Methods to Detect Volatile Organic Compounds for Breath Biopsy Using Solid-Phase Microextraction and Gas Chromatography–Mass Spectrometry
Abstract
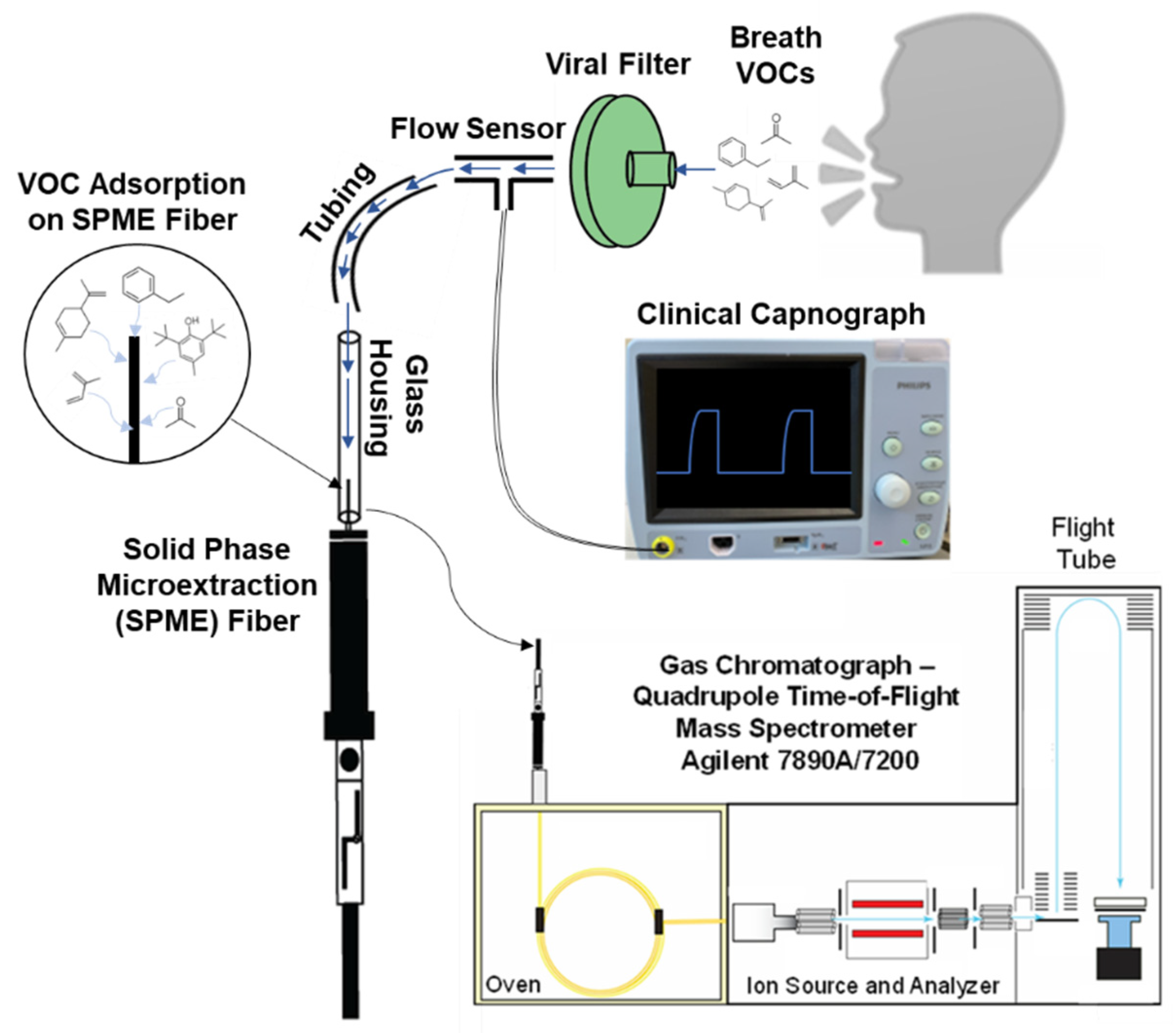
:1. Introduction
2. Results
2.1. DB–SPME-Method Optimization
2.2. Tedlar–SPME-Method Optimization
2.3. SPME-GC–MS-Method Comparison
3. Discussion
4. Materials and Methods
4.1. Materials and Instrumentation
4.2. DB-SPME Method Optimization
4.3. Tedlar–SPME and Cryotransfer Optimization
4.4. Comparison of DB–SPME, Tedlar–SPME, and Cryotransfer Methods
4.5. SPME GC-MS Protocol and Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Janfaza, S.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Digging deeper into volatile organic compounds associated with cancer. Biol. Methods Protoc. 2019, 4, bpz014. [Google Scholar] [CrossRef]
- Hardin, D.S.; Anderson, W.; Cattet, J. Dogs Can Be Successfully Trained to Alert to Hypoglycemia Samples from Patients with Type 1 Diabetes. Diabetes Ther. 2015, 6, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Rooney, N.J.; Guest, C.M.; Swanson, L.C.M.; Morant, S.V. How effective are trained dogs at alerting their owners to changes in blood glycaemic levels? Variations in performance of glycaemia alert dogs. PLoS ONE 2019, 14, e0210092. [Google Scholar] [CrossRef] [Green Version]
- Feil, C.; Staib, F.; Berger, M.R.; Stein, T.; Schmidtmann, I.; Forster, A.; Schimanski, C.C. Sniffer dogs can identify lung cancer patients from breath and urine samples. BMC Cancer 2021, 21, 917. [Google Scholar] [CrossRef]
- Guest, C.; Harris, R.; Sfanos, K.S.; Shrestha, E.; Partin, A.W.; Trock, B.; Mangold, L.; Bader, R.; Kozak, A.; McLean, S.; et al. Feasibility of integrating canine olfaction with chemical and microbial profiling of urine to detect lethal prostate cancer. PLoS ONE 2021, 16, e0245530. [Google Scholar] [CrossRef]
- Angle, T.C.; Passler, T.; Waggoner, P.L.; Fischer, T.D.; Rogers, B.; Galik, P.K.; Maxwell, H.S. Real-Time Detection of a Virus Using Detection Dogs. Front. Vet. Sci. 2016, 2, 79. [Google Scholar] [CrossRef] [Green Version]
- ten Hagen, N.A.; Twele, F.; Meller, S.; Jendrny, P.; Schulz, C.; von Köckritz-Blickwede, M.; Osterhaus, A.; Ebbers, H.; Pink, I.; Welte, T.; et al. Discrimination of SARS-CoV-2 Infections from Other Viral Respiratory Infections by Scent Detection Dogs. Front. Med. 2021, 8, 749588. [Google Scholar] [CrossRef]
- Pathak, A.K.; Viphavakit, C. VOC Biomarker Monitoring for Diabetes Through Exhaled Breath Using Ag/P-TiO2 Composite Plasmonic Sensor. IEEE Sens. J. 2021, 21, 22631–22637. [Google Scholar] [CrossRef]
- Woollam, M.; Teli, M.; Liu, S.; Daneshkhah, A.; Siegel, A.P.; Yokota, H.; Agarwal, M. Urinary Volatile Terpenes Analyzed by Gas Chromatography–Mass Spectrometry to Monitor Breast Cancer Treatment Efficacy in Mice. J. Proteome Res. 2020, 19, 1913–1922. [Google Scholar] [CrossRef]
- Adiguzel, Y.; Kulah, H. Breath sensors for lung cancer diagnosis. Biosens. Bioelectron. 2015, 65, 121–138. [Google Scholar] [CrossRef]
- Antoniou, S.X.; Gaude, E.; Ruparel, M.; van der Schee, M.P.; Janes, S.M.; Rintoul, R.C. The potential of breath analysis to improve outcome for patients with lung cancer. J. Breath Res. 2019, 13, 034002. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.D. Application of Electronic-Nose Technologies and VOC-Biomarkers for the Noninvasive Early Diagnosis of Gastrointestinal Diseases. Sensors 2018, 18, 2613. [Google Scholar] [CrossRef] [Green Version]
- Spinelle, L.; Gerboles, M.; Kok, G.; Sauerwald, T. 02—Sensitivity of VOC Sensors for Air Quality Monitoring within the EURAMET Key-VOC project. In Proceedings of the Fourth Scientific Meeting EuNetAir, Linkoping, Sweden, 3–5 June 2015; pp. 6–9. [Google Scholar] [CrossRef]
- Dospinescu, V.-M.; Tiele, A.; Covington, J.A. Sniffing Out Urinary Tract Infection—Diagnosis Based on Volatile Organic Compounds and Smell Profile. Biosensors 2020, 10, 83. [Google Scholar] [CrossRef]
- Amann, A.; Costello, B.d.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef]
- Smolinska, A.; Klaassen, E.M.M.; Dallinga, J.W.; van de Kant, K.D.G.; Jobsis, Q.; Moonen, E.J.C.; van Schayck, O.C.P.; Dompeling, E.; van Schooten, F.J. Profiling of Volatile Organic Compounds in Exhaled Breath As a Strategy to Find Early Predictive Signatures of Asthma in Children. PLoS ONE 2014, 9, e95668. [Google Scholar] [CrossRef] [Green Version]
- Fenske, J.D.; Paulson, S.E. Human breath emissions of VOCs. J. Air Waste Manag. Assoc. 1999, 49, 594–598. [Google Scholar] [CrossRef]
- Franchina, F.A.; Purcaro, G.; Burklund, A.; Beccaria, M.; Hill, J.E. Evaluation of different adsorbent materials for the untargeted and targeted bacterial VOC analysis using GC×GC-MS. Anal. Chim. Acta 2019, 1066, 146–153. [Google Scholar] [CrossRef]
- Harshman, S.W.; Pitsch, R.L.; Davidson, C.N.; Lee, E.M.; Scott, A.M.; Hill, E.M.; Mainali, P.; Brooks, Z.E.; Strayer, K.E.; Schaeublin, N.M.; et al. Evaluation of a standardized collection device for exhaled breath sampling onto thermal desorption tubes. J. Breath Res. 2020, 14, 036004. [Google Scholar] [CrossRef]
- Van Hoeck, E.; David, F.; Sandra, P. Stir bar sorptive extraction for the determination of pyrethroids in water samples: A comparison between thermal desorption in a dedicated thermal desorber, in a split/splitless inlet and by liquid desorption. J. Chromatogr. A 2007, 1157, 1–9. [Google Scholar] [CrossRef]
- Yuan, Z.-C.; Zhang, Y.; Cai, S.-H.; Chen, W.; Hu, B. Solid phase microextraction for human breath analysis of environmental and occupational exposures: A review. Adv. Sample Prep. 2022, 3, 100023. [Google Scholar] [CrossRef]
- Monedeiro, F.; Monedeiro-Milanowski, M.; Ratiu, I.A.; Brożek, B.; Ligor, T.; Buszewski, B. Needle Trap Device-GC-MS for Characterization of Lung Diseases Based on Breath VOC Profiles. Molecules 2021, 26, 1789. [Google Scholar] [CrossRef] [PubMed]
- Trefz, P.; Rösner, L.; Hein, D.; Schubert, J.K.; Miekisch, W. Evaluation of needle trap micro-extraction and automatic alveolar sampling for point-of-care breath analysis. Anal. Bioanal. Chem. 2013, 405, 3105–3115. [Google Scholar] [CrossRef]
- Martin, A.N.; Farquar, G.R.; Jones, A.D.; Frank, M. Human breath analysis: Methods for sample collection and reduction of localized background effects. Anal. Bioanal. Chem. 2010, 396, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.D.; Fowler, S.J.; Montpetit, A.J. Exhaled breath testing—A tool for the clinician and researcher. Paediatr. Respir. Rev. 2019, 29, 37–41. [Google Scholar] [CrossRef]
- Beauchamp, J.; Herbig, J.; Gutmann, R.; Hansel, A. On the use of Tedlar® bags for breath-gas sampling and analysis. J. Breath Res. 2008, 2, 046001. [Google Scholar] [CrossRef] [PubMed]
- Woollam, M.; Siegel, A.P.; Grocki, P.; Saunders, J.L.; Sanders, D.B.; Agarwal, M.; Davis, M.D. Preliminary method for profiling volatile organic compounds in breath that correlate with pulmonary function and other clinical traits of subjects diagnosed with cystic fibrosis: A pilot study. J. Breath Res. 2022, 16, 027103. [Google Scholar] [CrossRef]
- Phillips, C.; Mac Parthaláin, N.; Syed, Y.; Deganello, D.; Claypole, T.; Lewis, K. Short-Term Intra-Subject Variation in Exhaled Volatile Organic Compounds (VOCs) in COPD Patients and Healthy Controls and Its Effect on Disease Classification. Metabolites 2014, 4, 300–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Gilio, A.; Palmisani, J.; Ventrella, G.; Facchini, L.; Catino, A.; Varesano, N.; Pizzutilo, P.; Galetta, D.; Borelli, M.; Barbieri, P.; et al. Breath Analysis: Comparison among Methodological Approaches for Breath Sampling. Molecules 2020, 25, 5823. [Google Scholar] [CrossRef]
- Yuan, Z.-C.; Li, W.; Wu, L.; Huang, D.; Wu, M.; Hu, B. Solid-Phase Microextraction Fiber in Face Mask for in Vivo Sampling and Direct Mass Spectrometry Analysis of Exhaled Breath Aerosol. Anal. Chem. 2020, 92, 11543–11547. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, W.; Chan, C.-K.; Jin, L.; Griffith, S.M.; Yu, J.Z.; Chan, W. Polyurethane Foam Face Masks as a Dosimeter for Quantifying Personal Exposure to Airborne Volatile and Semi-Volatile Organic Compounds. Chem. Res. Toxicol. 2022, 35, 1604–1613. [Google Scholar] [CrossRef]
- Cabanas-Garrido, E.C.; Ledesma-Escobar, C.A.; Priego-Capote, F. Use of surgical masks for sampling in the determination of volatile organic compounds. Talanta 2023, 253, 124105. [Google Scholar] [CrossRef]
- Dominelli, P.B.; Molgat-Seon, Y. Sex, gender and the pulmonary physiology of exercise. Eur. Respir. Rev. 2022, 31, 210074. [Google Scholar] [CrossRef] [PubMed]
- Schubert, J.K.; Spittler, K.H.; Braun, G.; Geiger, K.; Guttmann, J. CO2-controlled sampling of alveolar gas in mechanically ventilated patients. J. Appl. Physiol. 2001, 90, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Kreit, J.W. Volume Capnography in the Intensive Care Unit: Potential Clinical Applications. Ann. Am. Thorac. Soc. 2019, 16, 409–420. [Google Scholar] [CrossRef]
- Righettoni, M.; Tricoli, A.; Gass, S.; Schmid, A.; Amann, A.; Pratsinis, S.E. Breath acetone monitoring by portable Si:WO3 gas sensors. Anal. Chim. Acta 2012, 738, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhouri, N.; Singh, T.; Alsabbagh, E.; Guirguis, J.; Chami, T.; Hanouneh, I.; Grove, D.; Lopez, R.; Dweik, R. Isoprene in the Exhaled Breath is a Novel Biomarker for Advanced Fibrosis in Patients with Chronic Liver Disease: A Pilot Study. Clin. Transl. Gastroenterol. 2015, 6, e112. [Google Scholar] [CrossRef]
- van den Broek, J.; Güntner, A.T.; Pratsinis, S.E. Highly Selective and Rapid Breath Isoprene Sensing Enabled by Activated Alumina Filter. ACS Sens. 2018, 3, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Beitler, J.R.; Sands, S.A.; Loring, S.H.; Owens, R.L.; Malhotra, A.; Spragg, R.G.; Matthay, M.A.; Thompson, B.T.; Talmor, D. Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ARDS: The BREATHE criteria. Intensive Care Med. 2016, 42, 1427–1436. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.; Coats, T.; Monks, P.; White, I.; Pandya, H.; Beardsmore, C.; Skinner, J. A comparison of tidal and incentive breath collection methods for the determination of breath volatiles concentration. Emerg. Med. J. 2015, 32, 983. [Google Scholar] [CrossRef]
- Mazzone, P.J.; Wang, X.-F.; Lim, S.; Jett, J.; Choi, H.; Zhang, Q.; Beukemann, M.; Seeley, M.; Martino, R.; Rhodes, P. Progress in the Development of Volatile Exhaled Breath Signatures of Lung Cancer. Ann. Am. Thorac. Soc. 2015, 12, 752–757. [Google Scholar] [CrossRef] [Green Version]
- Lawal, O.; Ahmed, W.M.; Nijsen, T.M.E.; Goodacre, R.; Fowler, S.J. Exhaled breath analysis: A review of ‘breath-taking’ methods for off-line analysis. Metabolomics 2017, 13, 110. [Google Scholar] [CrossRef]
- Mochalski, P.; King, J.; Unterkofler, K.; Amann, A. Stability of selected volatile breath constituents in Tedlar, Kynar and Flexfilm sampling bags. Analyst 2013, 138, 1405–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Raihala, T.S.; Jackman, A.P.; St. John, R. Use of Tedlar Bags in VOC Testing and Storage: Evidence of Significant VOC Losses. Environ. Sci. Technol. 1996, 30, 3115–3117. [Google Scholar] [CrossRef]
- Siegel, A.P.; Daneshkhah, A.; Hardin, D.S.; Shrestha, S.; Varahramyan, K.; Agarwal, M. Analyzing breath samples of hypoglycemic events in type 1 diabetes patients: Towards developing an alternative to diabetes alert dogs. J. Breath Res. 2017, 11, 026007. [Google Scholar] [CrossRef]
- Woollam, M.; Angarita-Rivera, P.; Siegel, A.P.; Kalra, V.; Kapoor, R.; Agarwal, M. Exhaled VOCs can discriminate subjects with COVID-19 from healthy controls. J. Breath Res. 2022, 16, 036002. [Google Scholar] [CrossRef]
- Guerra, B.; Recio, C.; Aranda-Tavío, H.; Guerra-Rodríguez, M.; García-Castellano, J.M.; Fernández-Pérez, L. The Mevalonate Pathway, a Metabolic Target in Cancer Therapy. Front. Oncol. 2021, 11, 626971. [Google Scholar] [CrossRef]
- Liu, Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006, 9, 230–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harshman, S.W.; Mani, N.; Geier, B.A.; Kwak, J.; Shepard, P.; Fan, M.; Sudberry, G.L.; Mayes, R.S.; Ott, D.K.; Martin, J.A.; et al. Storage stability of exhaled breath on Tenax TA. J. Breath Res. 2016, 10, 046008. [Google Scholar] [CrossRef]
- Peralbo-Molina, A.; Calderón-Santiago, M.; Priego-Capote, F.; Jurado-Gámez, B.; Luque de Castro, M.D. Identification of metabolomics panels for potential lung cancer screening by analysis of exhaled breath condensate. J. Breath Res. 2016, 10, 026002. [Google Scholar] [CrossRef]
- O’Hara, M.E.; Fernández del Río, R.; Holt, A.; Pemberton, P.; Shah, T.; Whitehouse, T.; Mayhew, C.A. Limonene in exhaled breath is elevated in hepatic encephalopathy. J. Breath Res. 2016, 10, 046010. [Google Scholar] [CrossRef]
- Marcondes-Braga, F.G.; Gutz, I.G.R.; Batista, G.L.; Saldiva, P.H.N.; Ayub-Ferreira, S.M.; Issa, V.S.; Mangini, S.; Bocchi, E.A.; Bacal, F. Exhaled Acetone as a New Biomarker of Heart Failure Severity. Chest 2012, 142, 457–466. [Google Scholar] [CrossRef]
- Rydosz, A. Sensors for Enhanced Detection of Acetone as a Potential Tool for Noninvasive Diabetes Monitoring. Sensors 2018, 18, 2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, D.; Jamnig, H.; Heininger, P.; Klieber, M.; Schroecksnadel, S.; Fiegl, M.; Hackl, M.; Denz, H.; Amann, A. Decline of exhaled isoprene in lung cancer patients correlates with immune activation. J. Breath Res. 2012, 6, 027101. [Google Scholar] [CrossRef]
- Lee, J.-H.; Hwang, S.-M.; Lee, D.-W.; Heo, G.-S. Determination of Volatile Organic Compounds (VOCs) Using Tedlar Bag/Solid-phase Microextraction/Gas Chromatography/Mass Spectrometry (SPME/GC/MS) in Ambient and Workplace Air. Bull. Korean Chem. Soc. 2002, 23, 488–496. [Google Scholar] [CrossRef] [Green Version]
- Ligor, T.; Ligor, M.; Amann, A.; Ager, C.; Bachler, M.; Dzien, A.; Buszewski, B. The analysis of healthy volunteers’ exhaled breath by the use of solid-phase microextraction and GC-MS. J. Breath Res. 2008, 2, 046006. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, E.; Woollam, M.; Grocki, P.; Davis, M.D.; Agarwal, M. Methods to Detect Volatile Organic Compounds for Breath Biopsy Using Solid-Phase Microextraction and Gas Chromatography–Mass Spectrometry. Molecules 2023, 28, 4533. https://doi.org/10.3390/molecules28114533
Schulz E, Woollam M, Grocki P, Davis MD, Agarwal M. Methods to Detect Volatile Organic Compounds for Breath Biopsy Using Solid-Phase Microextraction and Gas Chromatography–Mass Spectrometry. Molecules. 2023; 28(11):4533. https://doi.org/10.3390/molecules28114533
Chicago/Turabian StyleSchulz, Eray, Mark Woollam, Paul Grocki, Michael D. Davis, and Mangilal Agarwal. 2023. "Methods to Detect Volatile Organic Compounds for Breath Biopsy Using Solid-Phase Microextraction and Gas Chromatography–Mass Spectrometry" Molecules 28, no. 11: 4533. https://doi.org/10.3390/molecules28114533





