Pterospartum tridentatum Liqueur Using Spirits Aged with Almond Shells: Chemical Characterization and Phenolic Profile
Abstract
1. Introduction
2. Results & Discussion
2.1. Physicochemical Analysis of the Spirits and the Liqueur
2.2. Total Phenolic Compounds, Flavonoids, and Tannins
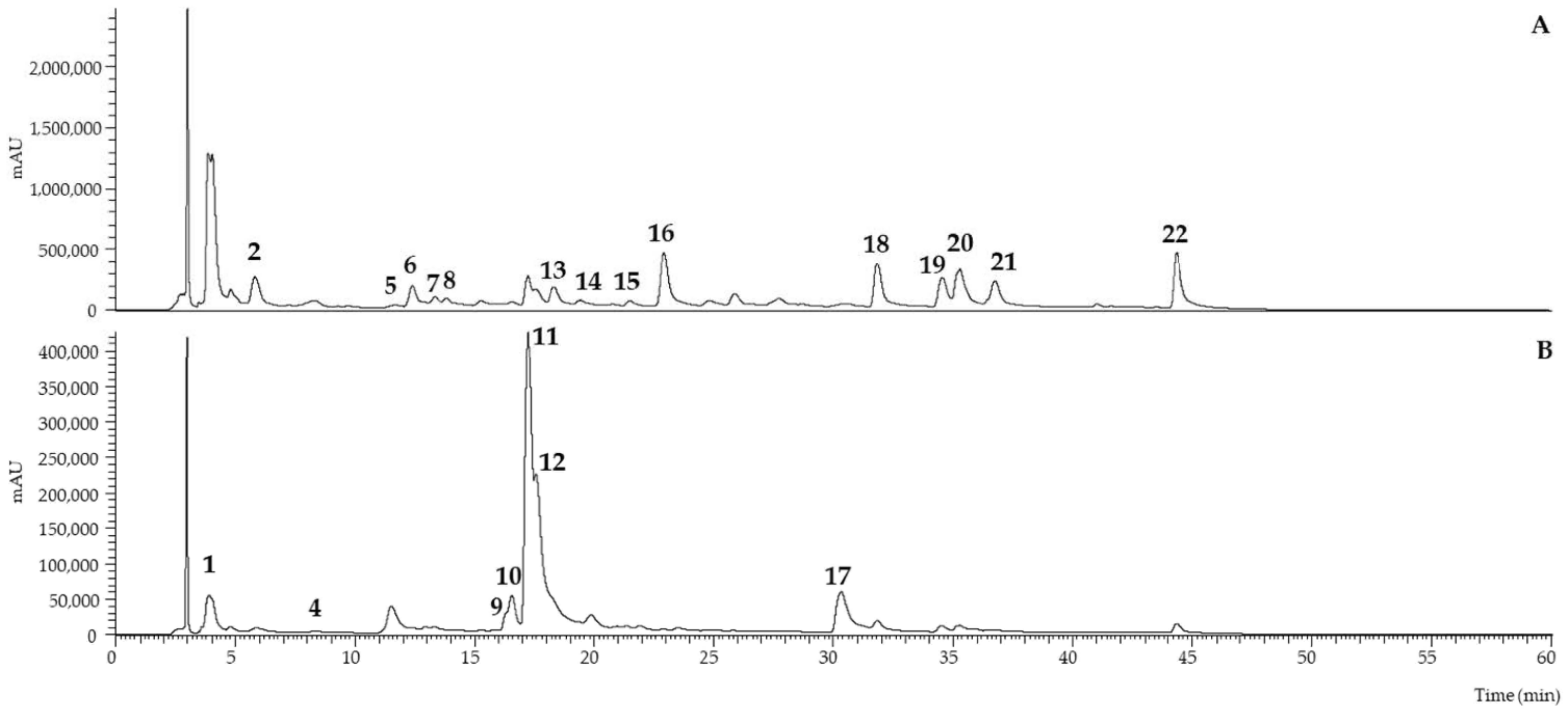
2.3. Analysis of the Phenolic Compounds by HPLC
| Peak | Rt (min) | λmax (nm) | [M − H]− (m/z) | MS2 (m/z) | Tentative Identification | Reference |
|---|---|---|---|---|---|---|
| 1 | 4.82 | 291, sh339 | 465 | 447 (9), 375 (12), 357 (5), 345 (100), 327 (11), 317 (5), 167 (7) | Dihydroquercetin-C-hexoside | [7] |
| 2 | 5.84 | 284, sh340 | 479 | 359 (100), 341 (5), 221 (5), 167 (5) | Myricetin-C-hexoside | [7] |
| 3 | 6.68 | 292, sh338 | - | - | Unknown compound | - |
| 4 | 8.29 | 258/301 | 465 | 447 (9), 375 (13), 357 (5), 345 (100), 327 (15), 317 (5), 167 (7) | Dihydroquercetin-C-hexoside | [7] |
| 5 | 11.54 | 367 | 413 | 311 (100), 269 (25) | Genistein derivative | DAD/MS |
| 6 | 12.41 | 255/320 | 491 | 445 (10), 283 (100), 269 (60) | 3′-Methoxy daidzin | [26] |
| 7 | 13.36 | 261/320 | 431 | 311 (100), 283 (10) | Genistein-C-hexoside | DAD/MS |
| 8 | 13.84 | 261/320 | 431 | 311 (100), 283 (12) | Genistein-C-hexoside | DAD/MS |
| 9 | 16.4 | 352 | 609 | 301 (100) | Quercetin-O-deoxylhexosyl-hexoside | DAD/MS |
| 10 | 16.55 | 357 | 609 | 301 (100) | Quercetin-O-deoxyhexosyl-hexoside | DAD/MS |
| 11 | 17.25 | 354 | 463 | 301 (100) | Quercetin-O-hexoside | DAD/MS |
| 12 | 17.56 | 353 | 463 | 301 (100) | Quercetin-O-hexoside | DAD/MS |
| 13 | 18.33 | 260/329 | 463 | 301 (100) | Ellagic acid hexoside | DAD/MS |
| 14 | 19.85 | 261/321 | 433 | 301 (100) | Ellagic acid pentoside | DAD/MS |
| 15 | 21.5 | 260/322 | 431 | 311 (10), 269 (100) | Genistein 7-O-glucoside (Genistein) | Composto Padrão |
| 16 | 22.92 | 256/320 | 505 | 459 (5), 297 (100), 282 (76) | Methylbiochanin A/methylprunetin O-hexoside | [7] |
| 17 | 30.33 | 368 | 301 | 179 (100), 151 (78) | Quercetin | DAD/MS |
| 18 | 31.82 | 260/320 | 491 | 445 (3), 283 (100) | Biochanin A O-hexoside | [7] |
| 19 | 34.53 | 260/320 | 269 | 241 (4), 225 (6), 201 (5), 181 (2), 133 (7) | Genistein | DAD/MS |
| 20 | 35.26 | 260/320 | 283 | 268 (100), 239 (7), 224 (5), 195 (2), 135 (2) | 4′-O-Methylgenistein (biochanin A) | [7] |
| 21 | 36.72 | 260/320 | 297 | 282 (100) | Methylbiochanin A/methylprunetin | [7] |
| 22 | 44.33 | 260/320 | 283 | 268 (100) | 7-O-Methylgenistein (prunetin) | [7] |
| Compound | WS | WSA | CE | CL |
|---|---|---|---|---|
| Dihydroquercetin-C-hesoxide | nd | 18.3 ± 0.2 a | 68.4 ± 6.6 c | 28.9 ± 0.5 b |
| Myricetin-C-hexoside | nd | nd | 138.4 ± 11.0 * | 67.9 ± 4.5 * |
| Unknown compound | nq | nq | nd | nd |
| Dihydroquercetin-C-hexoside | nd | 7.0 ± 0.3 a | 57.6 ± 14.3 c | 16.3 ± 0.3 b |
| Genistein derivative | nd | nd | 11.1 ± 0.5 b | 2.5 ± 0.1 a |
| 3′-Methoxy daidzin | nd | 9.8 ± 0.1 * | 134.9 ± 8.4 * | nd |
| Genistein-C-hexoside | nd | tr | 23.8 ± 2.6 * | 9.4 ± 0.2 * |
| Genistein-C-hexoside | nd | nd | 22.2 ± 0.8 | nd |
| Quercetin-O-deoxylhexosyl-hexoside | nd | nd | 13.4 ± 1.3 * | 6.2 ± 0.1 * |
| Quercetin-O-deoxyhexosyl-hexoside | nd | nd | 24.7 ± 3.0 * | 6.2 ± 0.2 * |
| Quercetin-O-hexoside | nd | nd | 214.4 ± 14.3 * | 13.6 ± 0.2 * |
| Quercetin-O-hexoside | nd | nd | 109.3 ± 18.3 * | 9.1 ± 0.1 * |
| Ellagic acid hexoside | nd | nd | 151.8 ± 8.6 | nd |
| Ellagic acid pentoside | nd | 21.7 ± 0.04 b | 36.9 ± 1.9 c | 14.5 ± 0.1 b |
| Genistein 7-O-glucoside (Genistein) | nd | nd | 19.5 ± 1.0 | nd |
| Methylbiochanin A/methylprunetin O-hexoside | nd | nd | 257.6 ± 33.6 | nd |
| Quercetin | nd | 6.3 ± 0.05 a | 47.3 ± 3.9 b | 6.6 ± 0.3 a |
| Biochanin A O-hexoside | nd | nd | 201.4 ± 13.9 | nd |
| Genistein | nd | nd | 95.1 ± 6.6 * | 0.1 ± 0.01 * |
| 4′-O-Methylgenistein (biochanin A) | nd | nd | 180.5 ± 58.5 | tr |
| Methylbiochanin A/methylprunetin | nd | nd | 137.8 ± 26.1 | tr |
| 7-O-Methylgenistein (prunetin) | nd | nd | 146.3 ± 6.5 | nd |
| Total isoflavones | nd | tr | 1230.2 ± 139.2 * | 12.0 ± 0.3 * |
| Total ellagic acid derivatives | nd | 33.6 ± 0.04 b | 188.7 ± 7.0 c | 14.5 ± 0.10 a |
| Total flavonoids | nd | 54.5 ± 0.10 a | 673.6 ± 42.7 c | 154.7 ± 4.2 b |
| Total Phenolic Compounds | nd | 43.4 ± 0.04 a | 2092.4 ± 176.5 c | 181.2 ± 3.8 b |
2.4. Sensorial Analysis
3. Materials and Methods
3.1. Materials and Samples for Analysis
3.2. Physicochemical Analysis of Spirits and Liqueur
3.3. Determination of Total Phenolic Compounds
3.4. Determination of Total Flavonoids
3.5. Determination of Tannins
3.6. Analysis of the Phenolic Compounds by HPLC
3.7. Sensorial Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Martins, J. Erva Carqueja—Um Chá Para Todos os Males; Universidade Fernando Pessoa: Porto, Portugal, 2011. [Google Scholar]
- Simões, M.A.M.; Pinto, D.C.G.A.; Neves, B.M.R.; Silva, A.M.S. Flavonoid Profile of the Genista tridentata L., a Species Used Traditionally to Treat Inflammatory Processes. Molecules 2020, 25, 812. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.M.; Dinis, L.T.; Azedo, P.; Galhano, C.I.C.; Simões, A.; Cardoso, S.M.; Domingues, M.R.M.; Pereira, O.R.; Palmeira, C.M.; Peixoto, F.P. Antioxidant capacity and toxicological evaluation of Pterospartum tridentatum flower extracts. CYTA—J. Food 2012, 10, 92–102. [Google Scholar] [CrossRef]
- Pimenta, A.C.M. Extractos aquosos de Pterospartum tridentatum L. Teor de Compostos Fenólicos Totais e Actividade Antioxidante. Master’s Thesis, Universidade Técnica de Lisboa, Lisbon, Portugal, 2012. Available online: http://hdl.handle.net/10400.5/5310 (accessed on 29 November 2021).
- Vitor, R.F.; Mota-Filipe, H.; Teixeira, G.; Borges, C.; Rodrigues, A.I.; Teixeira, A.; Paulo, A. Flavonoids of an extract of Pterospartum tridentatum showing endothelial protection against oxidative injury. J. Ethnopharmacol. 2004, 93, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Marrinhas, E.; Carvalho, R.; Dias, C.; Saavedra, M.J. Phytochemical composition and antibacterial activity of hydroalcoholic extracts of pterospartum tridentatum and mentha pulegium against staphylococcus aureus isolates. Biomed. Res. Int. 2016, 2016, 5201879. [Google Scholar] [CrossRef] [PubMed]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Pterospartum tridentatum, Gomphrena globosa and Cymbopogon citratus: A phytochemical study focused on antioxidant compounds. Food Res. Int. 2014, 62, 684–693. [Google Scholar] [CrossRef]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. HPLC-profiles of tocopherols, sugars, and organic acids in three medicinal plants consumed as infusions. Int. J. Food Sci. 2014, 2014, 5. [Google Scholar] [CrossRef]
- Pinto, T.; Vilela, A. Healthy drinks with lovely colors: Phenolic compounds as constituents of functional beverages. Beverages 2021, 7, 12. [Google Scholar] [CrossRef]
- SpiritsEurope Association. Internal Market. 2021. Available online: https://spirits.eu/issues/internal-market/introduction-3 (accessed on 7 October 2021).
- Regulation (EU) 2019/787 of the European Parliament and of the Council of 17 April 2019. Off. J. Eur. Union 2019, L130, 1–54.
- Teixeira, L.; Ramos, A.; Chaves, J.; Silva, P.; Stingheta, P. Avaliação tecnológica da extração alcoólica no processamento de licor de banana. Bol. CEPPA 2005, 23, 329–346. [Google Scholar] [CrossRef]
- Schmitzer, V.; Mikulic-Petkovsek, M.; Stampar, F. Traditional rose liqueur—A pink delight rich in phenolics. Food Chem. 2019, 272, 434–440. [Google Scholar] [CrossRef]
- Schuina, G.L.; Quelhas, J.O.F.; de Carvalho, G.B.M.; Del Bianchi, V.L. Use of carqueja (Baccharis trimera (Less.) DC. Asteraceae) as a total substitute for hops in the production of lager beer. J. Food Process. Preserv. 2020, 44, e14730. [Google Scholar] [CrossRef]
- Sá, C.C.; Pombo, J.C.P.; Botelho, V.A. Elaboração do licor de romã e canela: Qualidade físico-química e microbiologica. In Ciência, Tecnologia e Inovação: Do Campo à; Mesa; Editora IIDV: Recife, Brasil, 2020; pp. 426–441. [Google Scholar] [CrossRef]
- Rodrigues, V.N. Licor de Guabiroba (Campomanesia xanthocarpa): Análise Mercadológica, Desenvolvimento e Caracterização Físico-Química e Sensorial; Universidade Federal da Fronteira Sul: Chapecó, Brazil, 2017; Available online: https://rd.uffs.edu.br/handle/prefix/521 (accessed on 29 November 2021).
- Amado, S.I.P. Efeitos de Diferentes Tecnologias de Envelhecimento no Perfil Sensorial de Aguardentes Vínicas; Instituto Politécnico de Castelo Branco: Castelo Branco, Portugal, 2014. [Google Scholar]
- Luís, Â.; Domingues, F.; Gil, C.; Duarte, A.P. Antioxidant activity of extracts of Portuguese shrubs: Pterospartum tridentatum, Cytisus scoparius and Erica spp. J. Med. Plants Res. 2009, 3, 886–893. [Google Scholar]
- Coelho, M.T.P.P.G.R. Estudos de Propagação In Vitro, Caracterização e Valorização de Carqueja (Pterospartum tridentatum (L.) Willk); Universidade de Lisboa: Lisbon, Portugal, 2015; Available online: http://hdl.handle.net/10400.5/9271 (accessed on 29 November 2021).
- Queirós, C.S.G.P.; Cardoso, S.; Lourenço, A.; Ferreira, J.; Miranda, I.; Lourenço, M.J.V.; Pereira, H. Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Convers. Biorefin. 2020, 10, 175–188. [Google Scholar] [CrossRef]
- Ebringerová, A.; Hromádková, Z.; Košťálová, Z.; Sasinková, V. Chemical valorization of agricultural by-products: Isolation and characterization of xylan-based antioxidants from almond shell biomass. BioResources 2008, 3, 60–70. [Google Scholar]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I.R.N.A. A Box-Behnken Design for Optimal Extraction of Phenolics from Almond By-products. Food Anal. Methods 2019, 12, 2009–2024. [Google Scholar] [CrossRef]
- Thebo, N.; Simair, A.; Sheikh, W.; Ar, A.; JabbarLaghari, A.; HassanNizamani, M. Clinical Study of the Prunus dulcis (Almond) Shell Extract on Tinea capitis Infection. Nat. Prod. Chem. Res. 2014, 2. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Duarte, A.P. Bioactive compounds, RP-HPLC analysis of phenolics, and antioxidant activity of some Portuguese shrub species extracts. Nat. Prod. Commun. 2011, 6, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.B.; Martins, N.G.S.; de Belluco, A.E.S.; Horii, J.; Alcarde, A.R. Perfil físico-químico de aguardente durante envelhecimento em tonéis de carvalho. Ciência Tecnol. Aliment. 2008, 28, 84–89. [Google Scholar] [CrossRef]
- Qiao, X.; Song, W.; Ji, S.; Li, Y.; Wang, Y.; Li, R.; An, R.; Guo, D.; Ye, M. Separation and detection of minor constituents in herbal medicines using a combination of heart-cutting and comprehensive two-dimensional liquid chromatography. J. Chromatogr. A 2014, 1362, 157–167. [Google Scholar] [CrossRef]
- Milbury, P.E.; Chen, C.Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of flavonoids and phenolics and their distribution in almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef]
- Oyeleye, S.I.; Adebayo, A.A.; Ogunsuyi, O.B.; Dada, F.A.; Oboh, G. Phenolic profile and Enzyme Inhibitory activities of Almond (Terminalia catappa) leaf and Stem bark. Int. J. Food Prop. 2018, 20, S2810–S2821. [Google Scholar] [CrossRef]
- Bolling, B.W. Almond Polyphenols: Methods of Analysis, Contribution to Food Quality, and Health Promotion. Compr. Rev. Food Sci. Food Saf. 2017, 16, 346–368. [Google Scholar] [CrossRef]
- Paulo, A.; Martins, S.; Branco, P.; Dias, T.; Borges, C.; Rodrigues, A.I.; do Costa, M.C.; Teixeira, A.; Mota-Filipe, H. The Opposing Effects of the Flavonoids Isoquercitrin and Sissotrin, isolated from Pterospartum tridentatum, on Oral Glucose Tolerance in Rats. Phyther. Res. 2008, 22, 539–543. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Spirituous Beverages of Vitivinicultural Origin; OIV: Dijon, France, 2019; p. 226.
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144 LP–158 LP. [Google Scholar] [CrossRef]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- AOAC International Titrimetric Method for tannins. In AOAC Official Methods of Analysis; AOAC: Rockville, MD, USA, 1980.
- Atanassova, M.; Christova-Bagdassarian, V. Determination of tannins content by titrimetric method for comparison of different plant species. J. Univ. Chem. Tecnol. Metall. 2009, 44, 413–415. [Google Scholar]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Cruz, S.I.A. Evolução da Composição Físico-Química da Aguardente Vínica Lourinhã nos Primeiros Seis Meses de Envelhecimento em Sistema Tradicional e em Sistema Alternativo; Universidade Lisboa: Lisbon, Portugal, 2016; Available online: http://hdl.handle.net/10400.5/13371 (accessed on 29 November 2021).

| Parameter | WS | WSA | CL | p-Value |
|---|---|---|---|---|
| pH | 2.09 ± 0.09 a | 3.29 ± 0.01 b | 4.17 ± 0.01 c | p < 0.001 |
| Alcohol content (%, vol) | 53.12 ± 0.38 | 51.57 ± 0.06 | 18.33 ± 0.23 a | p < 0.001 |
| Dry extract (g/100 mL) | 0.034 ± 0.280 a | 0.248 ± 0.036 b | 55.170 ± 2.832 c | p < 0.001 |
| Color intensity (Abs445 nm) | 0.095 ± 0.005 a | 0.481 ± 0.002 b | 1.509 ± 0.011 c | p < 0.001 |
| Color | ||||
| L* | 100.01 ± 0.00 c | 98.57 ± 0.01 b | 89.54 ± 0.27 a | p < 0.001 |
| a* | −5.25 ± 0.02 a | −6.18 ± 0.01 b | −6.58 ± 0.07 c | p < 0.001 |
| b* | 5.42 ± 0.01 a | 12.73 ± 0.01 b | 23.47 ± 0.10 c | p < 0.001 |
| Acidity (mg acetic acid/L) | ||||
| Total | 1138.40 ± 19.4 a,b | 1189.60 ± 1.39 b | 1111.20 ± 35.52 a | p < 0.05 |
| Fixed | 282.40 ± 8.43 a | 409.60 ± 12.32 b | 759.20 ± 1.39 c | p < 0.001 |
| Volatile | 856.00 ± 12.32 c | 780.00 ± 12.70 b | 352.00 ± 36.90 a ** | p < 0.05 ** p < 0.001 |
| Viscosity (cP) | - | - | 2.511 ± 0.002 | - |
| Parameter | WS | WSA | CE * | CL |
|---|---|---|---|---|
| TPC (mg GAE/L) | 23.03 ± 0.11 a | 211.29 ± 2.18 b | 1485.41± 60.69 c | 1640.92 ± 3.36 b |
| TF (mg EACT/L) | 49.13 ± 0.10 a | 601.60 ± 10.10 b | 7175.44 ± 70.16 c | 8642.68 ± 69.28 d |
| Tan (mg ET/100 g) | 0.61 ± 0.00 a | 30.60 ± 1.22 b | 811.51 ± 12.32 d | 116.66 ± 2.33 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, C.; Dias, M.I.; Henriques, M.H.F.; Barros, L.; Ramos, F. Pterospartum tridentatum Liqueur Using Spirits Aged with Almond Shells: Chemical Characterization and Phenolic Profile. Molecules 2023, 28, 4455. https://doi.org/10.3390/molecules28114455
Garcia C, Dias MI, Henriques MHF, Barros L, Ramos F. Pterospartum tridentatum Liqueur Using Spirits Aged with Almond Shells: Chemical Characterization and Phenolic Profile. Molecules. 2023; 28(11):4455. https://doi.org/10.3390/molecules28114455
Chicago/Turabian StyleGarcia, Cátia, Maria Inês Dias, Marta H. F. Henriques, Lillian Barros, and Fernando Ramos. 2023. "Pterospartum tridentatum Liqueur Using Spirits Aged with Almond Shells: Chemical Characterization and Phenolic Profile" Molecules 28, no. 11: 4455. https://doi.org/10.3390/molecules28114455
APA StyleGarcia, C., Dias, M. I., Henriques, M. H. F., Barros, L., & Ramos, F. (2023). Pterospartum tridentatum Liqueur Using Spirits Aged with Almond Shells: Chemical Characterization and Phenolic Profile. Molecules, 28(11), 4455. https://doi.org/10.3390/molecules28114455










