Site-Selective Solvation-Induced Conformational Switching of Heteroleptic Heteronuclear Tb(III) and Y(III) Trisphthalocyaninates for the Control of Their Magnetic Anisotropy
Abstract
:1. Introduction
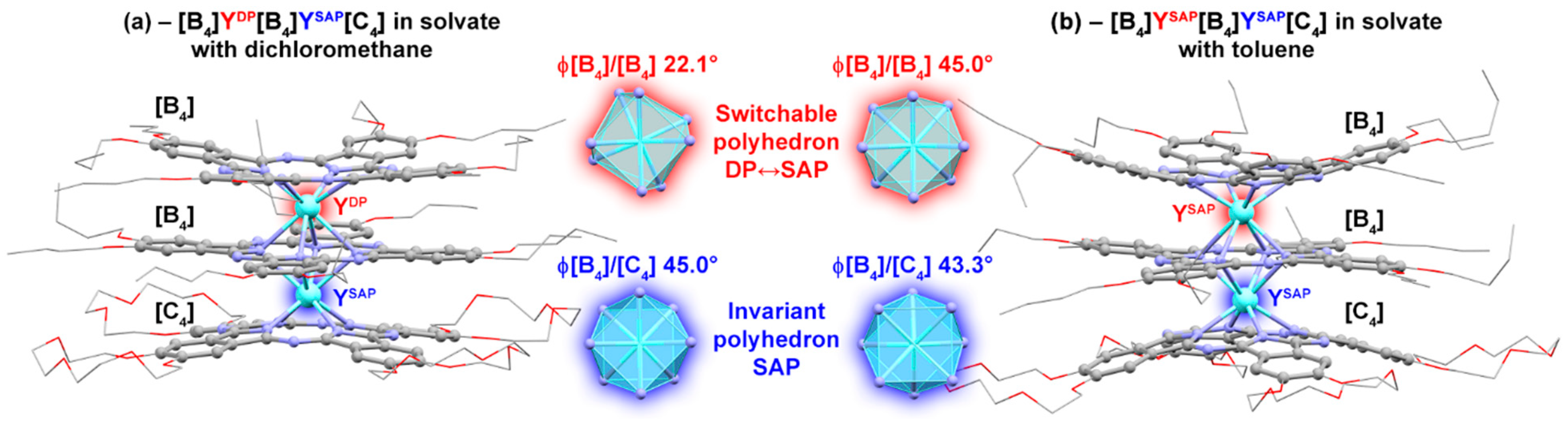
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.3. Synthesis and Characterization of the Triple-Decker Complexes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Feng, J.; Zhang, H. Hybrid Materials Based on Lanthanide Organic Complexes: A Review. Chem. Soc. Rev. 2013, 42, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Martynov, A.G.; Horii, Y.; Katoh, K.; Bian, Y.; Jiang, J.; Yamashita, M.; Gorbunova, Y.G. Rare-Earth Based Tetrapyrrolic Sandwiches: Chemistry, Materials and Applications. Chem. Soc. Rev. 2022, 51, 9262–9339. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Zhu, M.; Zhang, J.-L. Near-Infrared (NIR) Lanthanide Molecular Probes for Bioimaging and Biosensing. Coord. Chem. Rev. 2019, 399, 213028. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, M.; Li, X.-L.; Tang, J. Molecular Magnetism of Lanthanide: Advances and Perspectives. Coord. Chem. Rev. 2019, 378, 350–364. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Heffern, M.C.; Matosziuk, L.M.; Meade, T.J. Lanthanide Probes for Bioresponsive Imaging. Chem. Rev. 2014, 114, 4496–4539. [Google Scholar] [CrossRef]
- Lacerda, S.; Tóth, É. Lanthanide Complexes in Molecular Magnetic Resonance Imaging and Theranostics. ChemMedChem 2017, 12, 883–894. [Google Scholar] [CrossRef]
- Gamov, G.A.; Zavalishin, M.N.; Pimenov, O.A.; Klochkov, V.V.; Khodov, I.A. La(III), Ce(III), Gd(III), and Eu(III) Complexation with Tris(Hydroxymethyl)Aminomethane in Aqueous Solution. Inorg. Chem. 2020, 59, 17783–17793. [Google Scholar] [CrossRef]
- Utochnikova, V.V. The Use of Luminescent Spectroscopy to Obtain Information about the Composition and the Structure of Lanthanide Coordination Compounds. Coord. Chem. Rev. 2019, 398, 113006. [Google Scholar] [CrossRef]
- Liddle, S.T.; Van Slageren, J. Improving F-Element Single Molecule Magnets. Chem. Soc. Rev. 2015, 44, 6655–6669. [Google Scholar] [CrossRef]
- Piguet, C.; Geraldes, C.F.G.C. Paramagnetic NMR Lanthanide Induced Shifts for Extracting Solution Structures. In Handbook on the Physics and Chemistry of Rare Earths; Gschneidner, K.A., Bünzli, J.-C.G., Pecharsky, V.K., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2003; Volume 33, pp. 353–463. ISBN 9780444513236. [Google Scholar]
- Golding, R.; Halton, M. A Theoretical Study of the 14N and 17O N.M.R. Shifts in Lanthanide Complexes. Aust. J. Chem. 1972, 25, 2577. [Google Scholar] [CrossRef]
- Pinkerton, A.A.A.; Rossier, M.; Spiliadis, S.; Rower, M. Lanthanide-Induced Contact Shifts. the Average Electron Spin Polarization, Theory and Experiment. J. Magn. Reson. 1985, 64, 420–425. [Google Scholar] [CrossRef]
- Bleaney, B. Nuclear Magnetic Resonance Shifts in Solution Due to Lanthanide Ions. J. Magn. Reson. 1972, 8, 91–100. [Google Scholar] [CrossRef]
- Golding, R.M.; Pyykkö, P. On the Theory of Pseudocontact N.M.R. Shifts Due to Lanthanide Complexes. Mol. Phys. 1973, 26, 1389–1396. [Google Scholar] [CrossRef]
- Reilley, C.N.; Good, B.W.; Desreux, J.F. Structure-Independent Method for Dissecting Contact and Dipolar NMR Shifts in Lanthanide Complexes and Its Use in Structure Determination. Anal. Chem. 1975, 47, 2110–2116. [Google Scholar] [CrossRef]
- Reilley, C.N.; Good, B.W.; Allendoerfer, R.D. Separation of Contact and Dipolar Lanthanide Induced Nuclear Magnetic Resonance Shifts: Evaluation and Application of Some Structure Independent Methods. Anal. Chem. 1976, 48, 1446–1458. [Google Scholar] [CrossRef]
- Gorbunova, Y.G.; Martynov, A.G.; Birin, K.P.; Tsivadze, A.Y. NMR Spectroscopy—A Versatile Tool for Studying the Structure and Magnetic Properties of Paramagnetic Lanthanide Complexes in Solutions (Review). Russ. J. Inorg. Chem. 2021, 66, 202–216. [Google Scholar] [CrossRef]
- Babailov, S.P. Lanthanide Paramagnetic Probes for NMR Spectroscopic Studies of Molecular Conformational Dynamics in Solution: Applications to Macrocyclic Molecules. Prog. Nucl. Magn. Reson. Spectrosc. 2008, 52, 1–21. [Google Scholar] [CrossRef]
- Allegrozzi, M.; Bertini, I.; Janik, M.B.L.; Lee, Y.-M.; Liu, G.; Luchinat, C. Lanthanide-Induced Pseudocontact Shifts for Solution Structure Refinements of Macromolecules in Shells up to 40 Å from the Metal Ion. J. Am. Chem. Soc. 2000, 122, 4154–4161. [Google Scholar] [CrossRef]
- Müntener, T.; Joss, D.; Häussinger, D.; Hiller, S. Pseudocontact Shifts in Biomolecular NMR Spectroscopy. Chem. Rev. 2022, 122, 9422–9467. [Google Scholar] [CrossRef]
- Peters, J.A.; Huskens, J.; Raber, D.J. Lanthanide Induced Shifts and Relaxation Rate Enhancements. Prog. Nucl. Magn. Reson. Spectrosc. 1996, 28, 283–350. [Google Scholar] [CrossRef]
- Ishikawa, N.; Iino, T.; Kaizu, Y. Determination of Ligand-Field Parameters and f-Electronic Structures of Hetero-Dinuclear Phthalocyanine Complexes with a Diamagnetic Yttrium(III) and a Paramagnetic Trivalent Lanthanide Ion. J. Phys. Chem. A 2002, 106, 9543–9550. [Google Scholar] [CrossRef]
- Hiller, M.; Krieg, S.; Ishikawa, N.; Enders, M. Ligand-Field Energy Splitting in Lanthanide-Based Single-Molecule Magnets by NMR Spectroscopy. Inorg. Chem. 2017, 56, 15285–15294. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, N.; Sugita, M.; Okubo, T.; Tanaka, N.; Iino, T.; Kaizu, Y. Determination of Ligand-Field Parameters and f-Electronic Structures of Double-Decker Bis(Phthalocyaninato)Lanthanide Complexes. Inorg. Chem. 2003, 42, 2440–2446. [Google Scholar] [CrossRef]
- Ishikawa, N. Simultaneous Determination of Ligand-Field Parameters of Isostructural Lanthanide Complexes by Multidimensional Optimization. J. Phys. Chem. A 2003, 107, 5831–5835. [Google Scholar] [CrossRef]
- Martynov, A.G.; Sinelshchikova, A.A.; Dorovatovskii, P.V.; Polovkova, M.A.; Ovchenkova, A.E.; Birin, K.P.; Kirakosyan, G.A.; Gorbunova, Y.G.; Tsivadze, A.Y. Solvation-Induced Conformational Switching of Trisphthalocyanates for Control of Their Magnetic Properties. Inorg. Chem. 2023, Submitted. [Google Scholar]
- Martynov, A.G.; Polovkova, M.A.; Gorbunova, Y.G.; Tsivadze, A.Y. Redox-Triggered Switching of Conformational State in Triple-Decker Lanthanide Phthalocyaninates. Molecules 2022, 27, 6498. [Google Scholar] [CrossRef]
- Martynov, A.G.; Yagodin, A.V.; Birin, K.P.; Gorbunova, Y.G.; Tsivadze, A.Y. Solvation-Induced Switching of the Conformational State of Alkoxy- and Crown-Substituted Trisphthalocyaninates Studied by UV-Vis and 1 H-NMR Spectroscopy. J. Porphyr. Phthalocyanines 2023, 27, 414–422. [Google Scholar] [CrossRef]
- Babailov, S.P.; Polovkova, M.A.; Kirakosyan, G.A.; Martynov, A.G.; Zapolotsky, E.N.; Gorbunova, Y.G. NMR Thermosensing Properties on Binuclear Triple-Decker Complexes of Terbium(III) and Dysprosium(III) with 15-Crown-5-Phthalocyanine. Sens. Actuators A Phys. 2021, 331, 112933. [Google Scholar] [CrossRef]
- Martynov, A.G.; Polovkova, M.A.; Kirakosyan, G.A.; Zapolotsky, E.N.; Babailov, S.P.; Gorbunova, Y.G. 1H NMR Spectral Analysis of Structural Features in a Series of Paramagnetic Homoleptic Binuclear Triple-Decker Phthalocyaninato Lanthanide Complexes. Polyhedron 2022, 219, 115792. [Google Scholar] [CrossRef]
- Babailov, S.P.; Polovkova, M.A.; Zapolotsky, E.N.; Kirakosyan, G.A.; Martynov, A.G.; Gorbunova, Y.G. Nuclear Magnetic Resonance Thermosensing Properties of Holmium(III) and Thulium(III) Tris(Tetra-15-Crown-5-Phthalocyaninato) Complexes. J. Porphyr. Phthalocyanines 2022, 26, 334–339. [Google Scholar] [CrossRef]
- Polovkova, M.A.; Martynov, A.G.; Birin, K.P.; Nefedov, S.E.; Gorbunova, Y.G.; Tsivadze, A.Y. Determination of the Structural Parameters of Heteronuclear (Phthalocyaninato)Bis(Crownphthalocyaninato)Lanthanide(III) Triple-Deckers in Solution by Simultaneous Analysis of NMR and Single-Crystal X-Ray Data. Inorg. Chem. 2016, 55, 9258–9269. [Google Scholar] [CrossRef]
- Ishikawa, N.; Iino, T.; Kaizu, Y. Study of 1 H NMR Spectra of Dinuclear Complexes of Heavy Lanthanides with Phthalocyanines Based on Separation of the Effects of Two Paramagnetic Centers. J. Phys. Chem. A 2003, 107, 7879–7884. [Google Scholar] [CrossRef]
- Mironov, V.S.; Galyametdinov, Y.G.; Ceulemans, A.; Görller-Walrand, C.; Binnemans, K. Room-Temperature Magnetic Anisotropy of Lanthanide Complexes: A Model Study for Various Coordination Polyhedra. J. Chem. Phys. 2002, 116, 4673–4685. [Google Scholar] [CrossRef]
- Martynov, A.G.; Polovkova, M.A.; Berezhnoy, G.S.; Sinelshchikova, A.A.; Khrustalev, V.N.; Birin, K.P.; Kirakosyan, G.A.; Gorbunova, Y.G.; Tsivadze, A.Y. Heteroleptic Crown-Substituted Tris(Phthalocyaninates) as Dynamic Supramolecular Scaffolds with Switchable Rotational States and Tunable Magnetic Properties. Inorg. Chem. 2021, 60, 9110–9121. [Google Scholar] [CrossRef]
- Morita, T.; Damjanović, M.; Katoh, K.; Kitagawa, Y.; Yasuda, N.; Lan, Y.; Wernsdorfer, W.; Breedlove, B.K.; Enders, M.; Yamashita, M. Comparison of the Magnetic Anisotropy and Spin Relaxation Phenomenon of Dinuclear Terbium(III) Phthalocyaninato Single-Molecule Magnets Using the Geometric Spin Arrangement. J. Am. Chem. Soc. 2018, 140, 2995–3007. [Google Scholar] [CrossRef]
- Novikov, V.V.; Pavlov, A.A.; Nelyubina, Y.V.; Boulon, M.-E.; Varzatskii, O.A.; Voloshin, Y.Z.; Winpenny, R.E.P. A Trigonal Prismatic Mononuclear Cobalt(II) Complex Showing Single-Molecule Magnet Behavior. J. Am. Chem. Soc. 2015, 137, 9792–9795. [Google Scholar] [CrossRef]
- Pavlov, A.A.; Nelyubina, Y.V.; Kats, S.V.; Penkova, L.V.; Efimov, N.N.; Dmitrienko, A.O.; Vologzhanina, A.V.; Belov, A.S.; Voloshin, Y.Z.; Novikov, V.V. Polymorphism in a Cobalt-Based Single-Ion Magnet Tuning Its Barrier to Magnetization Relaxation. J. Phys. Chem. Lett. 2016, 7, 4111–4116. [Google Scholar] [CrossRef]
- Horii, Y.; Damjanovic, M.; Ajayakumar, M.R.; Katoh, K.; Kitagawa, Y.; Chibotaru, L.; Ungur, L.; Mas-Torrent, M.; Wernsdorfer, W.; Breedlove, B.K.; et al. Highly Oxidized States of Phthalocyaninato Terbium(III) Multiple-Decker Complexes Showing Structural Deformations, Biradical Properties and Decreases in Magnetic Anisotropy. Chem. A Eur. J. 2020, 26, 8621–8630. [Google Scholar] [CrossRef]
- Katoh, K.; Kajiwara, T.; Nakano, M.; Nakazawa, Y.; Wernsdorfer, W.; Ishikawa, N.; Breedlove, B.K.; Yamashita, M. Magnetic Relaxation of Single-Molecule Magnets in an External Magnetic Field: An Ising Dimer of a Terbium(III)-Phthalocyaninate Triple-Decker Complex. Chem. A Eur. J. 2011, 17, 117–122. [Google Scholar] [CrossRef]
- Martynov, A.G.; Polovkova, M.A.; Berezhnoy, G.S.; Sinelshchikova, A.A.; Dolgushin, F.M.; Birin, K.P.; Kirakosyan, G.A.; Gorbunova, Y.G.; Tsivadze, A.Y. Cation-Induced Dimerization of Heteroleptic Crown-Substituted Trisphthalocyaninates as Revealed by X-Ray Diffraction and NMR Spectroscopy. Inorg. Chem. 2020, 59, 9424–9433. [Google Scholar] [CrossRef] [PubMed]
- Horii, Y.; Kishiue, S.; Damjanović, M.; Katoh, K.; Breedlove, B.K.; Enders, M.; Yamashita, M. Supramolecular Approach for Enhancing Single-Molecule Magnet Properties of Terbium(III)-Phthalocyaninato Double-Decker Complexes with Crown Moieties. Chem. A Eur. J. 2018, 24, 4320–4327. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tomita, Y.; Hada, Y.; Tsubota, K.; Handa, M.; Kasuga, K.; Sogabe, K.; Tokii, T. Preparation and Electrochemical Properties of the Green Ytterbium(III) and Lutetium(III) Sandwich Complexes of Octabutoxy-Substituted Phthalocyanine. Chem. Lett. 1992, 21, 759–762. [Google Scholar] [CrossRef]
- Martynov, A.G.; Berezhnoy, G.S.; Safonova, E.A.; Polovkova, M.A.; Gorbunova, Y.G.; Tsivadze, A.Y. Aromatic Nucleophilic Substitution as a Side Process in the Synthesis of Alkoxy- and Crown-Substituted (Na)Phthalocyanines. Macroheterocycles 2019, 12, 75–81. [Google Scholar] [CrossRef]





| [B4]Tb[B4]Y[C4], | [B4]Y[B4]Tb[C4], | |||||||
|---|---|---|---|---|---|---|---|---|
| Toluene-d8 | CD2Cl2 | Toluene-d8 | CD2Cl2 | |||||
| Proton | , Å−3 | δ, ppm | , Å−3 | δ, ppm | , Å−3 | δ, ppm | , Å−3 | δ, ppm |
| bHPco | −2.85 × 10−3 | −51.0 | −3.28 × 10−3 | −69.7 | 8.36 × 10−4 | 25.2 | 7.10 × 10−4 | 25.9 |
| bHPci | −3.34 × 10−3 | −59.3 | −3.46 × 10−3 | −79.6 | −3.46 × 10−3 | −64.6 | −3.27 × 10−3 | −68.2 |
| cHPco | 8.72 × 10−4 | 24.8 | 7.19 × 10−4 | 26.7 | −2.70 × 10−3 | −52.1 | −3.23 × 10−3 | −67.0 |
| 1o | −8.28 × 10−4 | −13.8 | −1.26 × 10−3 | −21.8 | 2.94 × 10−4 | 9.4 | −6.11 × 10−5 | 9.1 |
| 1o’ | −1.53 × 10−3 | −25.7 | −1.55 × 10−3 | −33.2 | −4.08 × 10−4 | 1.5 | −1.86 × 10−4 | 0.0 |
| 1ib | −1.88 × 10−3 | −33.6 | −1.88 × 10−3 | −43.7 | −1.25 × 10−3 | −23.2 | −1.15 × 10−3 | −18.2 |
| 1ic | −1.31 × 10−3 | −20.9 | −1.36 × 10−3 | −28.9 | −1.85 × 10−3 | −36.5 | −1.66 × 10−3 | −31.0 |
| αo | −3.03 × 10−4 | 2.1 | −3.45 × 10−4 | −0.2 | −1.51 × 10−3 | −25.2 | −1.74 × 10−3 | −33.0 |
| αo’ | 2.89 × 10−4 | 9.9 | 1.23 × 10−4 | 10.7 | −8.77 × 10−4 | −13.3 | −1.18 × 10−3 | −20.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martynov, A.G.; Birin, K.P.; Kirakosyan, G.A.; Gorbunova, Y.G.; Tsivadze, A.Y. Site-Selective Solvation-Induced Conformational Switching of Heteroleptic Heteronuclear Tb(III) and Y(III) Trisphthalocyaninates for the Control of Their Magnetic Anisotropy. Molecules 2023, 28, 4474. https://doi.org/10.3390/molecules28114474
Martynov AG, Birin KP, Kirakosyan GA, Gorbunova YG, Tsivadze AY. Site-Selective Solvation-Induced Conformational Switching of Heteroleptic Heteronuclear Tb(III) and Y(III) Trisphthalocyaninates for the Control of Their Magnetic Anisotropy. Molecules. 2023; 28(11):4474. https://doi.org/10.3390/molecules28114474
Chicago/Turabian StyleMartynov, Alexander G., Kirill P. Birin, Gayane A. Kirakosyan, Yulia G. Gorbunova, and Aslan Yu. Tsivadze. 2023. "Site-Selective Solvation-Induced Conformational Switching of Heteroleptic Heteronuclear Tb(III) and Y(III) Trisphthalocyaninates for the Control of Their Magnetic Anisotropy" Molecules 28, no. 11: 4474. https://doi.org/10.3390/molecules28114474





