Design, Synthesis, and Biological Evaluation of Potent and Selective Inhibitors of Ataxia Telangiectasia Mutated and Rad3-Related (ATR) Kinase for the Efficient Treatment of Cancer
Abstract
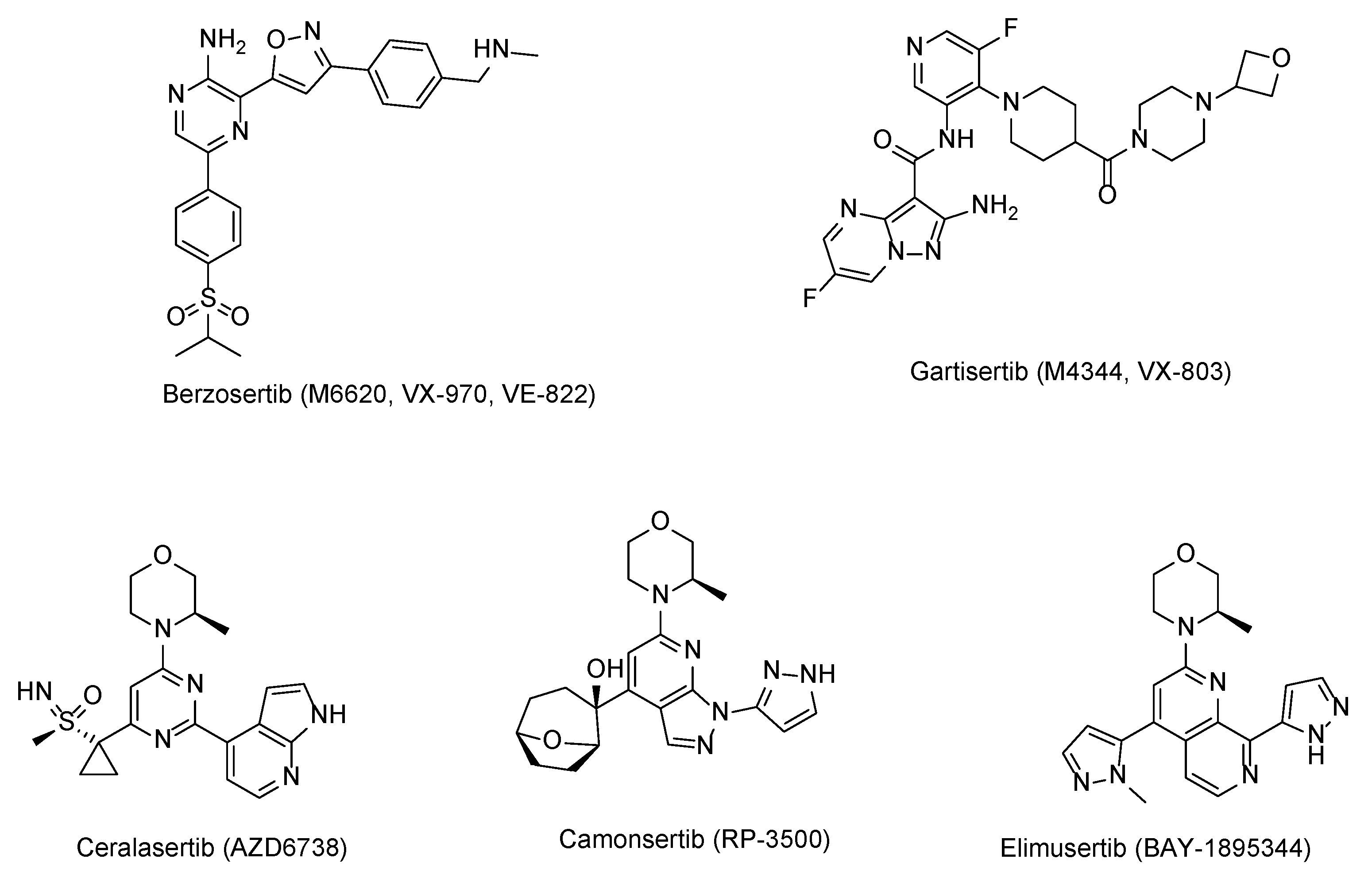
1. Introduction
2. Results and Discussion
2.1. Design of ATR Inhibitors
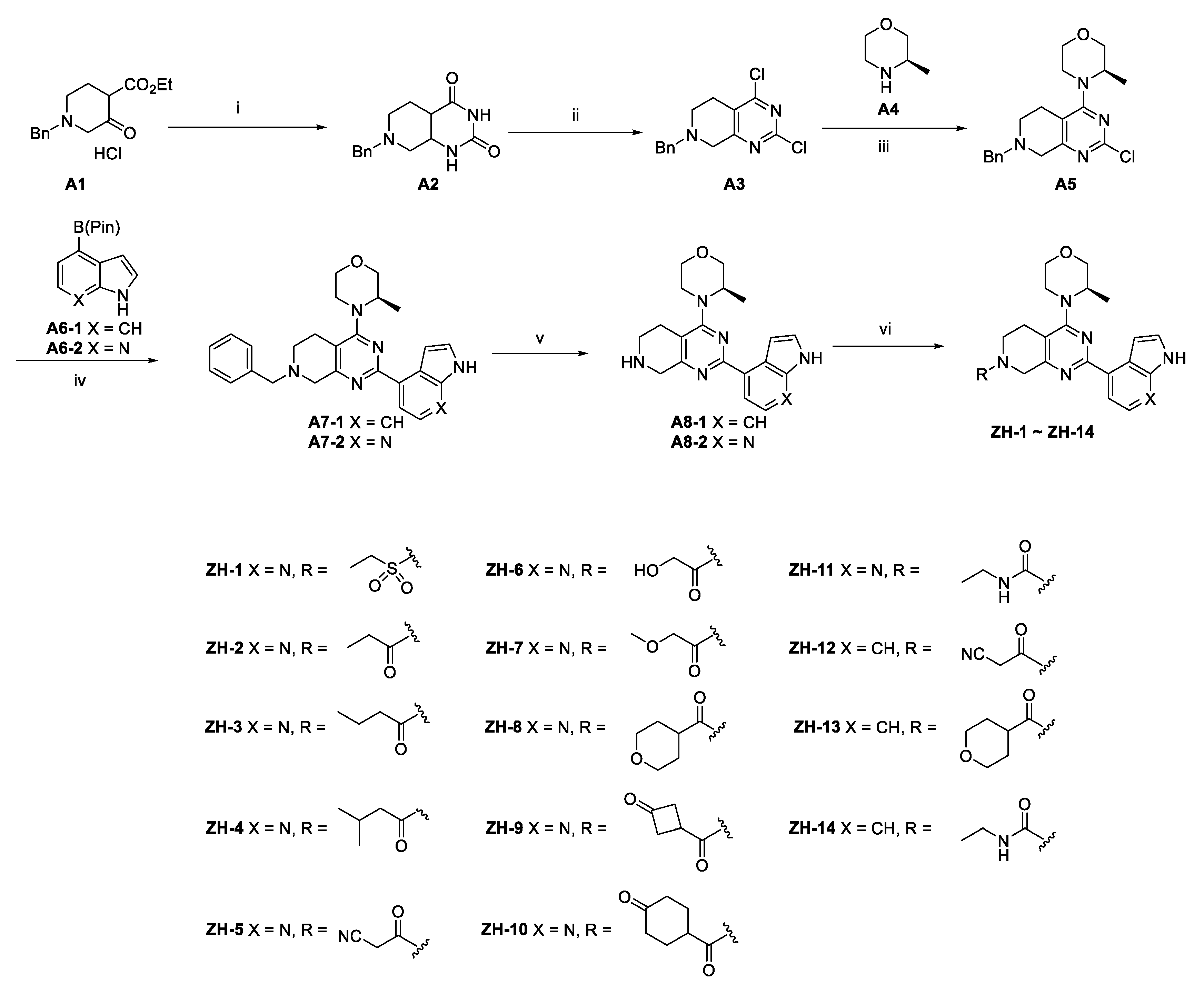
2.2. Chemistry
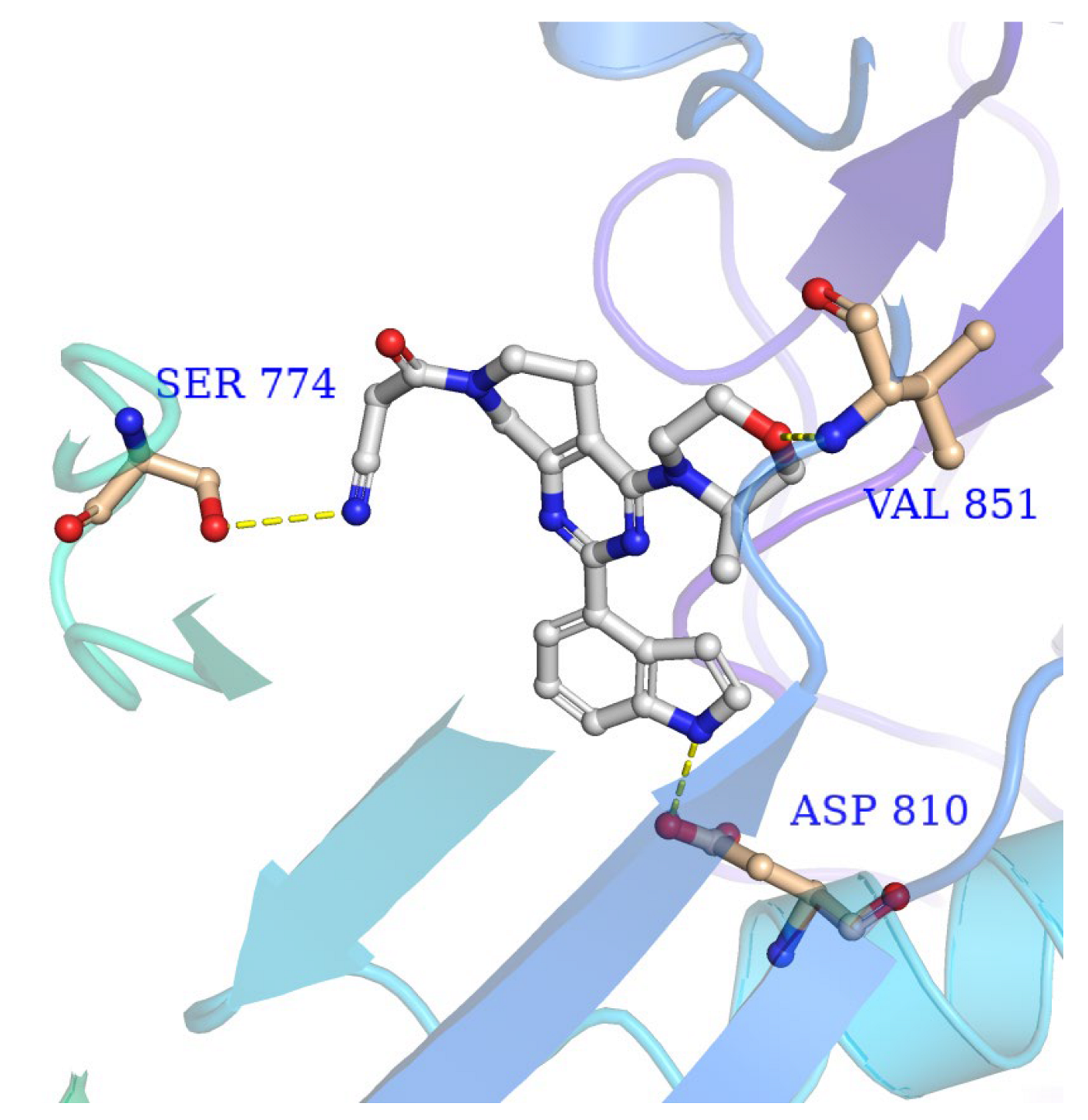
2.3. Molecular Docking of ZH-12
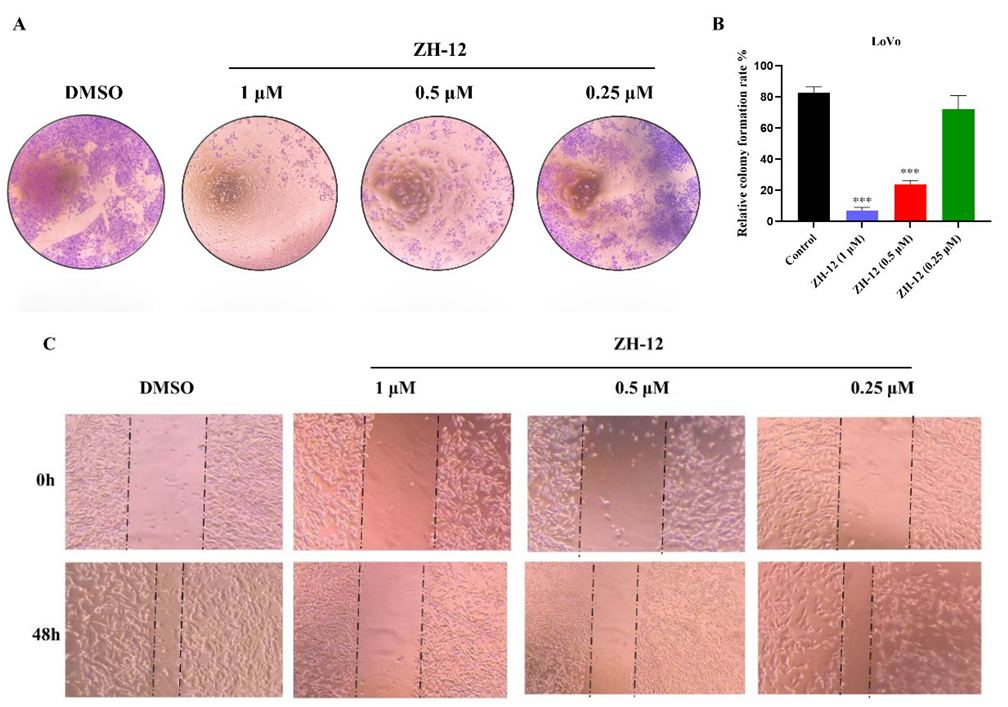
2.4. Antiproliferative Activity In Vitro
2.5. Cellular Mechanism of Action Studies
2.5.1. Comet Assay
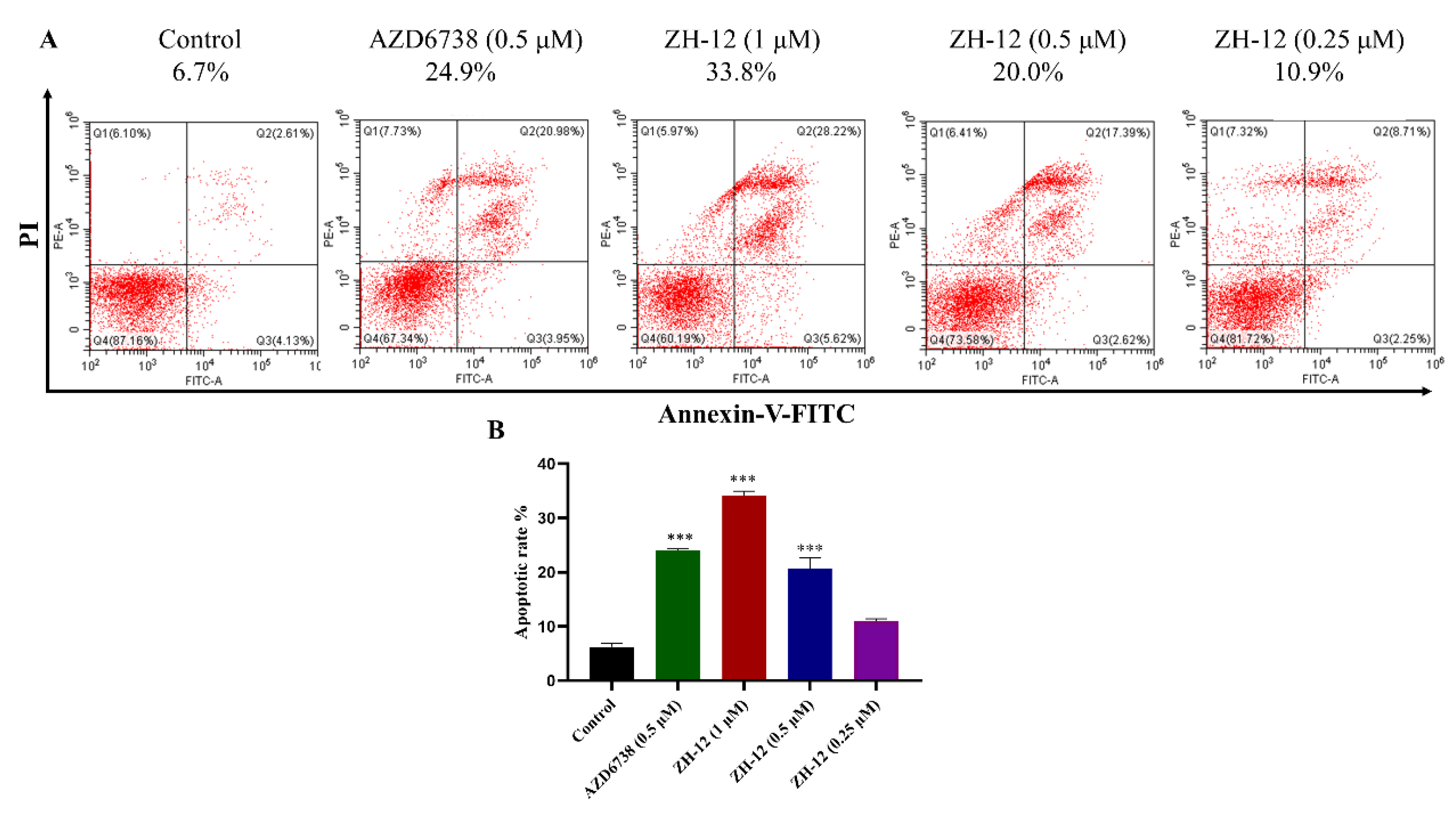
2.5.2. Cell Apoptosis Assay
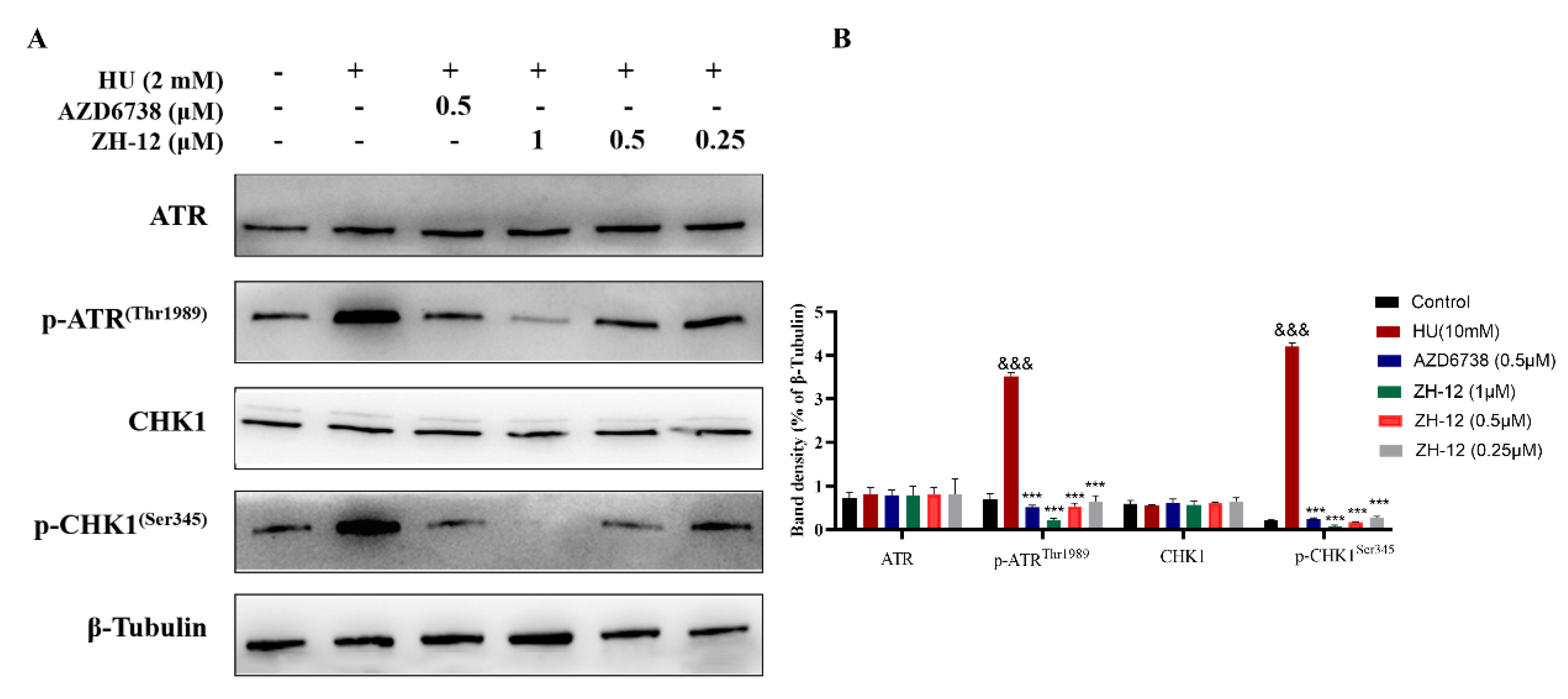
2.5.3. Western Blot Analysis of Protein Expression In Vitro
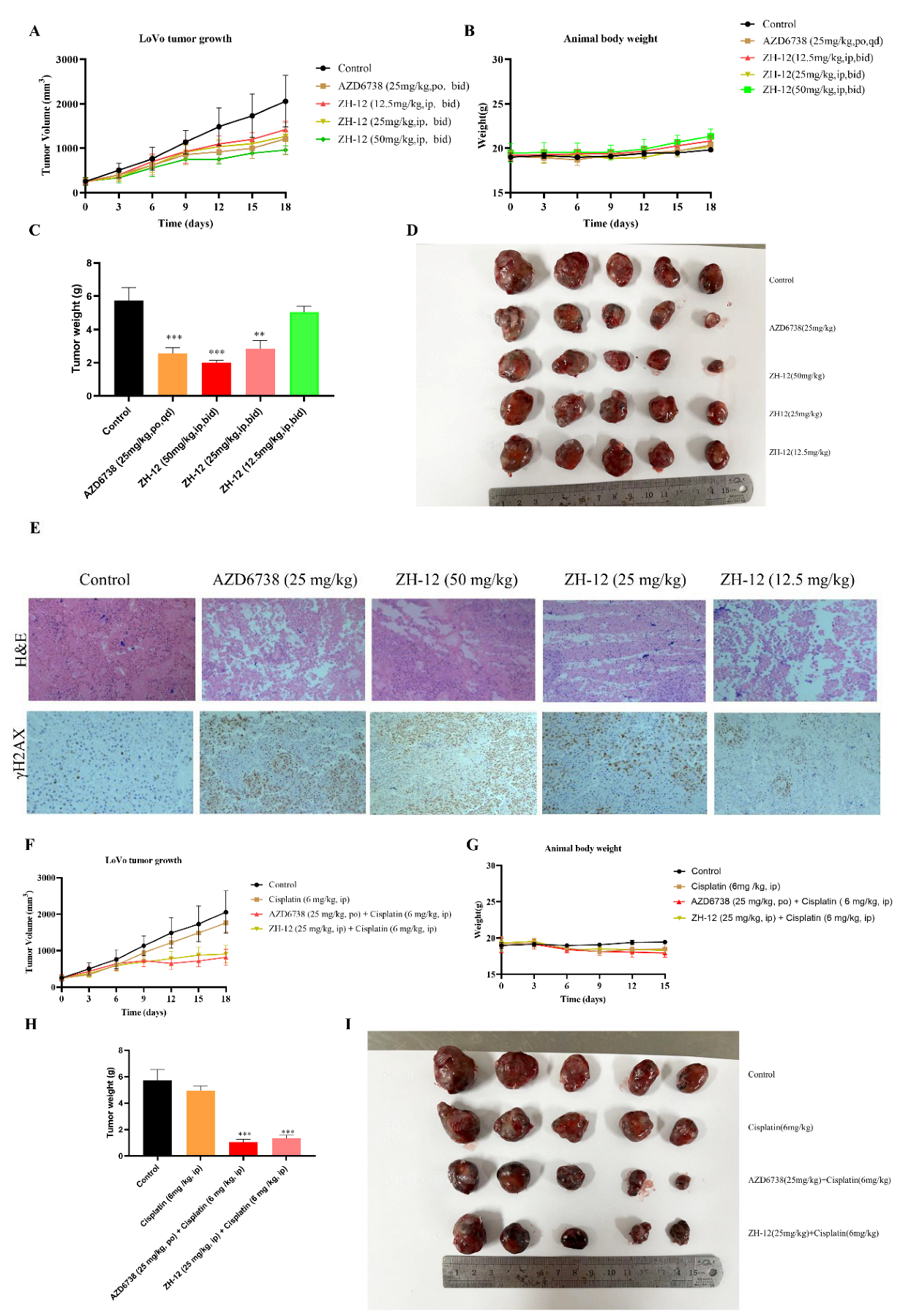
2.6. In Vivo Antitumor Effects of ZH-12
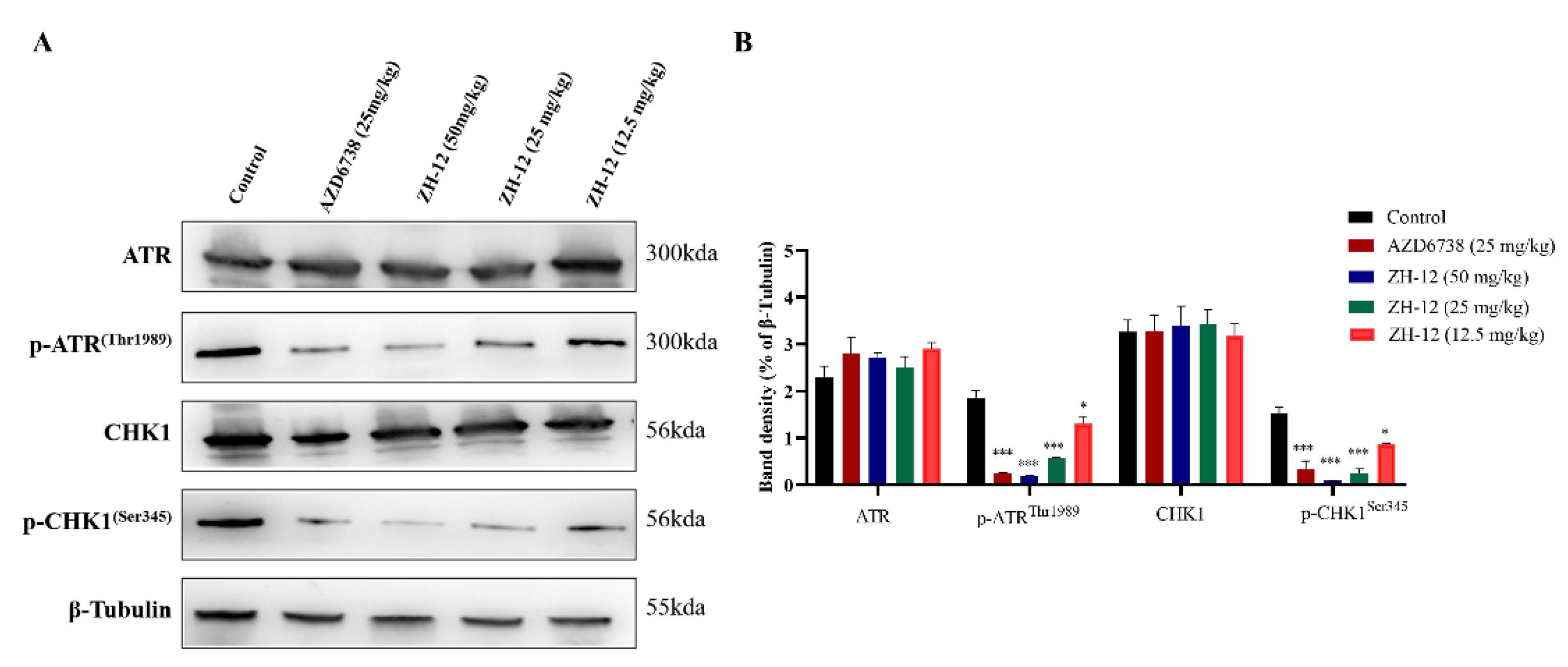
2.7. In Vivo Western Blot Analysis
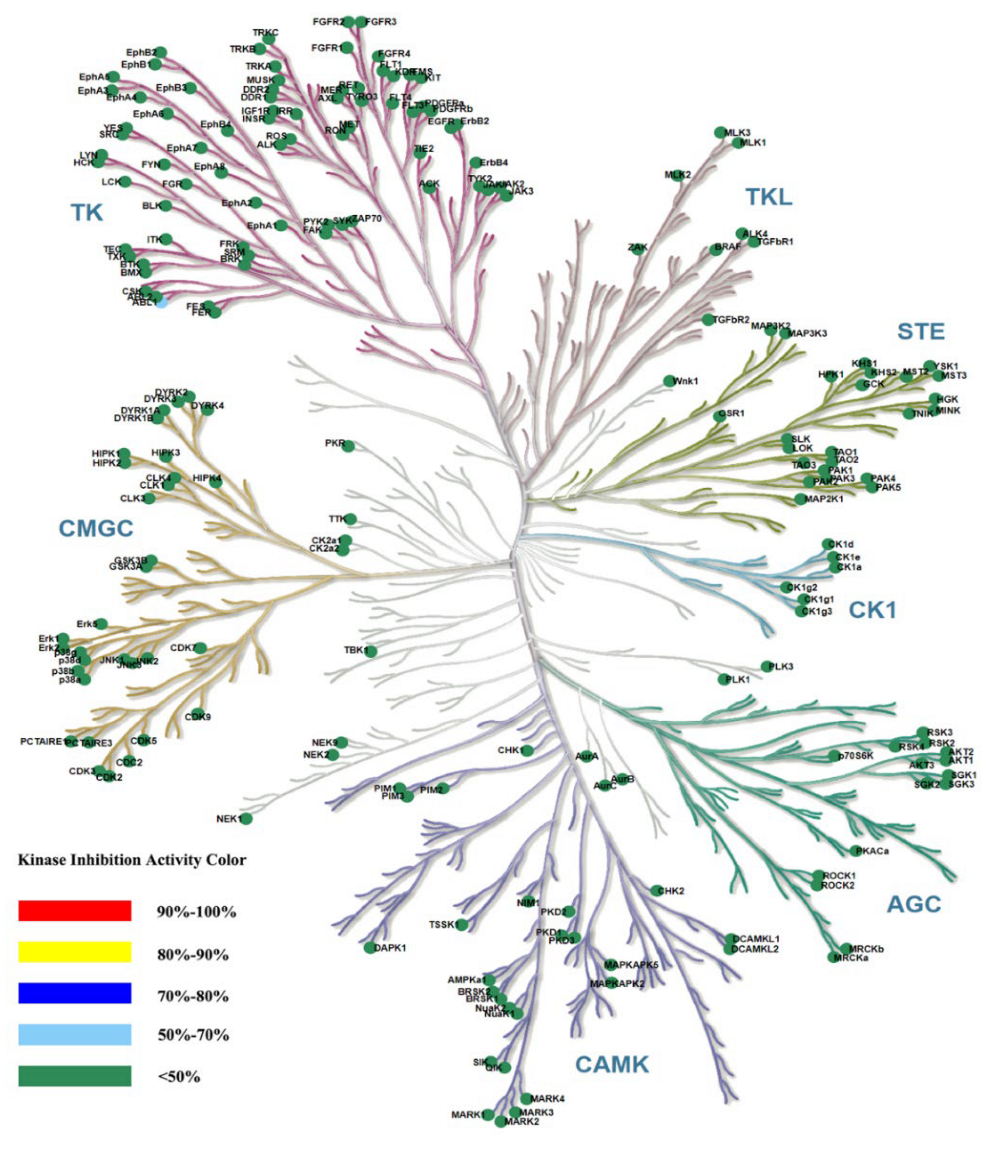
2.8. Kinase Selectivity of the Most Potent Compound ZH-12
3. Experiments
3.1. General Chemistry
3.1.1. General Procedure for the Synthesis of ZH-1~ZH-4, ZH-8, and ZH-13
3.1.2. General Procedure for the Synthesis of ZH-5~ZH-7, ZH-9~ZH-10, and ZH-12
3.1.3. General Procedure for the Synthesis of ZH-11 and ZH-14
3.2. Biological Activity Assay
3.2.1. Molecular Modeling
3.2.2. Cell Lines and Culture Methods
3.2.3. Cell Proliferation Assay
3.2.4. Colony Formation Assay
3.2.5. Migration Assays
3.2.6. Comet Assay
3.2.7. Cell Apoptosis
3.2.8. Western Blotting
3.2.9. Efficacy and Pharmacodynamics Studies in the LoVo Xenograft Model in Mice
3.2.10. H&E Staining
3.2.11. Immunohistochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pilie, P.G.; Tang, C.; Mills, G.B.; Yap, T.A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 81–104. [Google Scholar] [CrossRef]
- Baxter, J.S.; Zatreanu, D.; Pettitt, S.J.; Lord, C.J. Resistance to DNA repair inhibitors in cancer. Mol. Oncol. 2022, 16, 3811–3827. [Google Scholar] [CrossRef] [PubMed]
- Maleki Dana, P.; Sadoughi, F.; Mirzaei, H.; Asemi, Z.; Yousefi, B. DNA damage response and repair in the development and treatment of brain tumors. Eur. J. Pharmacol. 2022, 924, 174957. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K. Branching the Tel2 pathway for exact fit on phosphatidylinositol 3-kinase-related kinases. Curr. Genet. 2018, 64, 965–970. [Google Scholar] [CrossRef]
- Schlam-Babayov, S.; Ziv, Y.; Shiloh, Y. It takes three to the DNA damage response tango. Mol. Cell Oncol. 2021, 8, 1881395. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, T.A.; Tainer, J.A.; Lees-Miller, S.P. A structural model for regulation of NHEJ by DNA-PKcs autophosphorylation. DNA Repair 2010, 9, 1307–1314. [Google Scholar] [CrossRef]
- Qiu, Z.; Oleinick, N.L.; Zhang, J. ATR/CHK1 inhibitors and cancer therapy. Radiother. Oncol. 2018, 126, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, R.; Antoccia, A.; Leone, S.; Ascenzi, P.; di Masi, A. Hsp90alpha regulates ATM and NBN functions in sensing and repair of DNA double-strand breaks. FEBS J. 2017, 284, 2378–2395. [Google Scholar] [CrossRef]
- Kamp, J.A.; Lemmens, B.; Romeijn, R.J.; Gonzalez-Prieto, R.; Olsen, J.V.; Vertegaal, A.C.O.; van Schendel, R.; Tijsterman, M. THO complex deficiency impairs DNA double-strand break repair via the RNA surveillance kinase SMG-1. Nucleic Acids Res. 2022, 50, 6235–6250. [Google Scholar] [CrossRef]
- Cheng, A.; Tse, K.H.; Chow, H.M.; Gan, Y.; Song, X.; Ma, F.; Qian, Y.X.Y.; She, W.; Herrup, K. ATM loss disrupts the autophagy-lysosomal pathway. Autophagy 2021, 17, 1998–2010. [Google Scholar] [CrossRef]
- Neeb, A.; Herranz, N.; Arce-Gallego, S.; Miranda, S.; Buroni, L.; Yuan, W.; Athie, A.; Casals, T.; Carmichael, J.; Rodrigues, D.N.; et al. Advanced Prostate Cancer with ATM Loss: PARP and ATR Inhibitors. Eur. Urol. 2021, 79, 200–211. [Google Scholar] [CrossRef]
- Marechal, A.; Zou, L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, S.; Ballif, B.A.; Smogorzewska, A.; McDonald, E.R., 3rd; Hurov, K.E.; Luo, J.; Bakalarski, C.E.; Zhao, Z.; Solimini, N.; Lerenthal, Y.; et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007, 316, 1160–1166. [Google Scholar] [CrossRef]
- Faulhaber, E.M.; Jost, T.; Symank, J.; Scheper, J.; Burkel, F.; Fietkau, R.; Hecht, M.; Distel, L.V. Kinase Inhibitors of DNA-PK, ATM and ATR in Combination with Ionizing Radiation Can Increase Tumor Cell Death in HNSCC Cells While Sparing Normal Tissue Cells. Genes 2021, 12, 925. [Google Scholar] [CrossRef]
- Bi, X.; Srikanta, D.; Fanti, L.; Pimpinelli, S.; Badugu, R.; Kellum, R.; Rong, Y.S. Drosophila ATM and ATR checkpoint kinases control partially redundant pathways for telomere maintenance. Proc. Natl. Acad. Sci. USA 2005, 102, 15167–15172. [Google Scholar] [CrossRef] [PubMed]
- Knegtel, R.; Charrier, J.D.; Durrant, S.; Davis, C.; O’Donnell, M.; Storck, P.; MacCormick, S.; Kay, D.; Pinder, J.; Virani, A.; et al. Rational Design of 5-(4-(Isopropylsulfonyl)phenyl)-3-(3-(4-((methylamino)methyl)phenyl)isoxazol-5-yl)pyrazin-2-amine (VX-970, M6620): Optimization of Intra- and Intermolecular Polar Interactions of a New Ataxia Telangiectasia Mutated and Rad3-Related (ATR) Kinase Inhibitor. J. Med. Chem. 2019, 62, 5547–5561. [Google Scholar] [PubMed]
- Foote, K.M.; Nissink, J.W.M.; McGuire, T.; Turner, P.; Guichard, S.; Yates, J.W.T.; Lau, A.; Blades, K.; Heathcote, D.; Odedra, R.; et al. Discovery and Characterization of AZD6738, a Potent Inhibitor of Ataxia Telangiectasia Mutated and Rad3 Related (ATR) Kinase with Application as an Anticancer Agent. J. Med. Chem. 2018, 61, 9889–9907. [Google Scholar] [CrossRef]
- Jo, U.; Senatorov, I.S.; Zimmermann, A.; Saha, L.K.; Murai, Y.; Kim, S.H.; Rajapakse, V.N.; Elloumi, F.; Takahashi, N.; Schultz, C.W.; et al. Novel and Highly Potent ATR Inhibitor M4344 Kills Cancer Cells With Replication Stress, and Enhances the Chemotherapeutic Activity of Widely Used DNA Damaging Agents. Mol. Cancer Ther. 2021, 20, 1431–1441. [Google Scholar] [CrossRef]
- Roulston, A.; Zimmermann, M.; Papp, R.; Skeldon, A.; Pellerin, C.; Dumas-Berube, E.; Dumais, V.; Dorich, S.; Fader, L.D.; Fournier, S.; et al. RP-3500: A Novel, Potent, and Selective ATR Inhibitor that is Effective in Preclinical Models as a Monotherapy and in Combination with PARP Inhibitors. Mol. Cancer Ther. 2022, 21, 245–256. [Google Scholar] [CrossRef]
- Lucking, U.; Wortmann, L.; Wengner, A.M.; Lefranc, J.; Lienau, P.; Briem, H.; Siemeister, G.; Bomer, U.; Denner, K.; Schafer, M.; et al. Damage Incorporated: Discovery of the Potent, Highly Selective, Orally Available ATR Inhibitor BAY 1895344 with Favorable Pharmacokinetic Properties and Promising Efficacy in Monotherapy and in Combination Treatments in Preclinical Tumor Models. J. Med. Chem. 2020, 63, 7293–7325. [Google Scholar] [CrossRef]
- Kwok, M.; Davies, N.; Agathanggelou, A.; Smith, E.; Oldreive, C.; Petermann, E.; Stewart, G.; Brown, J.; Lau, A.; Pratt, G.; et al. ATR inhibition induces synthetic lethality and overcomes chemoresistance in TP53- or ATM-defective chronic lymphocytic leukemia cells. Blood 2016, 127, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Menezes, D.L.; Holt, J.; Tang, Y.; Feng, J.; Barsanti, P.; Pan, Y.; Ghoddusi, M.; Zhang, W.; Thomas, G.; Holash, J.; et al. A synthetic lethal screen reveals enhanced sensitivity to ATR inhibitor treatment in mantle cell lymphoma with ATM loss-of-function. Mol. Cancer Res. 2015, 13, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Min, A.; Im, S.A.; Jang, H.; Kim, S.; Lee, M.; Kim, D.K.; Yang, Y.; Kim, H.J.; Lee, K.H.; Kim, J.W.; et al. AZD6738, A Novel Oral Inhibitor of ATR, Induces Synthetic Lethality with ATM Deficiency in Gastric Cancer Cells. Mol. Cancer Ther. 2017, 16, 566–577. [Google Scholar] [CrossRef]
- Kim, H.J.; Min, A.; Im, S.A.; Jang, H.; Lee, K.H.; Lau, A.; Lee, M.; Kim, S.; Yang, Y.; Kim, J.; et al. Anti-tumor activity of the ATR inhibitor AZD6738 in HER2 positive breast cancer cells. Int. J. Cancer 2017, 140, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Knapp, M.; Crawford, K.; Warne, R.; Elling, R.; Yan, K.; Doyle, M.; Pardee, G.; Zhang, L.; Ma, S.; et al. Rationally Designed PI3Kalpha Mutants to Mimic ATR and Their Use to Understand Binding Specificity of ATR Inhibitors. J. Mol. Biol. 2017, 429, 1684–1704. [Google Scholar] [CrossRef]
- Bin, H.; Chen, P.; Wu, M.; Wang, F.; Lin, G.; Pan, S.; Liu, J.; Mu, B.; Nan, J.; Huang, Q.; et al. Discovery of a potent and highly selective inhibitor of ataxia telangiectasia mutated and Rad3-Related (ATR) kinase: Structural activity relationship and antitumor activity both in vitro and in vivo. Eur. J. Med. Chem. 2022, 232, 114187. [Google Scholar] [CrossRef]
- Chen, S.; Wu, J.L.; Liang, Y.; Tang, Y.G.; Song, H.X.; Wu, L.L.; Xing, Y.F.; Yan, N.; Li, Y.T.; Wang, Z.Y.; et al. Arsenic Trioxide Rescues Structural p53 Mutations through a Cryptic Allosteric Site. Cancer Cell 2021, 39, 225–239.e8. [Google Scholar] [CrossRef]
- Saxena, S.; Zou, L. Hallmarks of DNA replication stress. Mol. Cell 2022, 82, 2298–2314. [Google Scholar] [CrossRef]
- Gomes, L.R.; Rocha, C.R.R.; Martins, D.J.; Fiore, A.; Kinker, G.S.; Bruni-Cardoso, A.; Menck, C.F.M. ATR mediates cisplatin resistance in 3D-cultured breast cancer cells via translesion DNA synthesis modulation. Cell Death Dis. 2019, 10, 459. [Google Scholar] [CrossRef]
- Chao, Y.Y.; Huang, B.M.; Peng, I.C.; Lee, P.R.; Lai, Y.S.; Chiu, W.T.; Lin, Y.S.; Lin, S.C.; Chang, J.H.; Chen, P.S.; et al. ATM- and ATR-induced primary ciliogenesis promotes cisplatin resistance in pancreatic ductal adenocarcinoma. J. Cell. Physiol. 2022, 237, 4487–4503. [Google Scholar] [CrossRef]



| Compounds | X | R | ATR IC50 ± SD (nM) a | clogP b |
|---|---|---|---|---|
| ZH-1 | N | 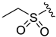 | 53.26 ± 1.08 | 2.27 |
| ZH-2 | N | 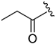 | 41.60 ± 1.38 | 2.80 |
| ZH-3 | N |  | 42.49 ± 1.05 | 3.22 |
| ZH-4 | N |  | 7.31 ± 0.16 | 3.55 |
| ZH-5 | N | 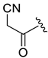 | 10.81 ± 0.72 | 2.35 |
| ZH-6 | N | 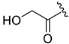 | 34.03 ± 0.27 | 1.45 |
| ZH-7 | N | 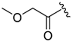 | 9.23 ± 0.16 | 1.81 |
| ZH-8 | N | 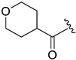 | 27.47 ± 1.04 | 2.44 |
| ZH-9 | N | 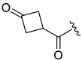 | 16.04 ± 1.08 | 2.21 |
| ZH-10 | N | 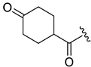 | 11.92 ± 0.19 | 3.05 |
| ZH-11 | N | 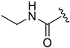 | 11.42 ± 0.25 | 2.39 |
| ZH-12 | CH | 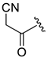 | 6.81 ± 0.04 | 2.94 |
| ZH-13 | CH | 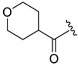 | 214.66 ± 0.04 | 2.49 |
| ZH-14 | CH |  | 14.86 ± 0.29 | 2.99 |
| AZD6738 | / | / | 6.50 ± 0.28 |
| Compound | IC50 ± SD (μM) a | |||
|---|---|---|---|---|
| A549 | HCT116 | NCI-H23 | LoVo | |
| ZH-4 | 20.85 ± 1.21 | 5.17 ± 0.58 | 1.74 ± 0.05 | 18.00 ± 1.69 |
| ZH-7 | 25.63 ± 1.62 | 6.02 ± 0.26 | 6.77 ± 0.43 | 5.71 ±0.81 |
| ZH-12 | 4.74 ± 0.27 | 1.61 ± 0.08 | 0.77 ± 0.13 | 0.29 ± 0.01 |
| AZD6738 | 6.60 ± 0.24 | 4.32 ± 0.16 | 0.50 ± 0.06 | 0.41 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, J.; Huang, L.; Lai, W.; Zou, Y.; Zhu, Q. Design, Synthesis, and Biological Evaluation of Potent and Selective Inhibitors of Ataxia Telangiectasia Mutated and Rad3-Related (ATR) Kinase for the Efficient Treatment of Cancer. Molecules 2023, 28, 4521. https://doi.org/10.3390/molecules28114521
Shao J, Huang L, Lai W, Zou Y, Zhu Q. Design, Synthesis, and Biological Evaluation of Potent and Selective Inhibitors of Ataxia Telangiectasia Mutated and Rad3-Related (ATR) Kinase for the Efficient Treatment of Cancer. Molecules. 2023; 28(11):4521. https://doi.org/10.3390/molecules28114521
Chicago/Turabian StyleShao, Jialu, Lei Huang, Wenwen Lai, Yi Zou, and Qihua Zhu. 2023. "Design, Synthesis, and Biological Evaluation of Potent and Selective Inhibitors of Ataxia Telangiectasia Mutated and Rad3-Related (ATR) Kinase for the Efficient Treatment of Cancer" Molecules 28, no. 11: 4521. https://doi.org/10.3390/molecules28114521
APA StyleShao, J., Huang, L., Lai, W., Zou, Y., & Zhu, Q. (2023). Design, Synthesis, and Biological Evaluation of Potent and Selective Inhibitors of Ataxia Telangiectasia Mutated and Rad3-Related (ATR) Kinase for the Efficient Treatment of Cancer. Molecules, 28(11), 4521. https://doi.org/10.3390/molecules28114521





