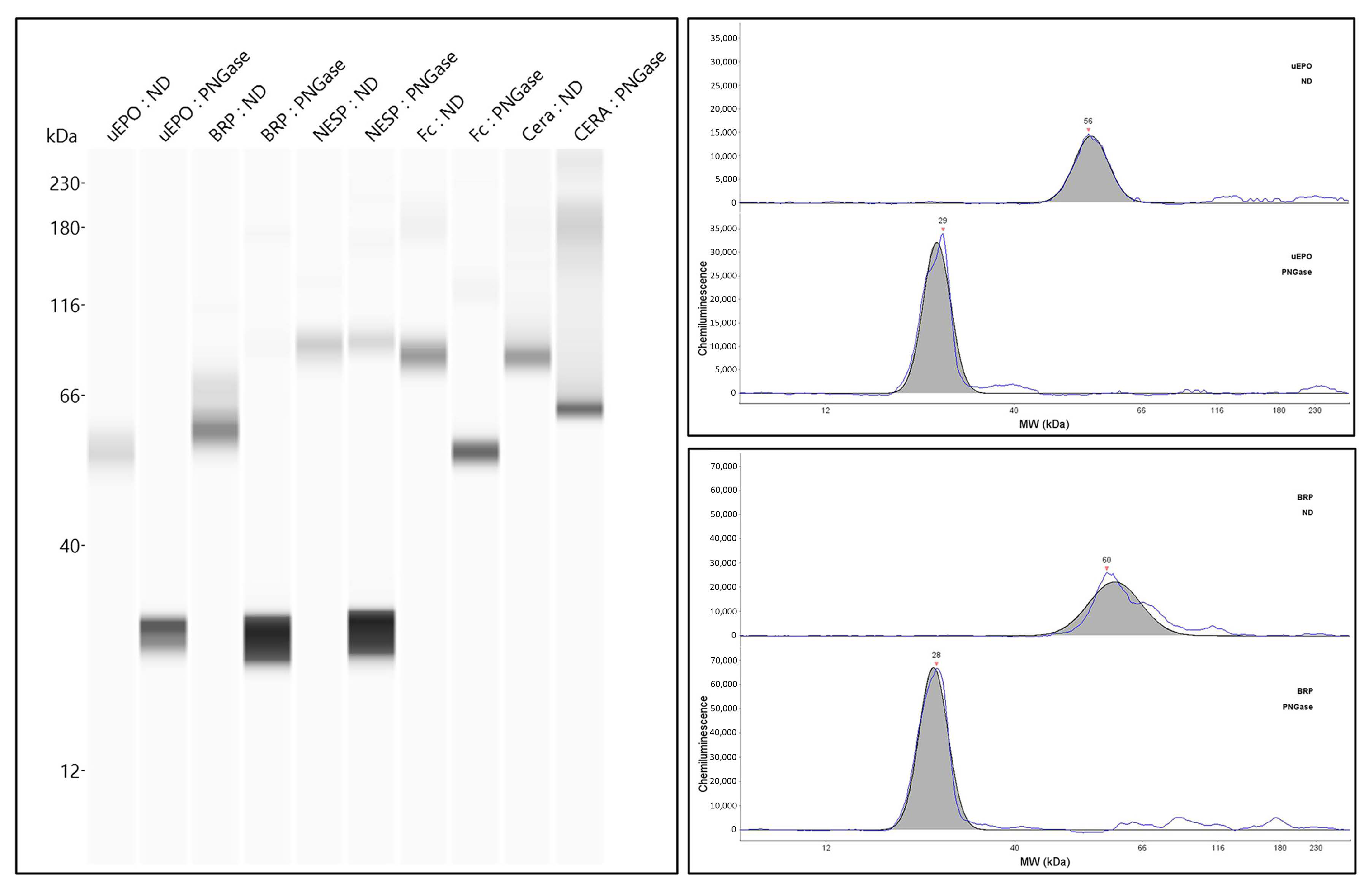
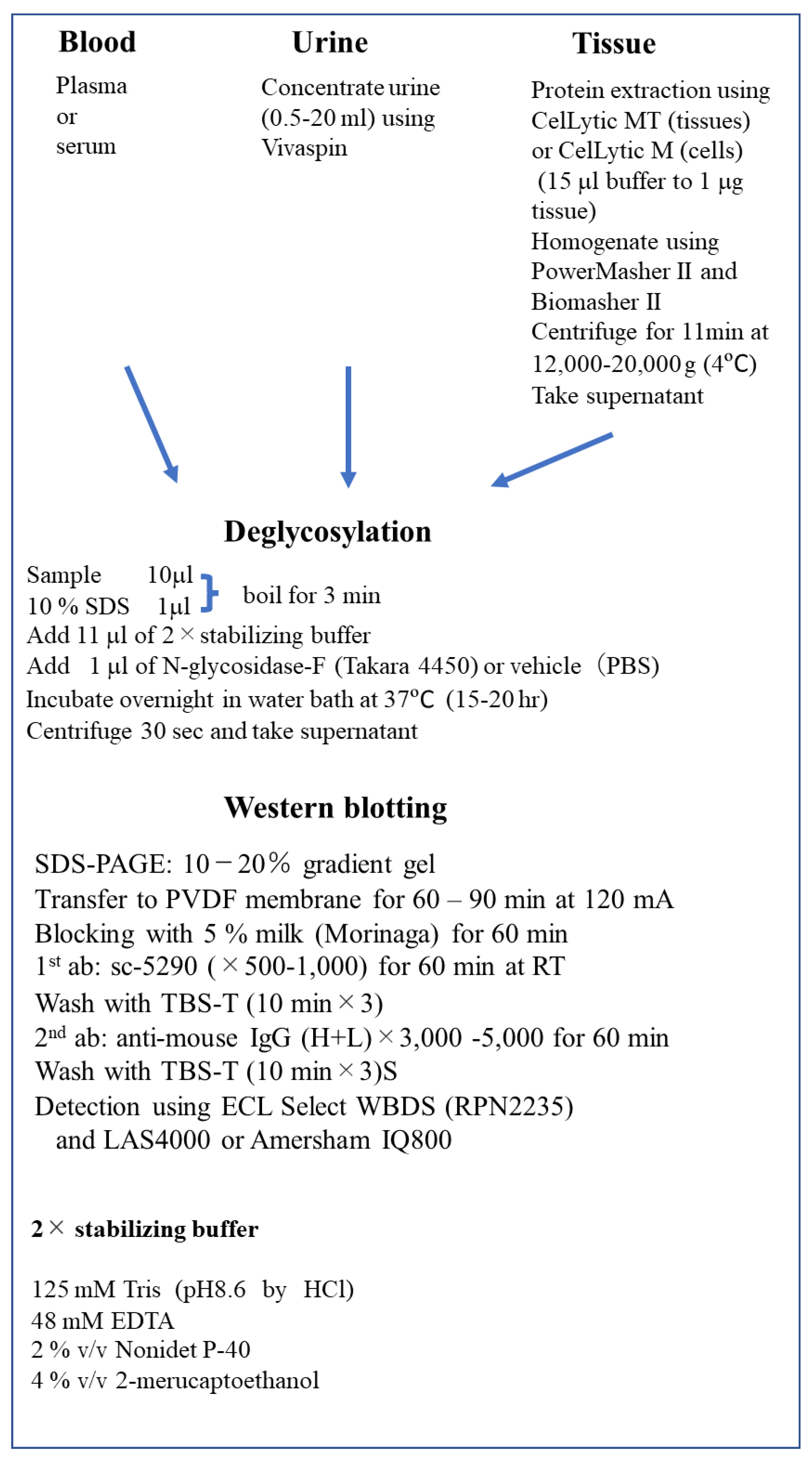
2.6. Deglycosylation-Coupled Western Blotting
2.7. Methods of Epo Detection by Deglycosylation-Coupled Western Blotting
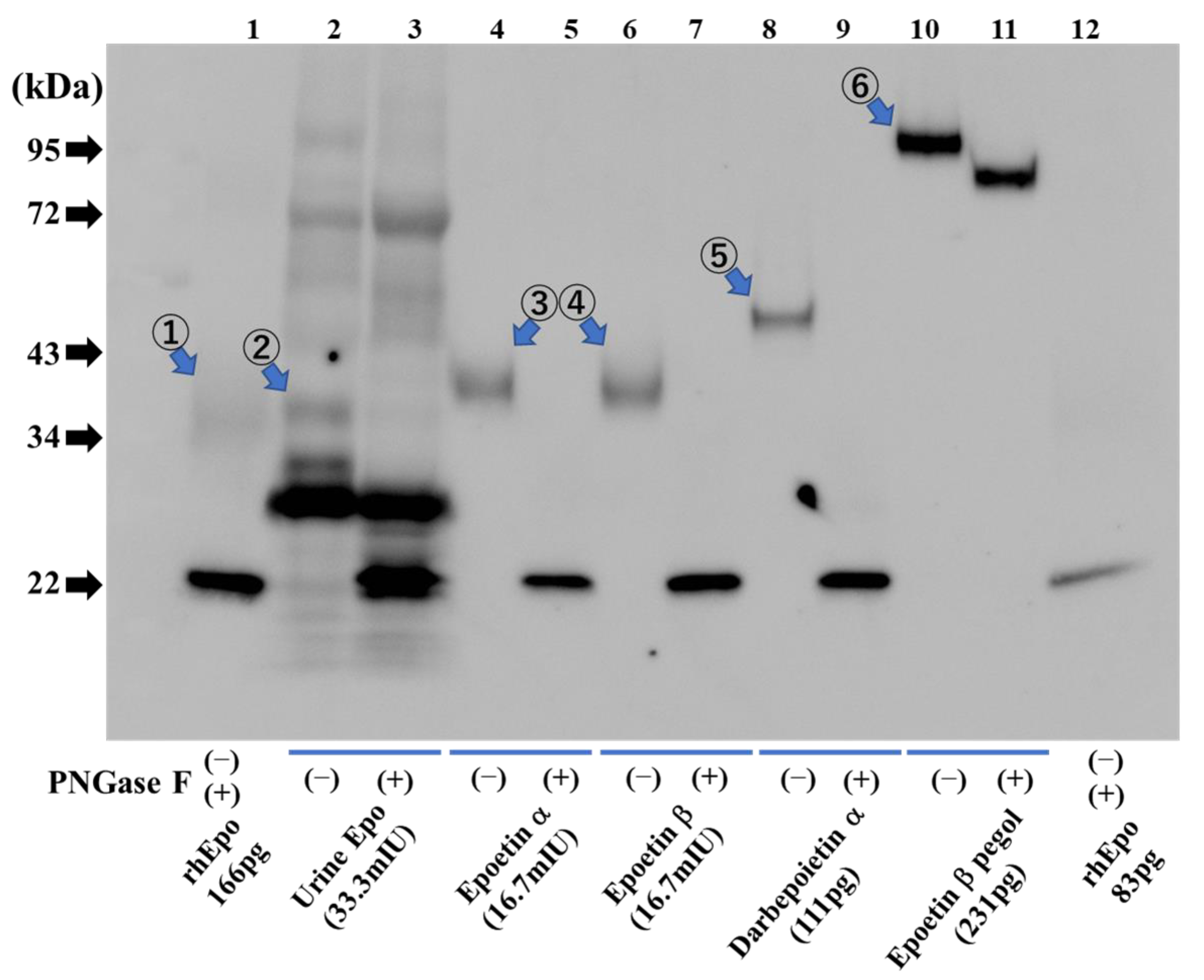
2.8. Detection of Recombinant Epo Protein and ESAs
2.9. Detection of Epo Protein in Blood and Urine: CLEIA and Western Blotting (Figure 6)
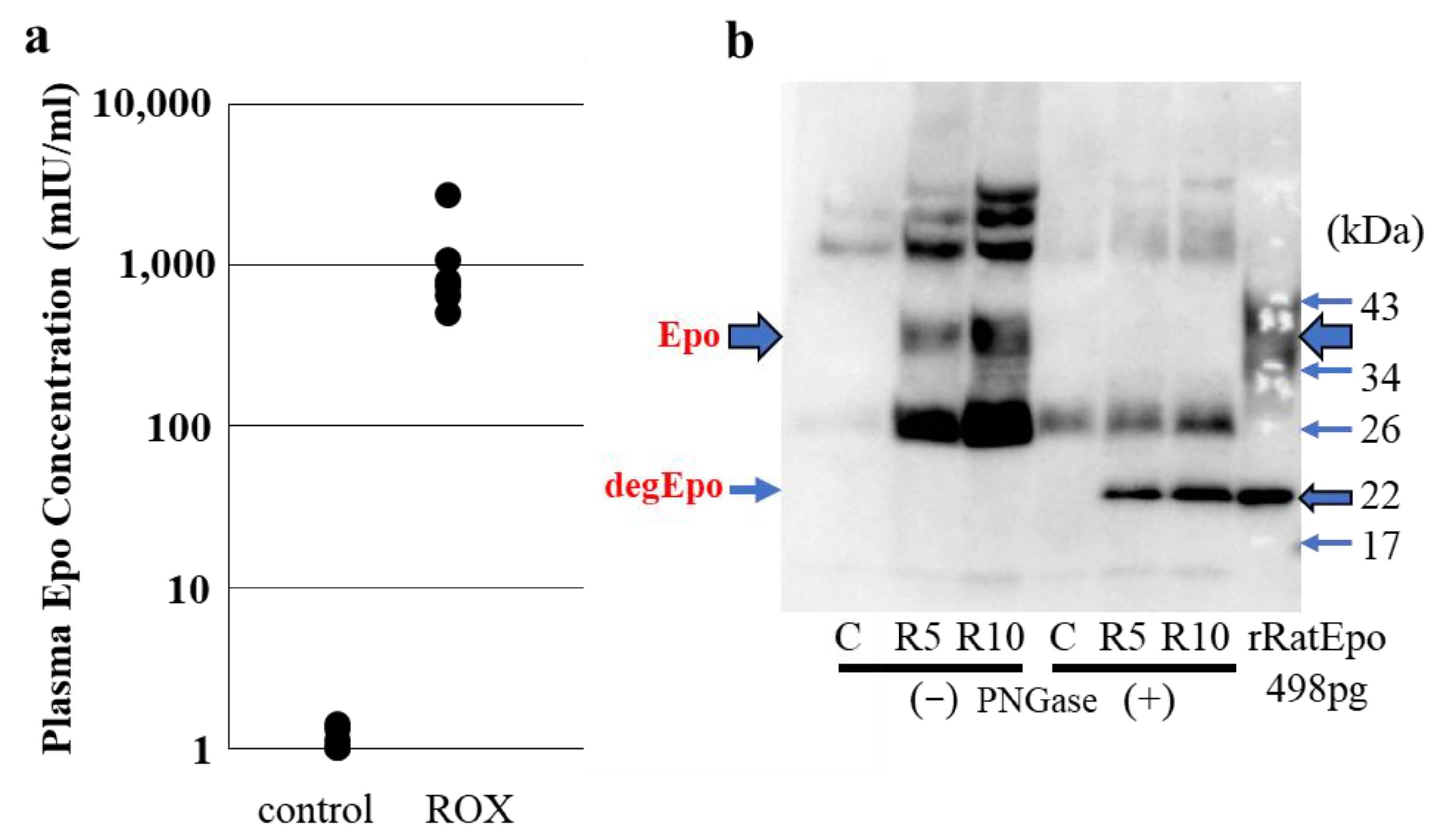
3. Detection of Epo Protein in Tissue
3.1. Methods for the Detection of Epo Protein in Tissue
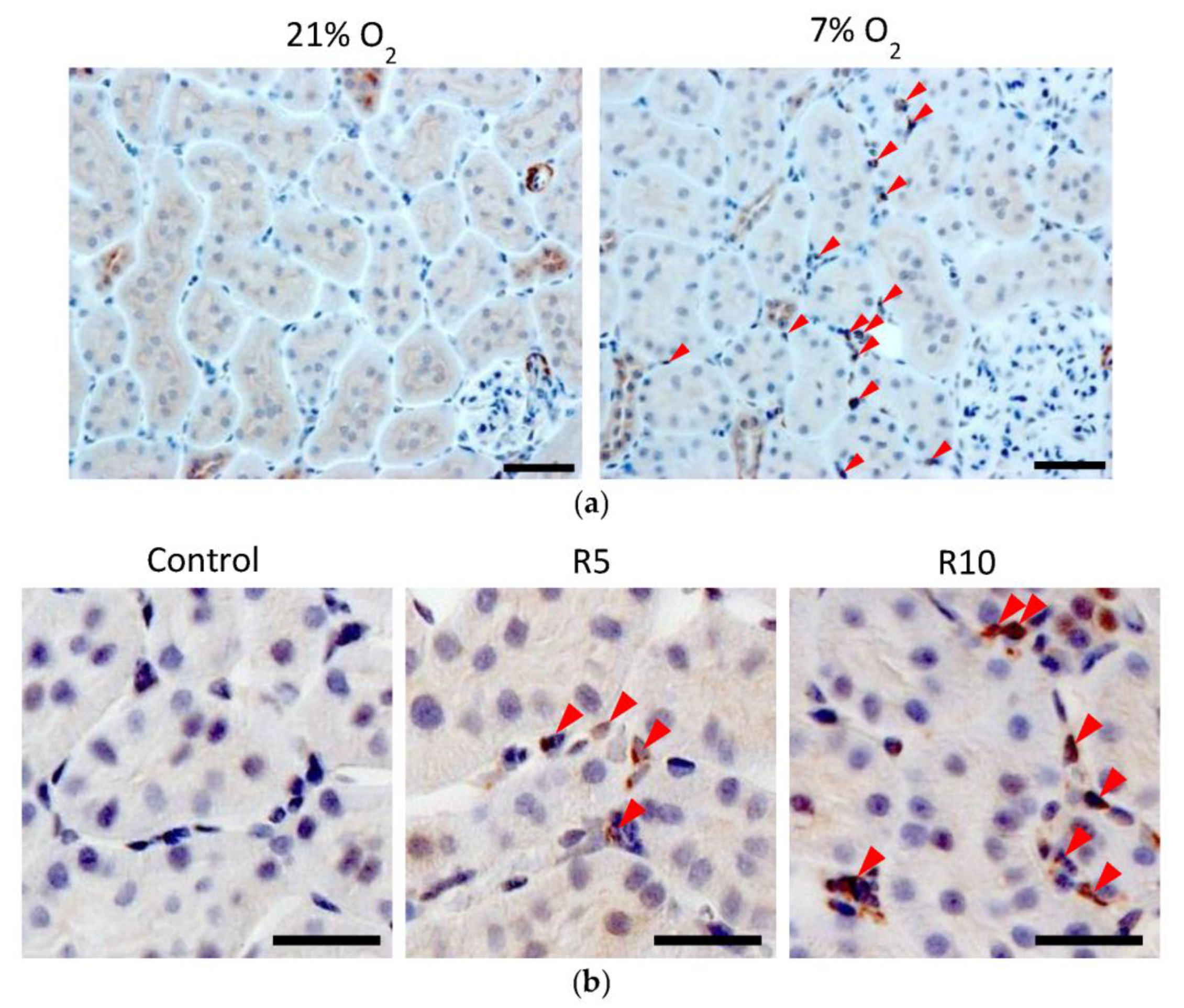
3.2. Epo Production by the Kidneys and Liver (Figure 8 and Figure 9)
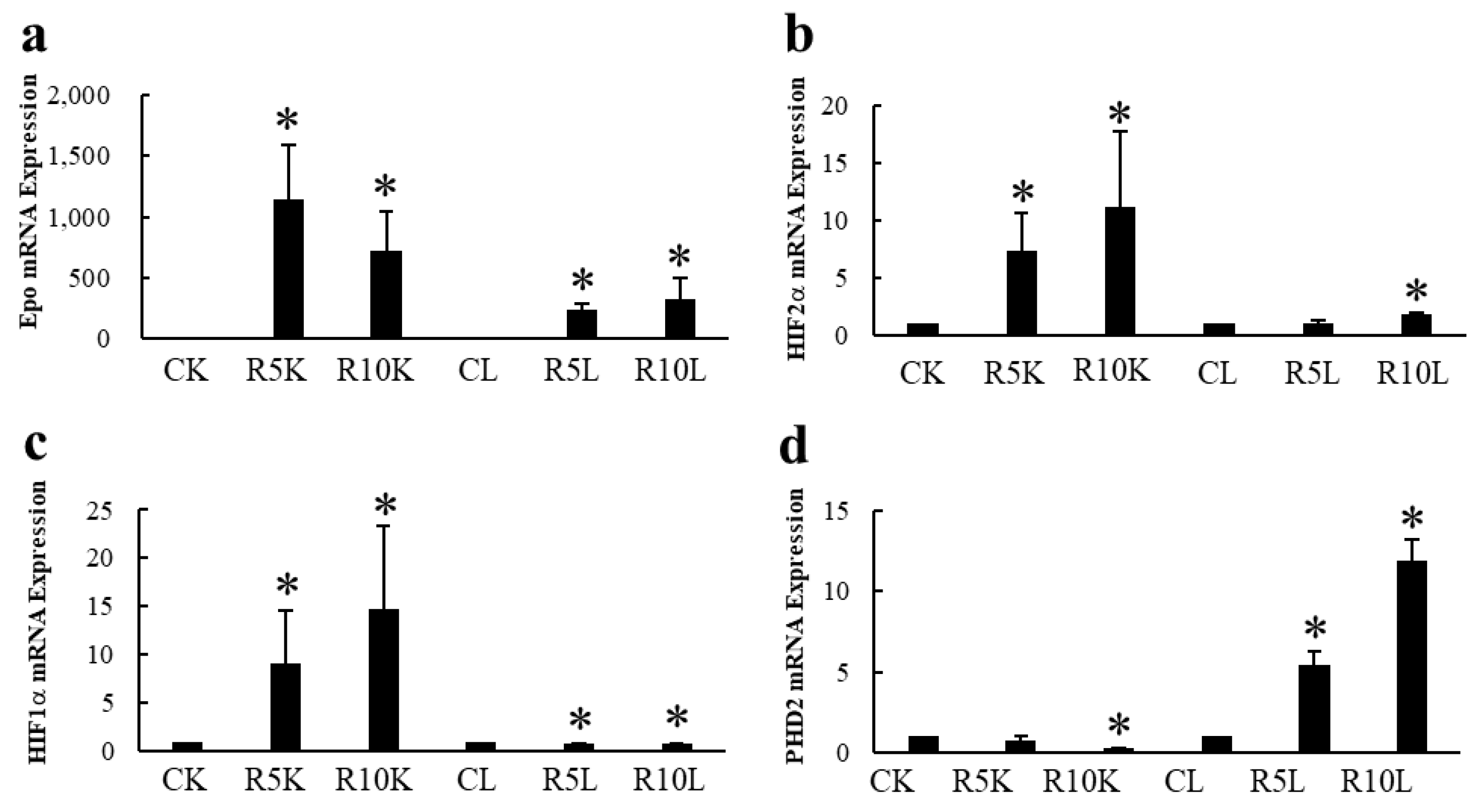
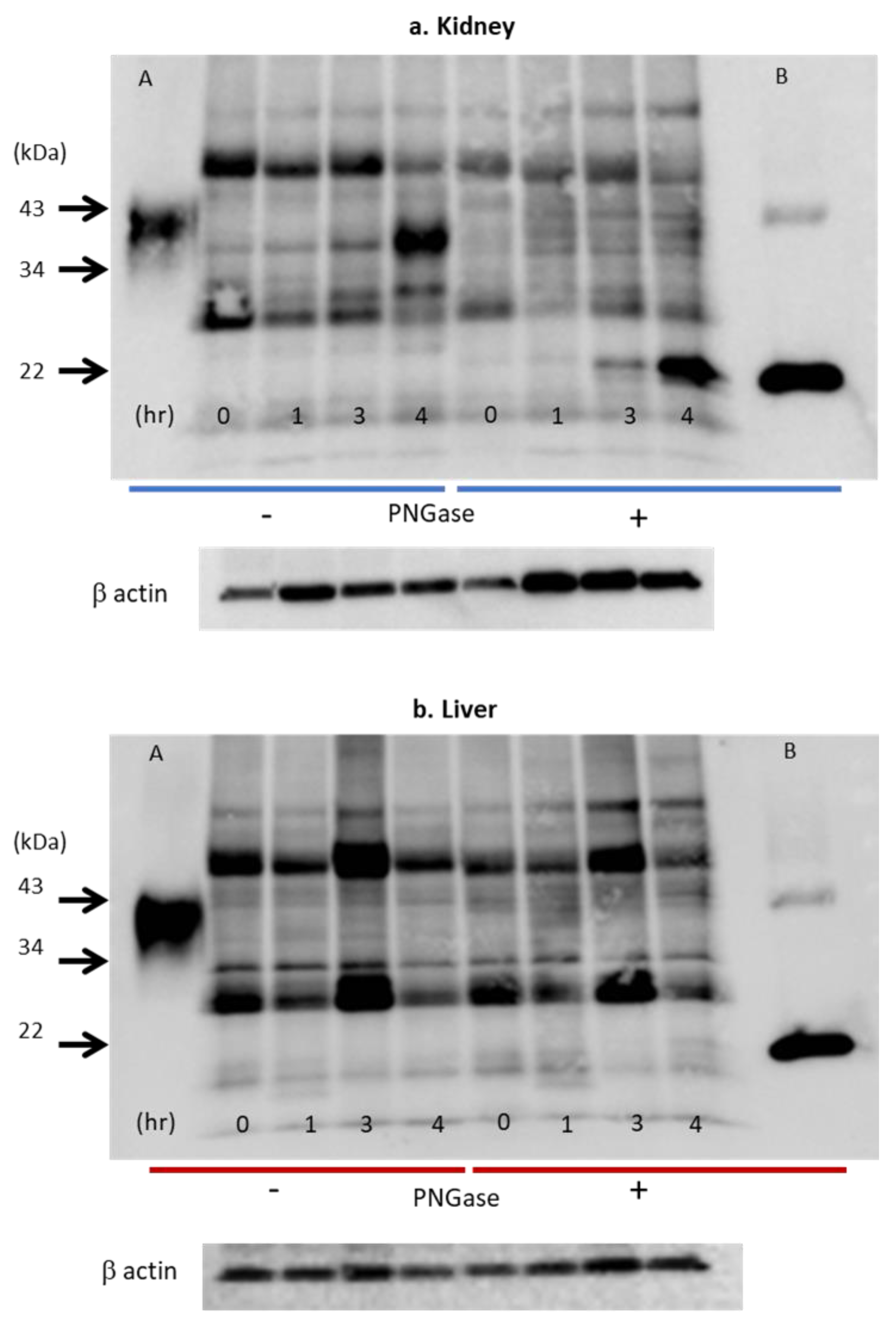
3.3. Immunohistochemistry (IHC) of Epo Production
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Kung, C.K.; Goldwasser, E. Purification of human erythropoietin. J. Biol. Chem. 1977, 252, 5558–5564. [Google Scholar] [CrossRef]
- Jacobs, K.; Shoemaker, C.; Rudersdorf, R.; Neill, S.D.; Kaufman, R.J.; Mufson, A.; Seehra, J.; Jones, S.S.; Hewick, R.; Fritsch, E.F.; et al. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature 1985, 313, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Haase, V.H. Hypoxic regulation of erythropoiesis and iron metabolism. Am. J. Physiol. Ren. Physiol. 2010, 299, F1–F13. [Google Scholar] [CrossRef]
- Jelkmann, W. Regulation of erythropoietin production. J. Physiol. 2011, 589, 1251–1258. [Google Scholar] [CrossRef]
- Koury, M.J.; Haase, V.H. Anaemia in kidney disease: Harnessing hypoxia responses for therapy. Nat. Rev. Nephrol. 2015, 11, 394–410. [Google Scholar] [CrossRef]
- Ebert, B.L.; Bunn, H.F. Regulation of the erythropoietin gene. Blood 1999, 94, 1864–1877. [Google Scholar] [CrossRef]
- Cole, J.; Ertoy, D.; Lin, H.; Sutliff, R.L.; Ezan, E.; Guyene, T.T.; Capecchi, M.; Corvol, P.; Bernstein, K.E. Lack of angiotensin II-facilitated erythropoiesis causes anemia in angiotensin-converting enzyme-deficient mice. J. Clin. Investig. 2000, 106, 1391–1398. [Google Scholar] [CrossRef]
- Kato, H.; Ishida, J.; Imagawa, S.; Saito, T.; Suzuki, N.; Matsuoka, T.; Sugaya, T.; Tanimoto, K.; Yokoo, T.; Ohneda, O.; et al. Enhanced erythropoiesis mediated by activation of the renin-angiotensin system via angiotensin II type 1a receptor. FASEB J. 2005, 19, 2023–2025. [Google Scholar] [CrossRef]
- Kato, H.; Ishida, J.; Matsusaka, T.; Ishimaru, T.; Tanimoto, K.; Sugiyama, F.; Yagami, K.; Nangaku, M.; Fukamizu, A. Erythropoiesis and Blood Pressure Are Regulated via AT1 Receptor by Distinctive Pathways. PLoS ONE 2015, 10, e0129484. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Mungunsukh, O.; Day, R.M. Erythropoietin Regulation by Angiotensin II. Vitam. Horm. 2017, 105, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Mungunsukh, O.; McCart, E.A.; Roehrich, P.J.; Yee, D.K.; Day, R.M. Mechanism of erythropoietin regulation by angiotensin II. Mol. Pharmacol. 2014, 85, 898–908. [Google Scholar] [CrossRef]
- Babitt, J.L.; Lin, H.Y. Mechanisms of anemia in CKD. J. Am. Soc. Nephrol. 2012, 23, 1631–1634. [Google Scholar] [CrossRef]
- Akizawa, T.; Iwasaki, M.; Otsuka, T.; Reusch, M.; Misumi, T. Roxadustat Treatment of Chronic Kidney Disease-Associated Anemia in Japanese Patients Not on Dialysis: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Trial. Adv. Ther. 2019, 36, 1438–1454. [Google Scholar] [CrossRef]
- Besarab, A.; Provenzano, R.; Hertel, J.; Zabaneh, R.; Klaus, S.J.; Lee, T.; Leong, R.; Hemmerich, S.; Yu, K.H.; Neff, T.B. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol. Dial. Transplant. 2015, 30, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Del Balzo, U.; Signore, P.E.; Walkinshaw, G.; Seeley, T.W.; Brenner, M.C.; Wang, Q.; Guo, G.; Arend, M.P.; Flippin, L.A.; Chow, F.A.; et al. Nonclinical Characterization of the Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat, a Novel Treatment of Anemia of Chronic Kidney Disease. J. Pharmacol. Exp. Ther. 2020, 374, 342–353. [Google Scholar] [CrossRef]
- Gupta, N.; Wish, J.B. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors: A Potential New Treatment for Anemia in Patients with CKD. Am. J. Kidney Dis. 2017, 69, 815–826. [Google Scholar] [CrossRef]
- Ogawa, C.; Tsuchiya, K.; Maeda, K. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors and Iron Metabolism. Int. J. Mol. Sci. 2023, 24, 3037. [Google Scholar] [CrossRef]
- de Seigneux, S.; Lundby, A.K.; Berchtold, L.; Berg, A.H.; Saudan, P.; Lundby, C. Increased Synthesis of Liver Erythropoietin with CKD. J. Am. Soc. Nephrol. 2016, 27, 2265–2269. [Google Scholar] [CrossRef]
- Hendler, E.D.; Goffinet, J.A.; Ross, S.; Longnecker, R.E.; Bakovic, V. Controlled study of androgen therapy in anemia of patients on maintenance hemodialysis. N. Engl. J. Med. 1974, 291, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Deicher, R.; Hörl, W.H. Hormonal adjuvants for the treatment of renal anaemia. Eur. J. Clin. Investig. 2005, 35 (Suppl. S3), 75–84. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.F.; Mora, C. In-depth review effect of androgens on anemia and malnutrition in renal failure: Implications for patients on peritoneal dialysis. Perit. Dial. Int. 2001, 21, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Sexauer, C.L.; Matson, J.R. Anemia of chronic renal failure. Ann. Clin. Lab. Sci. 1981, 11, 484–487. [Google Scholar]
- Courtney, A.E.; Maxwell, A.P. Critiques of clinical guidelines in nephrology: Anaemia. Nephron Clin. Pract. 2008, 110, c115–c125. [Google Scholar] [CrossRef]
- Greeviroj, P.; Lertussavavivat, T.; Thongsricome, T.; Takkavatakarn, K.; Phannajit, J.; Avihingsanon, Y.; Praditpornsilpa, K.; Eiam-Ong, S.; Susantitaphong, P. The world prevalence, associated risk factors and mortality of hepatitis C virus infection in hemodialysis patients: A meta-analysis. J. Nephrol. 2022, 35, 2269–2282. [Google Scholar] [CrossRef]
- Rahnavardi, M.; Hosseini Moghaddam, S.M.; Alavian, S.M. Hepatitis C in hemodialysis patients: Current global magnitude, natural history, diagnostic difficulties, and preventive measures. Am. J. Nephrol. 2008, 28, 628–640. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Kiyokawa, H.; Machida, J.; Obayashi, A.; Nojiri, N.; Ueda, S.; Takatsuki, K. Seroepidemiology of hepatitis C virus infection in Japan and HCV infection in haemodialysis patients. FEMS Microbiol. Rev. 1994, 14, 253–258. [Google Scholar] [CrossRef]
- Zacks, S.L.; Fried, M.W. Hepatitis B and C and renal failure. Infect. Dis. Clin. N. Am. 2001, 15, 877–899. [Google Scholar] [CrossRef]
- Mima, A. Hypoxia-inducible factor-prolyl hydroxylase inhibitors for renal anemia in chronic kidney disease: Advantages and disadvantages. Eur. J. Pharmacol. 2021, 912, 174583. [Google Scholar] [CrossRef]
- Sanghani, N.S.; Haase, V.H. Hypoxia-Inducible Factor Activators in Renal Anemia: Current Clinical Experience. Adv. Chronic Kidney Dis. 2019, 26, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Pergola, P.E.; Farag, Y.M.K.; Agarwal, R.; Arnold, S.; Bako, G.; Block, G.A.; Burke, S.; Castillo, F.P.; Jardine, A.G.; et al. Vadadustat in Patients with Anemia and Non-Dialysis-Dependent CKD. N. Engl. J. Med. 2021, 384, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S.; Schiller, B.; Locatelli, F.; Covic, A.C.; Provenzano, R.; Wiecek, A.; Levin, N.W.; Kaplan, M.; Macdougall, I.C.; Francisco, C.; et al. Peginesatide in patients with anemia undergoing hemodialysis. N. Engl. J. Med. 2013, 368, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Jörg, D.J.; Fuertinger, D.H.; Kotanko, P. Mechanisms of hemoglobin cycling in anemia patients treated with erythropoiesis-stimulating agents. PLoS Comput. Biol. 2023, 19, e1010850. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Provenzano, R.; Sharma, A.; Spinowitz, B.S.; Schmidt, R.J.; Pergola, P.E.; Zabaneh, R.I.; Tong-Starksen, S.; Mayo, M.R.; Tang, H.; et al. Peginesatide for anemia in patients with chronic kidney disease not receiving dialysis. N. Engl. J. Med. 2013, 368, 320–332. [Google Scholar] [CrossRef]
- Singh, A.K.; Carroll, K.; Perkovic, V.; Solomon, S.; Jha, V.; Johansen, K.L.; Lopes, R.D.; Macdougall, I.C.; Obrador, G.T.; Waikar, S.S.; et al. Daprodustat for the Treatment of Anemia in Patients Undergoing Dialysis. N. Engl. J. Med. 2021, 385, 2325–2335. [Google Scholar] [CrossRef]
- Semenza, G.L.; Agani, F.; Booth, G.; Forsythe, J.; Iyer, N.; Jiang, B.H.; Leung, S.; Roe, R.; Wiener, C.; Yu, A. Structural and functional analysis of hypoxia-inducible factor 1. Kidney Int. 1997, 51, 553–555. [Google Scholar] [CrossRef]
- Haase, V.H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013, 27, 41–53. [Google Scholar] [CrossRef]
- Rosenberger, C.; Mandriota, S.; Jürgensen, J.S.; Wiesener, M.S.; Hörstrup, J.H.; Frei, U.; Ratcliffe, P.J.; Maxwell, P.H.; Bachmann, S.; Eckardt, K.-U. Expression of Hypoxia-Inducible Factor-1α and -2α in Hypoxic and Ischemic Rat Kidneys. J. Am. Soc. Nephrol. 2002, 13, 1721–1732. [Google Scholar] [CrossRef]
- Fried, W. The liver as a source of extrarenal erythropoietin production. Blood 1972, 40, 671–677. [Google Scholar] [CrossRef]
- Eckardt, K.U.; Ratcliffe, P.J.; Tan, C.C.; Bauer, C.; Kurtz, A. Age-dependent expression of the erythropoietin gene in rat liver and kidneys. J. Clin. Investig. 1992, 89, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.C.; Eckardt, K.U.; Firth, J.D.; Ratcliffe, P.J. Feedback modulation of renal and hepatic erythropoietin mRNA in response to graded anemia and hypoxia. Am. J. Physiol. 1992, 263, F474–F481. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Takayama, N.; Takano, H.; Koretsune, H.; Koizumi, C.; Kunioka, E.I.; Uchida, S.; Takahashi, T.; Yamamoto, K. TP0463518, a novel inhibitor for hypoxia-inducible factor prolyl hydroxylases, increases erythropoietin in rodents and monkeys with a good pharmacokinetics-pharmacodynamics correlation. Eur. J. Pharmacol. 2018, 838, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Groenendaal-van de Meent, D.; Adel, M.D.; Noukens, J.; Rijnders, S.; Krebs-Brown, A.; Mateva, L.; Alexiev, A.; Schaddelee, M. Effect of Moderate Hepatic Impairment on the Pharmacokinetics and Pharmacodynamics of Roxadustat, an Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor. Clin. Drug Investig. 2016, 36, 743–751. [Google Scholar] [CrossRef]
- Kato, S.; Ochiai, N.; Takano, H.; Io, F.; Takayama, N.; Koretsune, H.; Kunioka, E.I.; Uchida, S.; Yamamoto, K. TP0463518, a Novel Prolyl Hydroxylase Inhibitor, Specifically Induces Erythropoietin Production in the Liver. J. Pharmacol. Exp. Ther. 2019, 371, 675–683. [Google Scholar] [CrossRef]
- WADA Science/EPO Working Group. Harmonization of Analysis and Reporting of Erythropoietin (EPO) and Other Epo-Receptor Agonists (ESAs) by Polyacrylamaide Gel Electrophoresis (PAGE) Analytical Methods. (TD2022EPO); Word Anti-Doping Agency: Montreal, QC, Canada, 2022. [Google Scholar]
- Kaneko, K.; Sato, Y.; Uchino, E.; Toriu, N.; Shigeta, M.; Kiyonari, H.; Endo, S.; Fukuma, S.; Yanagita, M. Lineage tracing analysis defines erythropoietin-producing cells as a distinct subpopulation of resident fibroblasts with unique behaviors. Kidney Int. 2022, 102, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.U.; Dittmer, J.; Neumann, R.; Bauer, C.; Kurtz, A. Decline of erythropoietin formation at continuous hypoxia is not due to feedback inhibition. Am. J. Physiol. 1990, 258, F1432–F1437. [Google Scholar] [CrossRef]
- Nishimura, K.; Goto, K.; Nakagawa, H. Effect of erythropoietin production induced by hypoxia on autophagy in HepG2 cells. Biochem. Biophys. Res. Commun. 2018, 495, 1317–1321. [Google Scholar] [CrossRef]
- Wognum, A.W.; Lansdorp, P.M.; Eaves, A.C.; Krystal, G. An enzyme-linked immunosorbent assay for erythropoietin using monoclonal antibodies, tetrameric immune complexes, and substrate amplification. Blood 1989, 74, 622–628. [Google Scholar] [CrossRef]
- Davidović, S.; Babić, N.; Jovanović, S.; Barišić, S.; Grković, D.; Miljković, A. Serum erythropoietin concentration and its correlation with stage of diabetic retinopathy. BMC Ophthalmol. 2019, 19, 227. [Google Scholar] [CrossRef]
- Grote Beverborg, N.; Verweij, N.; Klip, I.T.; van der Wal, H.H.; Voors, A.A.; van Veldhuisen, D.J.; Gansevoort, R.T.; Bakker, S.J.; van der Harst, P.; van der Meer, P. Erythropoietin in the general population: Reference ranges and clinical, biochemical and genetic correlates. PLoS ONE 2015, 10, e0125215. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Takasaki, S.; Shimada, M.; Kobata, A. Role of sugar chains in the in vitro biological activity of human erythropoietin produced in recombinant Chinese hamster ovary cells. J. Biol. Chem. 1990, 265, 12127–12130. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, E.; Kawanishi, G.; Ueda, M.; Masuda, S.; Sasaki, R. The role of carbohydrate in recombinant human erythropoietin. Eur. J. Biochem. 1990, 188, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Oh-eda, M.; Kuboniwa, H.; Tomonoh, K.; Shimonaka, Y.; Ochi, N. Role of sugar chains in the expression of the biological activity of human erythropoietin. J. Biol. Chem. 1992, 267, 7703–7709. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Akai, K.; Kawanishi, G.; Ueda, M.; Masuda, S.; Sasaki, R. Effects of site-directed removal of N-glycosylation sites in human erythropoietin on its production and biological properties. J. Biol. Chem. 1991, 266, 20434–20439. [Google Scholar] [CrossRef]
- Kodama, D.; Nishimiya, D.; Iwata, K.; Yamaguchi, K.; Yoshida, K.; Kawabe, Y.; Motono, M.; Watanabe, H.; Yamashita, T.; Nishijima, K.; et al. Production of human erythropoietin by chimeric chickens. Biochem. Biophys. Res. Commun. 2008, 367, 834–839. [Google Scholar] [CrossRef]
- Lasne, F.; Martin, L.; Martin, J.A.; de Ceaurriz, J. Isoelectric profiles of human erythropoietin are different in serum and urine. Int. J. Biol. Macromol. 2007, 41, 354–357. [Google Scholar] [CrossRef]
- Reichel, C.; Abzieher, F.; Geisendorfer, T. SARCOSYL-PAGE: A new method for the detection of MIRCERA- and EPO-doping in blood. Drug Test. Anal. 2009, 1, 494–504. [Google Scholar] [CrossRef]
- Capdeville, P.; Martin, L.; Cholet, S.; Damont, A.; Audran, M.; Ericsson, M.; Fenaille, F.; Marchand, A. Evaluation of erythropoietin biosimilars Epotin™, Hemax® and Jimaixin™ by electrophoretic methods used for doping control analysis and specific N-glycan analysis revealed structural differences from original epoetin alfa drug Eprex®. J. Pharm. Biomed. Anal. 2021, 194, 113750. [Google Scholar] [CrossRef]
- Heuberger, J.; van Eenoo, P.; Rotmans, J.I.; Gal, P.; Stuurman, F.E.; Post, T.E.; Daniels, J.M.A.; Ram, H.; de Hon, O.; Burggraaf, J.; et al. Sensitivity and specificity of detection methods for erythropoietin doping in cyclists. Drug Test. Anal. 2019, 11, 1290–1301. [Google Scholar] [CrossRef]
- Jelkmann, W. Erythropoietin. Front. Horm. Res. 2016, 47, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, W.; Lundby, C. Blood doping and its detection. Blood 2011, 118, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Leuenberger, N.; Reichel, C.; Lasne, F. Detection of erythropoiesis-stimulating agents in human anti-doping control: Past, present and future. Bioanalysis 2012, 4, 1565–1575. [Google Scholar] [CrossRef]
- Reichel, C. Recent developments in doping testing for erythropoietin. Anal. Bioanal. Chem. 2011, 401, 463–481. [Google Scholar] [CrossRef]
- Lasne, F.; de Ceaurriz, J. Recombinant erythropoietin in urine. Nature 2000, 405, 635. [Google Scholar] [CrossRef]
- Martin, L.; Martin, J.A.; Audran, M.; Marchand, A. An optimized SDS-PAGE protocol with a new blotting system for the initial testing procedure of ESAs in doping control. Drug Test. Anal. 2022, 14, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Reihlen, P.; Blobel, M.; Kempkes, R.; Reichel, C.; Völker Schänzer, E.; Majer, B.; Schänzer, W. Optimizing SAR-PAGE. Drug Test. Anal. 2015, 7, 1014–1016. [Google Scholar] [CrossRef]
- Reihlen, P.; Blobel, M.; Weiß, P.; Harth, J.; Wittmann, J.; Leenders, F.; Thevis, M. Introduction of a PEGylated EPO conjugate as internal standard for EPO analysis in doping controls. Drug Test. Anal. 2021, 1–7. [Google Scholar] [CrossRef]
- Lasne, F.; Thioulouse, J.; Martin, L.; de Ceaurriz, J. Detection of recombinant human erythropoietin in urine for doping analysis: Interpretation of isoelectric profiles by discriminant analysis. Electrophoresis 2007, 28, 1875–1881. [Google Scholar] [CrossRef]
- Reihlen, P.; Völker-Schänzer, E.; Majer, B.; Schänzer, W. Easy-to-use IEF compatible immunoaffinity purification of Erythropoietin from urine retentates. Drug Test. Anal. 2012, 4, 813–817. [Google Scholar] [CrossRef]
- Reichel, C. SARCOSYL-PAGE: A new electrophoretic method for the separation and immunological detection of PEGylated proteins. Methods Mol. Biol. 2012, 869, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhang, Y.; Wu, J.; Jia, L. Poly(norepinephrine)-coated open tubular column for the separation of proteins and recombination human erythropoietin by capillary electrochromatography. J. Sep. Sci. 2017, 40, 4636–4644. [Google Scholar] [CrossRef] [PubMed]
- Desharnais, P.; Naud, J.F.; Ayotte, C. Detection of erythropoiesis stimulating agents in urine samples using a capillary Western system. Drug Test. Anal. 2018, 10, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.J.; Zhang, X.X.; Li, X.; Chen, H.X. Isoforms analysis of recombinant human erythropoietin by polarity-reversed capillary isoelectric focusing. Electrophoresis 2020, 41, 2055–2061. [Google Scholar] [CrossRef]
- Li, X.; Yu, L.; Shi, X.; Rao, C.; Zhou, Y. Capillary isoelectric focusing with UV fluorescence imaging detection enables direct charge heterogeneity characterization of erythropoietin drug products. J. Chromatogr. A 2021, 1643, 462043. [Google Scholar] [CrossRef]
- Okano, M.; Sato, M.; Kageyama, S. Identification of the long-acting erythropoiesis-stimulating agent darbepoetin alfa in human urine by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 1317–1329. [Google Scholar] [CrossRef]
- Guan, F.; Uboh, C.E.; Soma, L.R.; Birks, E.; Chen, J.; Mitchell, J.; You, Y.; Rudy, J.; Xu, F.; Li, X.; et al. LC-MS/MS method for confirmation of recombinant human erythropoietin and darbepoetin alpha in equine plasma. Anal. Chem. 2007, 79, 4627–4635. [Google Scholar] [CrossRef]
- Yasuoka, Y.; Fukuyama, T.; Izumi, Y.; Yamashita, T.; Nakayama, Y.; Inoue, H.; Yanagita, K.; Oshima, T.; Yamazaki, T.; Uematsu, T.; et al. Differentiation of endogenous erythropoietin and exogenous ESAs by Western blotting. Heliyon 2020, 6, e05389. [Google Scholar] [CrossRef]
- Sugasawa, T.; Nakano, T.; Fujita, S.I.; Matsumoto, Y.; Ishihara, G.; Aoki, K.; Yanazawa, K.; Ono, S.; Tamai, S.; Manevich, L.; et al. Proof of Gene Doping in a Mouse Model with a Human Erythropoietin Gene Transferred Using an Adenoviral Vector. Genes 2021, 12, 1249. [Google Scholar] [CrossRef]
- Nag, S.; Resnick, A. Stabilization of hypoxia inducible factor by cobalt chloride can alter renal epithelial transport. Physiol. Rep. 2017, 5, e13531. [Google Scholar] [CrossRef]
- Fliedl, L.; Manhart, G.; Kast, F.; Katinger, H.; Kunert, R.; Grillari, J.; Wieser, M.; Grillari-Voglauer, R. Novel human renal proximal tubular cell line for the production of complex proteins. J. Biotechnol. 2014, 176, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Baraghithy, S.; Soae, Y.; Assaf, D.; Hinden, L.; Udi, S.; Drori, A.; Gabet, Y.; Tam, J. Renal Proximal Tubule Cell Cannabinoid-1 Receptor Regulates Bone Remodeling and Mass via a Kidney-to-Bone Axis. Cells 2021, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, H.; Yu, S.J.; Afedo, S.Y.; Bai, X.F. Effects of PHD and HSP90 on erythropoietin production in yak (Bos grunniens) renal interstitial fibroblast-like cells under hypoxia. J. Mol. Histol. 2022, 53, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Kiriyama, N.; Ogawa, K.; Inoue, R.; Haque, M.A.; Nakagawa, H. Effect of pentavalent inorganic arsenic salt on erythropoietin production and autophagy induction. Arch. Biochem. Biophys. 2023, 734, 109487. [Google Scholar] [CrossRef] [PubMed]
- Yasuoka, Y.; Fukuyama, T.; Izumi, Y.; Nakayama, Y.; Inoue, H.; Yanagita, K.; Oshima, T.; Yamazaki, T.; Uematsu, T.; Kobayashi, N.; et al. Erythropoietin production by the kidney and the liver in response to severe hypoxia evaluated by Western blotting with deglycosylation. Physiol. Rep. 2020, 8, e14485. [Google Scholar] [CrossRef]
- Yasuoka, Y.; Izumi, Y.; Fukuyama, T.; Inoue, H.; Oshima, T.; Yamazaki, T.; Uematsu, T.; Kobayashi, N.; Shimada, Y.; Nagaba, Y.; et al. Effects of Angiotensin II on Erythropoietin Production in the Kidney and Liver. Molecules 2021, 26, 5399. [Google Scholar] [CrossRef]
- Yasuoka, Y.; Izumi, Y.; Fukuyama, T.; Omiya, H.; Pham, T.D.; Inoue, H.; Oshima, T.; Yamazaki, T.; Uematsu, T.; Kobayashi, N.; et al. Effects of Roxadustat on Erythropoietin Production in the Rat Body. Molecules 2022, 27, 1119. [Google Scholar] [CrossRef]
- Sato, K.; Hirano, I.; Sekine, H.; Miyauchi, K.; Nakai, T.; Kato, K.; Ito, S.; Yamamoto, M.; Suzuki, N. An immortalized cell line derived from renal erythropoietin-producing (REP) cells demonstrates their potential to transform into myofibroblasts. Sci. Rep. 2019, 9, 11254. [Google Scholar] [CrossRef]
- Yin, H.; Blanchard, K.L. DNA methylation represses the expression of the human erythropoietin gene by two different mechanisms. Blood 2000, 95, 111–119. [Google Scholar] [CrossRef]
- Rössler, J.; Stolze, I.; Frede, S.; Freitag, P.; Schweigerer, L.; Havers, W.; Fandrey, J. Hypoxia-induced erythropoietin expression in human neuroblastoma requires a methylation free HIF-1 binding site. J. Cell. Biochem. 2004, 93, 153–161. [Google Scholar] [CrossRef]
- Yasuoka, Y.; Izumi, Y.; Nagai, T.; Fukuyama, T.; Nakayama, Y.; Inoue, H.; Horikawa, K.; Kimura, M.; Nanami, M.; Yanagita, K.; et al. Fludrocortisone stimulates erythropoietin production in the intercalated cells of the collecting ducts. Biochem. Biophys. Res. Commun. 2018, 503, 3121–3127. [Google Scholar] [CrossRef] [PubMed]
- Bapst, A.M.; Knöpfel, T.; Nolan, K.A.; Imeri, F.; Schuh, C.D.; Hall, A.M.; Guo, J.; Katschinski, D.M.; Wenger, R.H. Neurogenic and pericytic plasticity of conditionally immortalized cells derived from renal erythropoietin-producing cells. J. Cell. Physiol. 2022, 237, 2420–2433. [Google Scholar] [CrossRef] [PubMed]
- Obara, N.; Suzuki, N.; Kim, K.; Nagasawa, T.; Imagawa, S.; Yamamoto, M. Repression via the GATA box is essential for tissue-specific erythropoietin gene expression. Blood 2008, 111, 5223–5232. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yamamoto, M. Roles of renal erythropoietin-producing (REP) cells in the maintenance of systemic oxygen homeostasis. Pflugers Arch. 2016, 468, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Davidoff, O.; Pujari-Palmer, S.; Drevin, M.; Haase, V.H. EPO synthesis induced by HIF-PHD inhibition is dependent on myofibroblast transdifferentiation and colocalizes with non-injured nephron segments in murine kidney fibrosis. Acta Physiol. 2022, 235, e13826. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Yasuoka, Y.; Izumi, Y.; Horikawa, K.; Kimura, M.; Nakayama, Y.; Uematsu, T.; Fukuyama, T.; Yamazaki, T.; Kohda, Y.; et al. Reevaluation of erythropoietin production by the nephron. Biochem. Biophys. Res. Commun. 2014, 449, 222–228. [Google Scholar] [CrossRef]
- Sue, J.M.; Sytkowski, A.J. Site-specific antibodies to human erythropoietin directed toward the NH2-terminal region. Proc. Natl. Acad. Sci. USA 1983, 80, 3651–3655. [Google Scholar] [CrossRef]
- Sytkowski, A.J.; Fisher, J.W. Isolation and characterization of an anti-peptide monoclonal antibody to human erythropoietin. J. Biol. Chem. 1985, 260, 14727–14731. [Google Scholar] [CrossRef]
- Lee, J.W.; Chou, C.L.; Knepper, M.A. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-Specific Transcriptomes. J. Am. Soc. Nephrol. 2015, 26, 2669–2677. [Google Scholar] [CrossRef]
- Ito, M.; Tanaka, T.; Ishii, T.; Wakashima, T.; Fukui, K.; Nangaku, M. Prolyl hydroxylase inhibition protects the kidneys from ischemia via upregulation of glycogen storage. Kidney Int. 2020, 97, 687–701. [Google Scholar] [CrossRef]
- Joharapurkar, A.A.; Patel, V.J.; Kshirsagar, S.G.; Patel, M.S.; Savsani, H.H.; Jain, M.R. Prolyl hydroxylase inhibitor desidustat protects against acute and chronic kidney injury by reducing inflammatory cytokines and oxidative stress. Drug Dev. Res. 2021, 82, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Miao, A.F.; Liang, J.X.; Yao, L.; Han, J.L.; Zhou, L.J. Hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) protects against renal ischemia/reperfusion injury by inhibiting inflammation. Ren. Fail. 2021, 43, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dong, R.; Yuan, J.; Da, J.; Zha, Y.; Long, Y. Roxadustat (FG-4592) protects against ischaemia/reperfusion-induced acute kidney injury through inhibiting the mitochondrial damage pathway in mice. Clin. Exp. Pharmacol. Physiol. 2022, 49, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Buisson, C.; Marchand, A.; Bailloux, I.; Lahaussois, A.; Martin, L.; Molina, A. Detection by LC-MS/MS of HIF stabilizer FG-4592 used as a new doping agent: Investigation on a positive case. J. Pharm. Biomed. Anal. 2016, 121, 181–187. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuoka, Y.; Izumi, Y.; Sands, J.M.; Kawahara, K.; Nonoguchi, H. Progress in the Detection of Erythropoietin in Blood, Urine, and Tissue. Molecules 2023, 28, 4446. https://doi.org/10.3390/molecules28114446
Yasuoka Y, Izumi Y, Sands JM, Kawahara K, Nonoguchi H. Progress in the Detection of Erythropoietin in Blood, Urine, and Tissue. Molecules. 2023; 28(11):4446. https://doi.org/10.3390/molecules28114446
Chicago/Turabian StyleYasuoka, Yukiko, Yuichiro Izumi, Jeff M. Sands, Katsumasa Kawahara, and Hiroshi Nonoguchi. 2023. "Progress in the Detection of Erythropoietin in Blood, Urine, and Tissue" Molecules 28, no. 11: 4446. https://doi.org/10.3390/molecules28114446
APA StyleYasuoka, Y., Izumi, Y., Sands, J. M., Kawahara, K., & Nonoguchi, H. (2023). Progress in the Detection of Erythropoietin in Blood, Urine, and Tissue. Molecules, 28(11), 4446. https://doi.org/10.3390/molecules28114446





