Efficient Adsorption Capacity of MgFe-Layered Double Hydroxide Loaded on Pomelo Peel Biochar for Cd (II) from Aqueous Solutions: Adsorption Behaviour and Mechanism
Abstract
:1. Introduction
2. Results
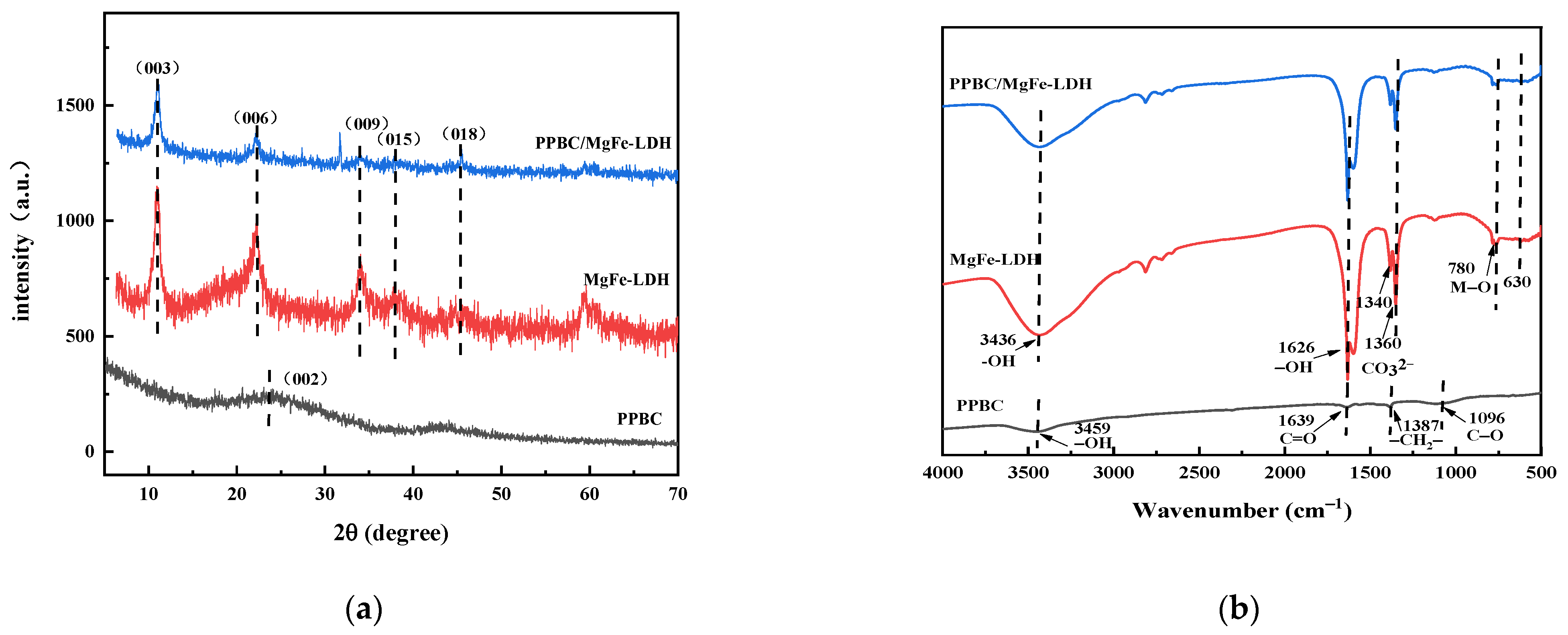
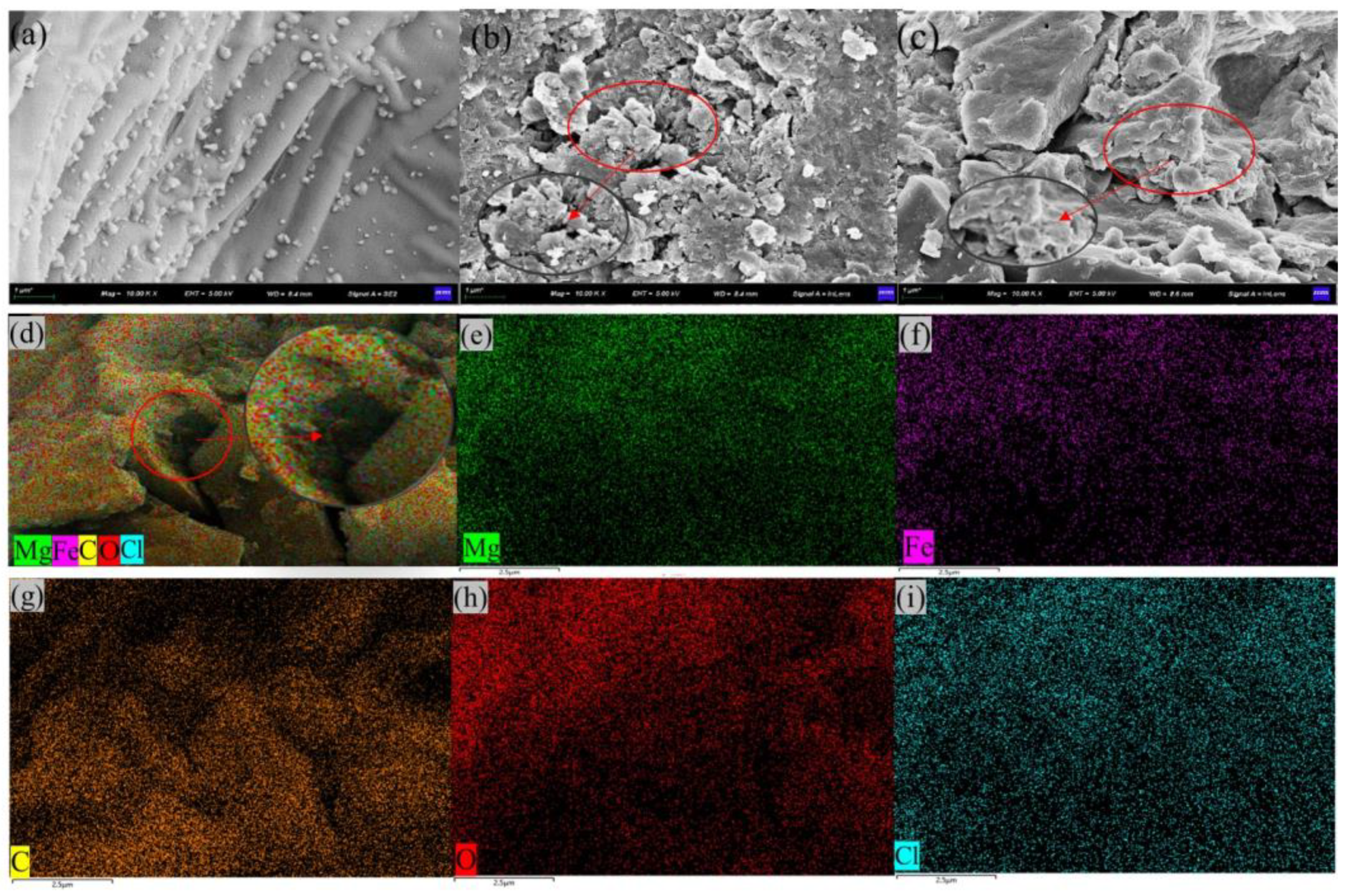
2.1. Structure Characterisation
2.2. Bath Experiments
2.2.1. Effect of Solution pH
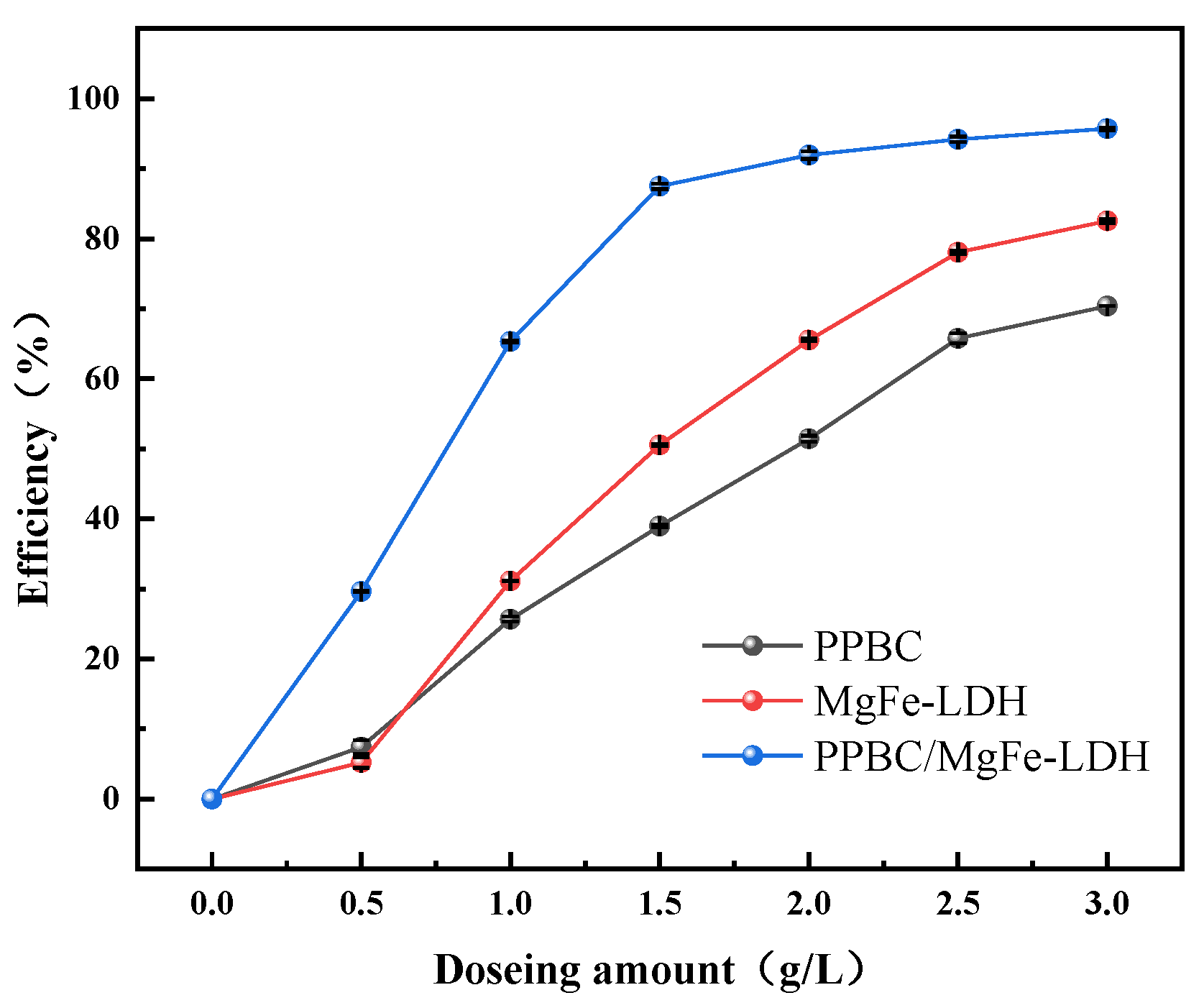
2.2.2. Effect of Dosage Amount
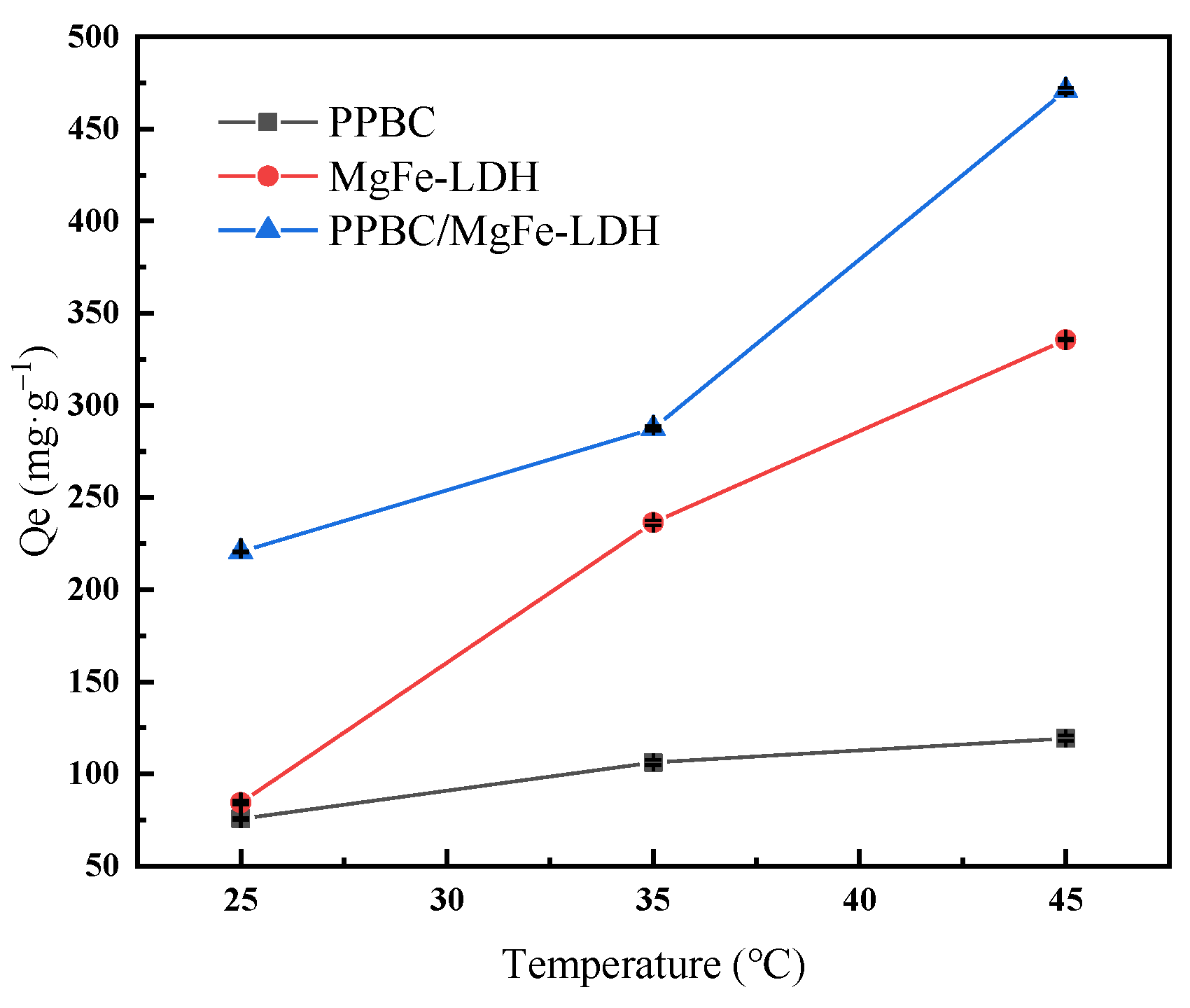
2.2.3. Effect of Reaction Temperature
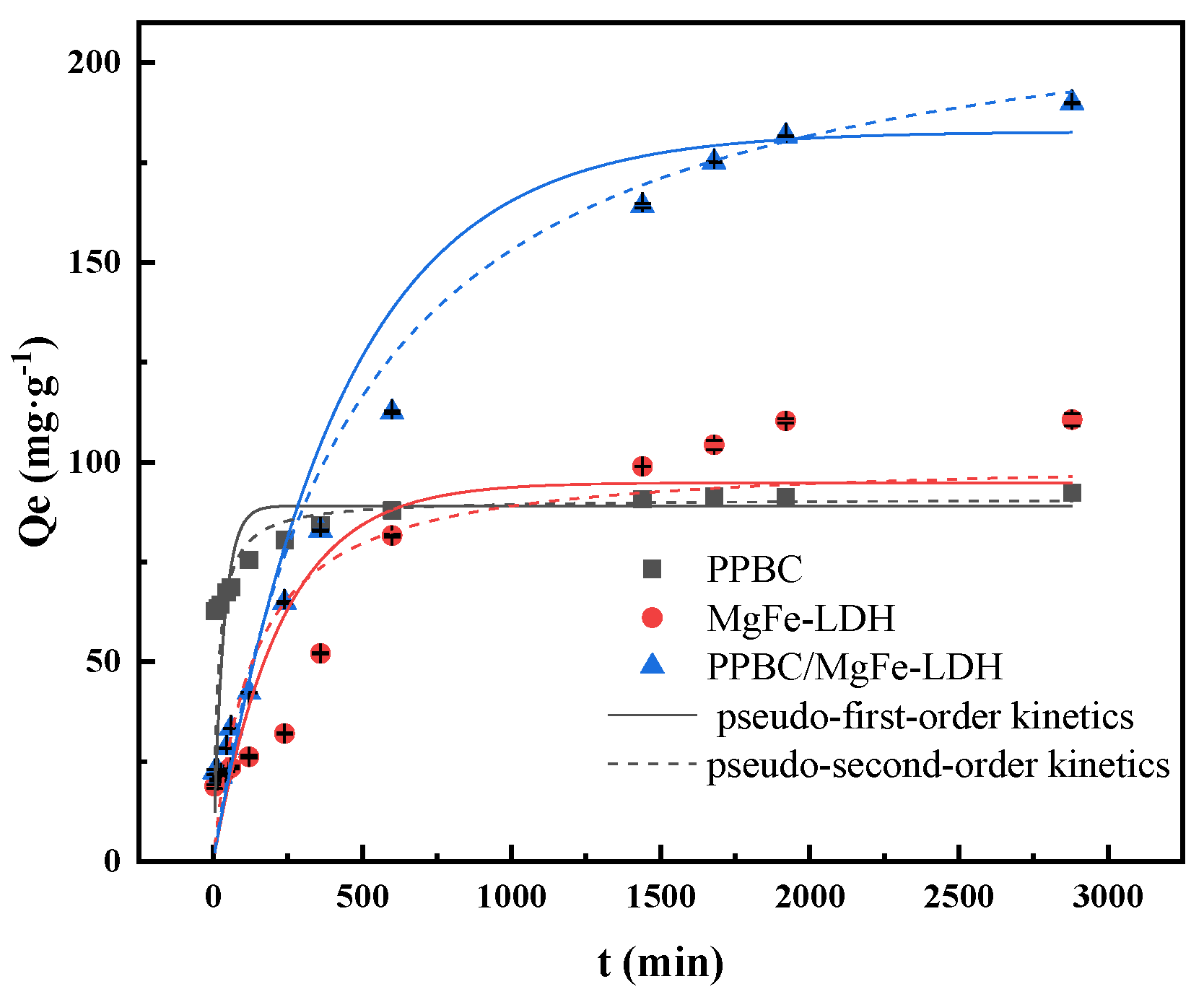
2.2.4. Sorption Kinetics
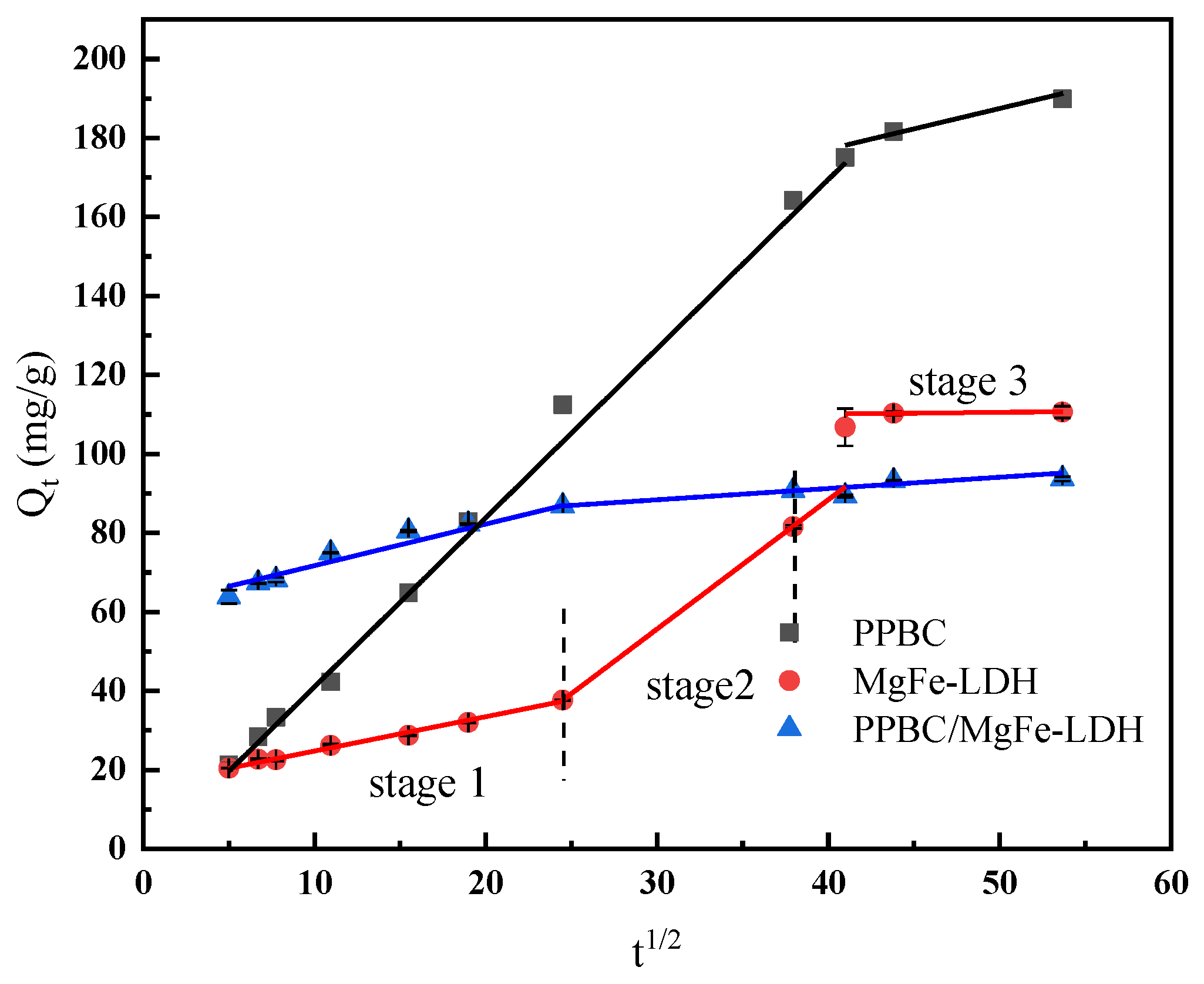
2.2.5. Intra-Particle Diffusion Model Analysis
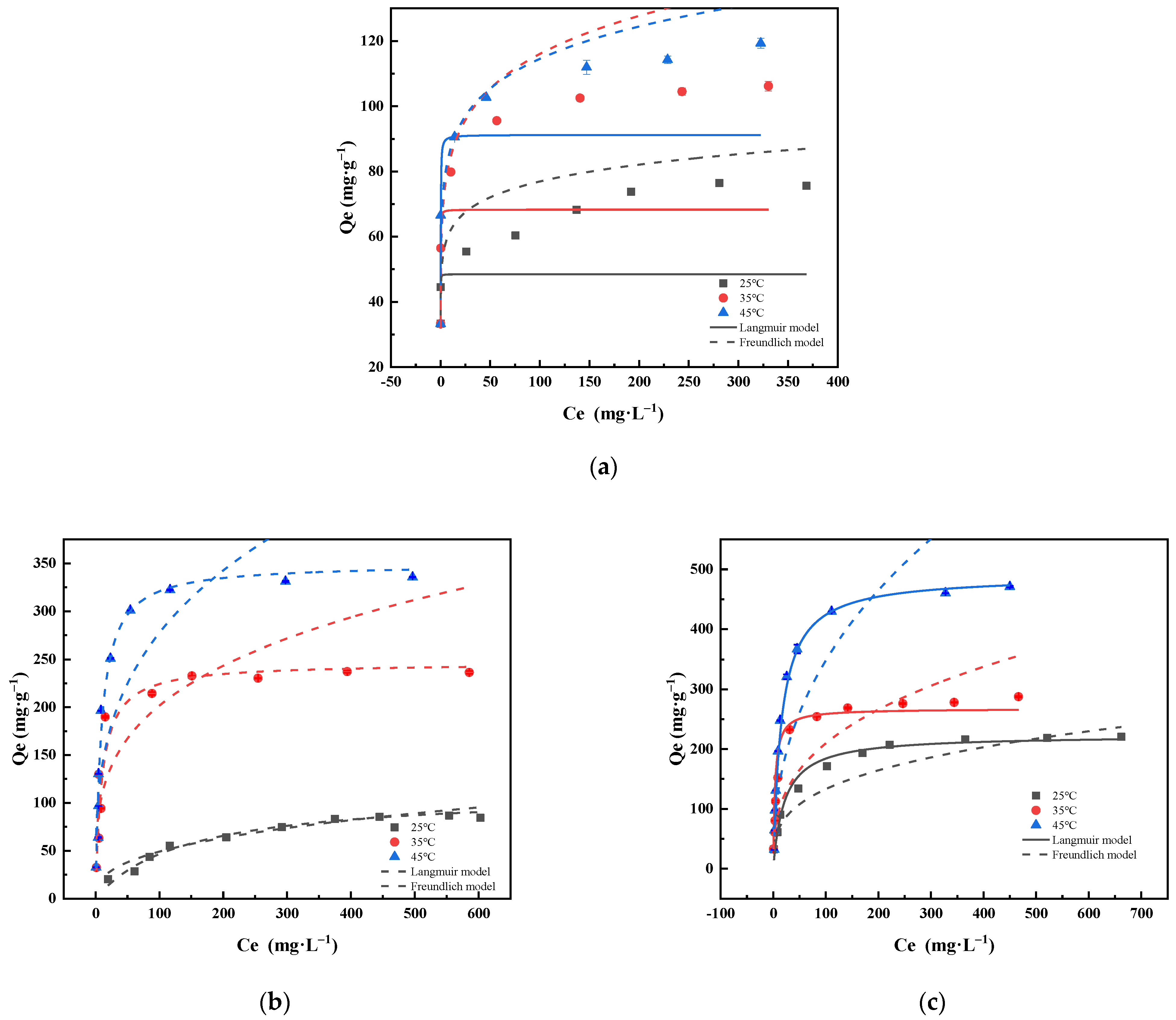
2.2.6. Sorption Isotherms
2.2.7. Thermodynamic Study
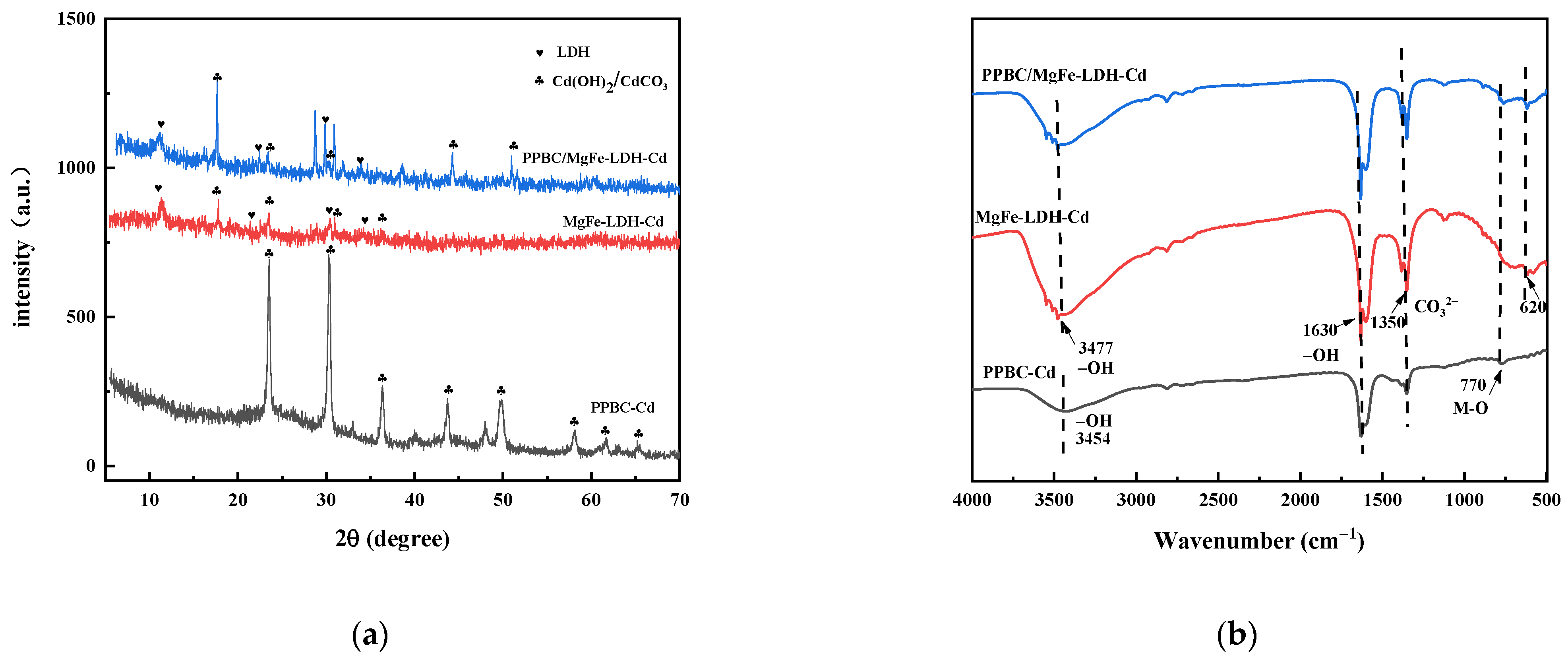
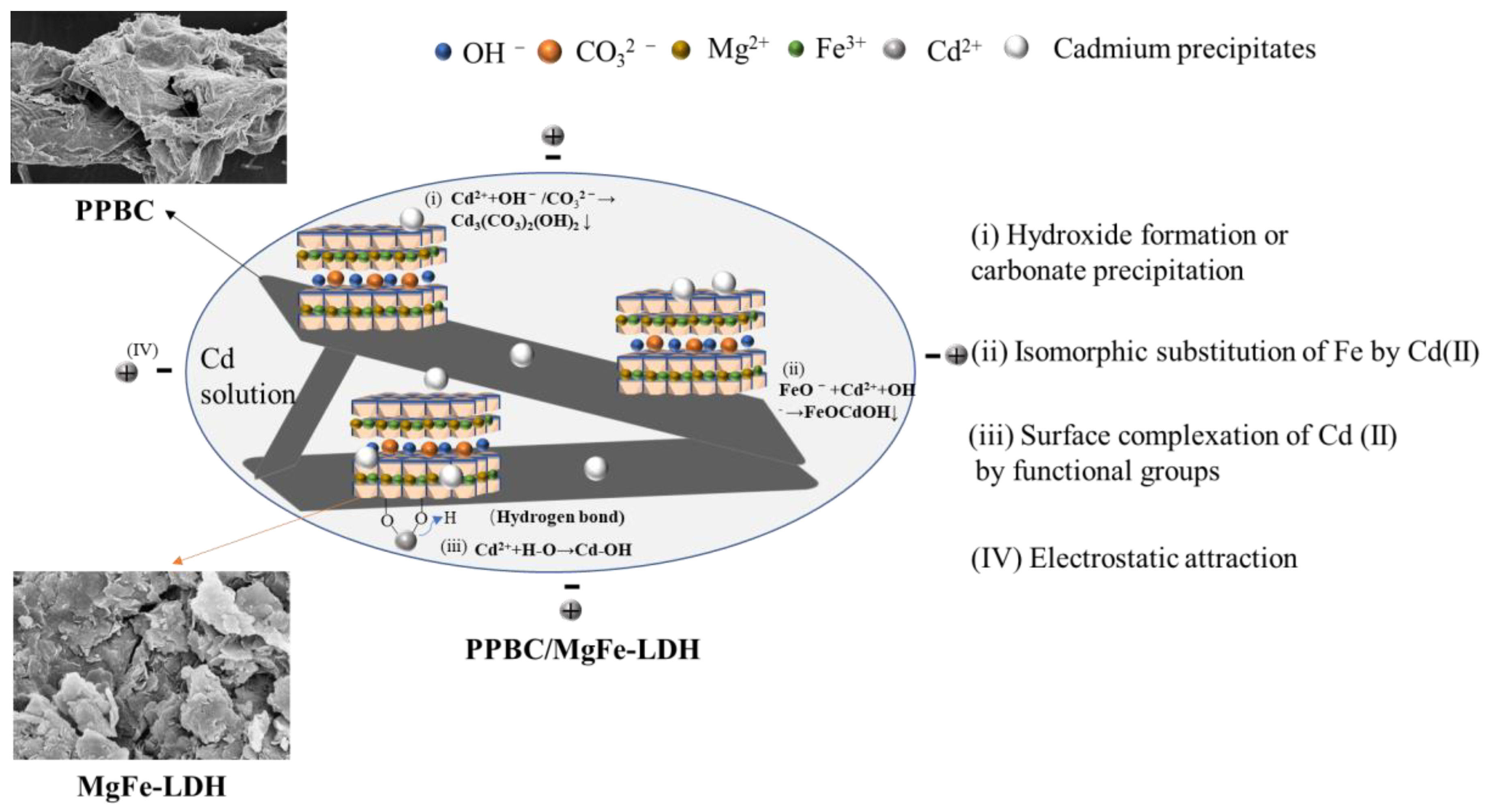
3. Adsorption Mechanisms
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Materials
4.3. Bath Adsorption Experiments
4.4. Adsorption Model Fitting
4.5. Characterisation Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Manzoor, R.; Zhang, T.; Zhang, X.; Wang, M.; Pan, J.; Wang, Z.; Zhang, B. Single and combined metal contamination in coastal environments in China: Current status and potential ecological risk evaluation. Environ. Sci. Pollut. Res. Int. 2018, 252, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Jin, Q.; Kavan, P. A Study of Heavy Metal Pollution in China: Current Status, Pollution-Control Policies and Countermeasures. Sustainability 2014, 69, 5820–5838. [Google Scholar] [CrossRef] [Green Version]
- Fan, T.; Sun, Q.; Cui, P.; Xuan, L.; Wang, Y. Sorption mechanism of cadmium on soils: A combination of batch experiment, path analysis, and EXAFS techniques. Geoderma 2022, 422, 115950. [Google Scholar] [CrossRef]
- Wang, X.; Dong, J.; Gong, C.; Zhang, S.; Yang, J.; Zhang, A.; Feng, Z. Bendable poly(vinylidene fluoride)/polydopamine/β-cyclodextrin composite electrospun membranes for highly efficient and bidirectional adsorption of cation and anion dyes from aqueous media. Compos. Sci. Technol. 2022, 219, 109256. [Google Scholar] [CrossRef]
- Cai, Y.R.; Song, Y.; Chang, C. Adsorption properties and mechanism of ginkgo biloba leaf-based materials for Cd (II) in aqueous solution. Environ. Sci. Pollut. Res. Int. 2022, 78499–78508. [Google Scholar] [CrossRef] [PubMed]
- Gharib, A.; Arab, A. Chemically prepared Pd-Cd alloy nanocatalysts as the highly active material for formic acid electrochemical oxidation. Mol. Catal. 2022, 528, 112434. [Google Scholar] [CrossRef]
- Alexander, J.A.; Ahmad Zaini, M.A.; Surajudeen, A.; Aliyu, E.U.; Omeiza, A.U. Surface modification of low-cost bentonite adsorbents—A review. Part. Sci. Technol. 2018, 375, 538–549. [Google Scholar] [CrossRef]
- Younes, K.; Kharboutly, Y.; Antar, M.; Chaouk, H.; Obeid, E.; Mouhtady, O.; Abu-samha, M.; Halwani, J.; Murshid, N. Application of Unsupervised Machine Learning for the Evaluation of Aerogels’ Efficiency towards Ion Removal-A Principal Component Analysis (PCA) Approach. Gels 2023, 94, 304. [Google Scholar] [CrossRef]
- Younes, K.; Kharboutly, Y.; Antar, M.; Chaouk, H.; Obeid, E.; Mouhtady, O.; Abu-samha, M.; Halwani, J.; Murshid, N. Application of Unsupervised Learning for the Evaluation of Aerogels’ Efficiency towards Dye Removal-A Principal Component Analysis (PCA) Approach. Gels 2023, 94, 327. [Google Scholar] [CrossRef]
- Dong, Y.; Kong, X.; Luo, X.; Wang, H. Adsorptive removal of heavy metal anions from water by layered double hydroxide: A review. Chemosphere 2022, 303, 134685. [Google Scholar] [CrossRef]
- Huang, Y.X.; Liu, C.M.; Rad, S.; He, H.J.; Qin, L.T. A Comprehensive Review of Layered Double Hydroxide-Based Carbon Composites as an Environmental Multifunctional Material for Wastewater Treatment. Processes 2022, 10, 617. [Google Scholar] [CrossRef]
- Chen, M.; Wu, P.; Huang, Z.; Liu, J.; Li, Y.; Zhu, N.; Dang, Z.; Bi, Y. Environmental application of MgMn-layered double oxide for simultaneous efficient removal of tetracycline and Cd pollution: Performance and mechanism. J. Environ. Manag. 2019, 246, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Deng, J.; Li, X.; Wei, X.; Shao, Y.; Zhao, Y. Layered double hydroxides loaded sludge biochar composite for adsorptive removal of benzotriazole and Pb(II) from aqueous solution. Chemosphere 2021, 287 Pt 1, 131966. [Google Scholar] [CrossRef]
- Guan, X.H.; Yuan, X.; Zhao, Y.; Bai, J.; Li, Y.; Cao, Y.; Chen, Y.; Xiong, T. Adsorption behaviors and mechanisms of Fe/Mg layered double hydroxide loaded on bentonite on Cd (II) and Pb (II) removal. J. Colloid. Interface Sci. 2022, 612, 572–583. [Google Scholar] [CrossRef]
- González, M.A.; Pavlovic, I.; Barriga, C. Cu(II), Pb(II) and Cd (II) sorption on different layered double hydroxides. A kinetic and thermodynamic study and competing factors. Chem. Eng. J. 2015, 269, 221–228. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Y.; Yu, H.; Yan, L.; Zhang, J.; Wang, B.; Du, B.; Xing, L. Magnetic graphene oxide/MgAl-layered double hydroxide nanocomposite: One-pot solvothermal synthesis, adsorption performance and mechanisms for Pb2+, Cd2+, and Cu2+. Chem. Eng. J. 2018, 341, 1–9. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Li, J.; Li, Y.; Song, W.; Li, X.; Yan, L.; Yu, H. Highly efficient removal of aqueous Cu(II) and Cd (II) by hydrothermal synthesized CaAl-layered double hydroxide. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128584. [Google Scholar] [CrossRef]
- Tran, H.N.; Lin, C.-C.; Chao, H.-P. Amino acids-intercalated Mg/Al layered double hydroxides as dual-electronic adsorbent for effective removal of cationic and oxyanionic metal ions. Sep. Purif. Technol. 2018, 192, 36–45. [Google Scholar] [CrossRef]
- Rahmanian, O.; Maleki, M.H.; Dinari, M. Ultrasonically assisted solvothermal synthesis of novel Ni/Al layered double hydroxide for capturing of Cd (II) from contaminated water. J. Phys. Chem. Solids 2017, 110, 195–201. [Google Scholar] [CrossRef]
- Barahuie, F.; Hussein, M.Z.; Arulselvan, P.; Fakurazi, S.; Zainal, Z. Drug delivery system for an anticancer agent, chlorogenate-Zn/Al-layered double hydroxide nanohybrid synthesised using direct co-precipitation and ion exchange methods. J. Solid. State Chem. 2014, 217, 31–41. [Google Scholar] [CrossRef]
- Lyu, F.; Yu, H.; Hou, T.; Yan, L.; Zhang, X.; Du, B. Efficient and fast removal of Pb(2+) and Cd(2+) from an aqueous solution using a chitosan/Mg-Al-layered double hydroxide nanocomposite. J. Colloid. Interface Sci. 2019, 539, 184–193. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; Bi, Y.; Rehman, S.U.; Dang, Z.; Wu, P. Multifunctional magnetic MgMn-oxide composite for efficient purification of Cd(2+) and paracetamol pollution: Synergetic effect and stability. J. Hazard. Mater. 2020, 388, 122078. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Hu, F.; Wang, M.; Peng, X.; Qiu, F.; Zhang, T.; Dai, H.; Liu, Z.; Cao, Z. High-efficient adsorption of phosphates from water by hierarchical CuAl/biomass carbon fiber layered double hydroxide. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 314–323. [Google Scholar] [CrossRef]
- Rahmanian, O.; Dinari, M.; Abdolmaleki, M.K. Carbon quantum dots/layered double hydroxide hybrid for fast and efficient decontamination of Cd (II): The adsorption kinetics and isotherms. Appl. Surf. Sci. 2018, 428, 272–279. [Google Scholar] [CrossRef]
- Tóth, V.; Sipiczki, M.; Bugris, V.; Kukovecz, Á.; Kónya, Z.; Sipos, P.; Pálinkó, I. Carbon nanotube-layered double hydroxide nanocomposites. Chem. Pap. 2014, 685, 650–655. [Google Scholar] [CrossRef] [Green Version]
- Blaisi, N.I.; Zubair, M.; Ihsanullah, I.; Ali, S.; Kazeem, T.S.; Manzar, M.S.; Al-Kutti, W.A.; Al Harthi, M.A. Date palm ash-MgAl-layered double hydroxide composite: Sustainable adsorbent for effective removal of methyl orange and eriochrome black-T from aqueous phase. Environ. Sci. Pollut. Res. Int. 2018, 2534, 34319–34331. [Google Scholar] [CrossRef]
- Zhang, F.; Song, Y.; Song, S.; Zhang, R.; Hou, W. Synthesis of magnetite-graphene oxide-layered double hydroxide composites and applications for the removal of Pb(II) and 2,4-dichlorophenoxyacetic acid from aqueous solutions. ACS Appl. Mater. Interfaces 2015, 713, 7251–7263. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, M.; Zhou, R.; Li, J.; Zhao, W.; Zhou, J. Adsorption characteristics and mechanism of norfloxacin in water by gamma-Fe(2)O(3)@BC. Water Sci. Technol. 2020, 822, 242–254. [Google Scholar]
- Yin, H.Z.; Hu, C.; Li, C.; Jianlong, Z.; Hong, H. Jiasheng. Physico-chemical characteristics of pummelo peel adsorbent. Environ. Sci. Technol. 2010, 33, 87–91. [Google Scholar]
- Yang, J.; Zhang, Y.; Fu, J.; Yuan, L.; Li, Z.; Liu, C.; Zhao, D.; Wang, X. A novel magnetic biochar/MgFe-layered double hydroxides composite removing Pb2+ from aqueous solution: Isotherms, kinetics and thermodynamics. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 278–287. [Google Scholar]
- Wang, T.; Li, C.; Wang, C.; Wang, H. Biochar/MnAl-LDH composites for Cu (ΙΙ) removal from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 443–450. [Google Scholar] [CrossRef]
- Zubair, M.; Aziz, H.A.; Ihsanullah, I.; Ahmad, M.A.; Al-harthi, M.A. Biochar supported CuFe layered double hydroxide composite as a sustainable adsorbent for efficient removal of anionic azo dye from water. Environ. Technol. Innov. 2021, 23, 101614. [Google Scholar] [CrossRef]
- Maamoun, I.; Falyouna, O.; Eljamal, R.; Bensaida, K.; Tosco, T.; Sugihara, Y.; Eljamal, O. Multi-functional magnesium hydroxide coating for iron nanoparticles towards prolonged reactivity in Cr(VI) removal from aqueous solutions. J. Environ. Chem. Eng. 2022, 103, 107431. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Gu, G. Ultrasound-assisted xanthation of cellulose from lignocellulosic biomass optimized by response surface methodology for Pb(II) sorption. Carbohydr. Polym. 2018, 182, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Jiang, Z.; Wei, S.; Liang, J. Adsorption of Cd (II) from Aqueous Solutions by a Novel Layered Double Hydroxide FeMnMg-LDH. Water Air Soil. Pollut. 2018, 229, 78. [Google Scholar] [CrossRef]
- Santos, R.M.; Gonçalves, R.G.; Constantino, V.R.; Costa, L.; Silva, L.H.; Tronto, J.; Pinto, F.G. Removal of Acid Green 68:1 from aqueous solutions by calcined and uncalcined layered double hydroxides. Appl. Clay Sci. 2013, 80, 189–195. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Inyang, M. Phosphate removal ability of biochar/MgAl-LDH ultra-fine composites prepared by liquid-phase deposition. Chemosphere 2013, 928, 1042–1047. [Google Scholar] [CrossRef]
- Deng, S.; An, Q.; Ran, B.; Yang, Z.; Xu, B.; Zhao, B.; Li, Z. Efficient remediation of Mn(2+) and NH4(+)-N in co-contaminated water and soil by Acinetobacter sp. AL-6 synergized with grapefruit peel biochar: Performance and mechanism. Water Res. 2022, 223, 118962. [Google Scholar] [CrossRef]
- Tran, L.; Wu, P.; Zhu, Y.; Yang, L.; Zhu, N. Highly enhanced adsorption for the removal of Hg(II) from aqueous solution by Mercaptoethylamine/Mercaptopropyltrimethoxysilane functionalized vermiculites. J. Colloid. Interface Sci. 2015, 445, 348–356. [Google Scholar] [CrossRef]
- Ajmal, M.; Siddiq, M.Y.; Aktas, N.; Sahiner, N. Magnetic Co–Fe bimetallic nanoparticle containing modifiable microgels for the removal of heavy metal ions, organic dyes and herbicides from aqueous media. RSC Adv. 2015, 554, 43873–43884. [Google Scholar] [CrossRef]
- Chen, M.; Wu, P.; Yu, L.; Liu, S.; Ruan, B.; Hu, H.; Zhu, N.; Lin, Z. FeOOH-loaded MnO(2) nano-composite: An efficient emergency material for thallium pollution incident. J. Environ. Manag. 2017, 192, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Qiu, Z.; Lu, S.; Shi, X. Functionalized layered double hydroxide applied to heavy metal ions absorption: A review. Nanotechnol. Rev. 2020, 91, 800–819. [Google Scholar] [CrossRef]
- Dotto, G.L.; Pinto, L.A. Adsorption of food dyes acid blue 9 and food yellow 3 onto chitosan: Stirring rate effect in kinetics and mechanism. J. Hazard. Mater. 2011, 1871, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Yan, L.; Yang, K.; Hao, Y.; Du, B. Adsorption of Cd (II) by Mg-Al-CO3- and magnetic Fe3O4/Mg-Al-CO3-layered double hydroxides: Kinetic, isothermal, thermodynamic and mechanistic studies. J. Hazard. Mater. 2015, 299, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Jia, S.; Chen, Y.; Wen, Y.; Du, C.; Guo, H.; Wang, Z. Adsorption of Pb(II), Cr (III), Cu (II), Cd (II) and Ni (II) onto a vanadium mine tailing from aqueous solution. J. Hazard. Mater. 2009, 1691, 838–846. [Google Scholar] [CrossRef]
- Lyu, P.; Li, L.; Huang, X.; Wang, G.; Zhu, C. Pre-magnetic bamboo biochar cross-linked CaMgAl layered double-hydroxide composite: High-efficiency removal of As(III) and Cd (II) from aqueous solutions and insight into the mechanism of simultaneous purification. Sci. Total Environ. 2022, 823, 153743. [Google Scholar] [CrossRef]
- Tofazzal Hossain, M.; Khandaker, S.; Mahbubul Bashar, M.; Islam, A.; Ahmed, M.; Akter, R.; Alsukaibi, A.K.; Munjur Hasan, M.; Alshammari, H.M.; Kuba, T.; et al. Simultaneous toxic Cd (II) and Pb (II) encapsulation from contaminated water using Mg/Al-LDH composite materials. J. Mol. Liq. 2022, 368, 120810. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Dai, C.; Zhou, X.; Zhang, W. Sequestration of Cd (II) with nanoscale zero-valent iron (nZVI): Characterization and test in a two-stage system. Chem. Eng. J. 2014, 244, 218–226. [Google Scholar] [CrossRef]
- Wang, G.; Luo, Q.; Dai, J.; Deng, N. Adsorption of dichromate ions from aqueous solution onto magnetic graphene oxide modified by beta-cyclodextrin. Environ. Sci. Pollut. Res. Int. 2020, 2724, 30778–30788. [Google Scholar] [CrossRef]


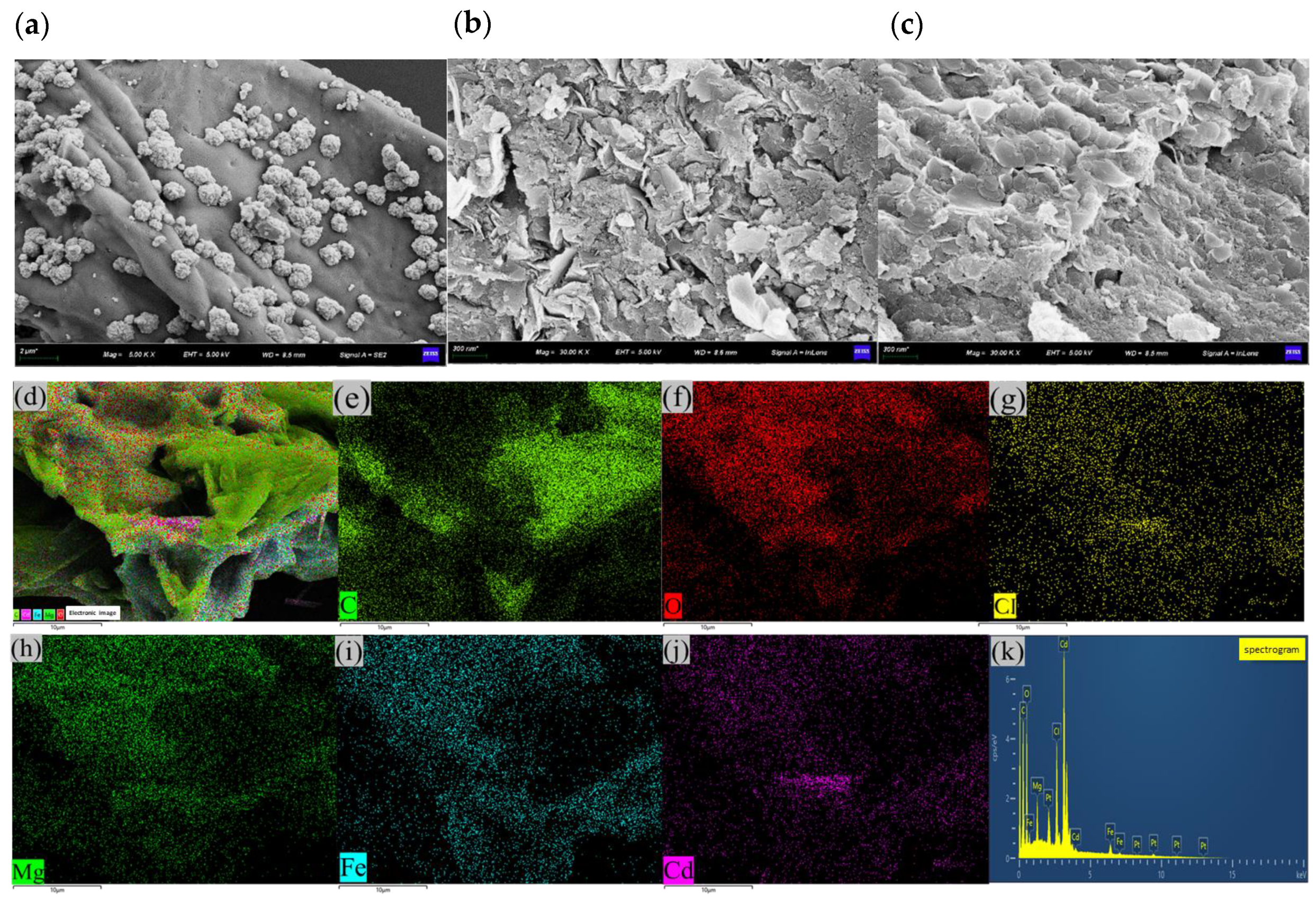
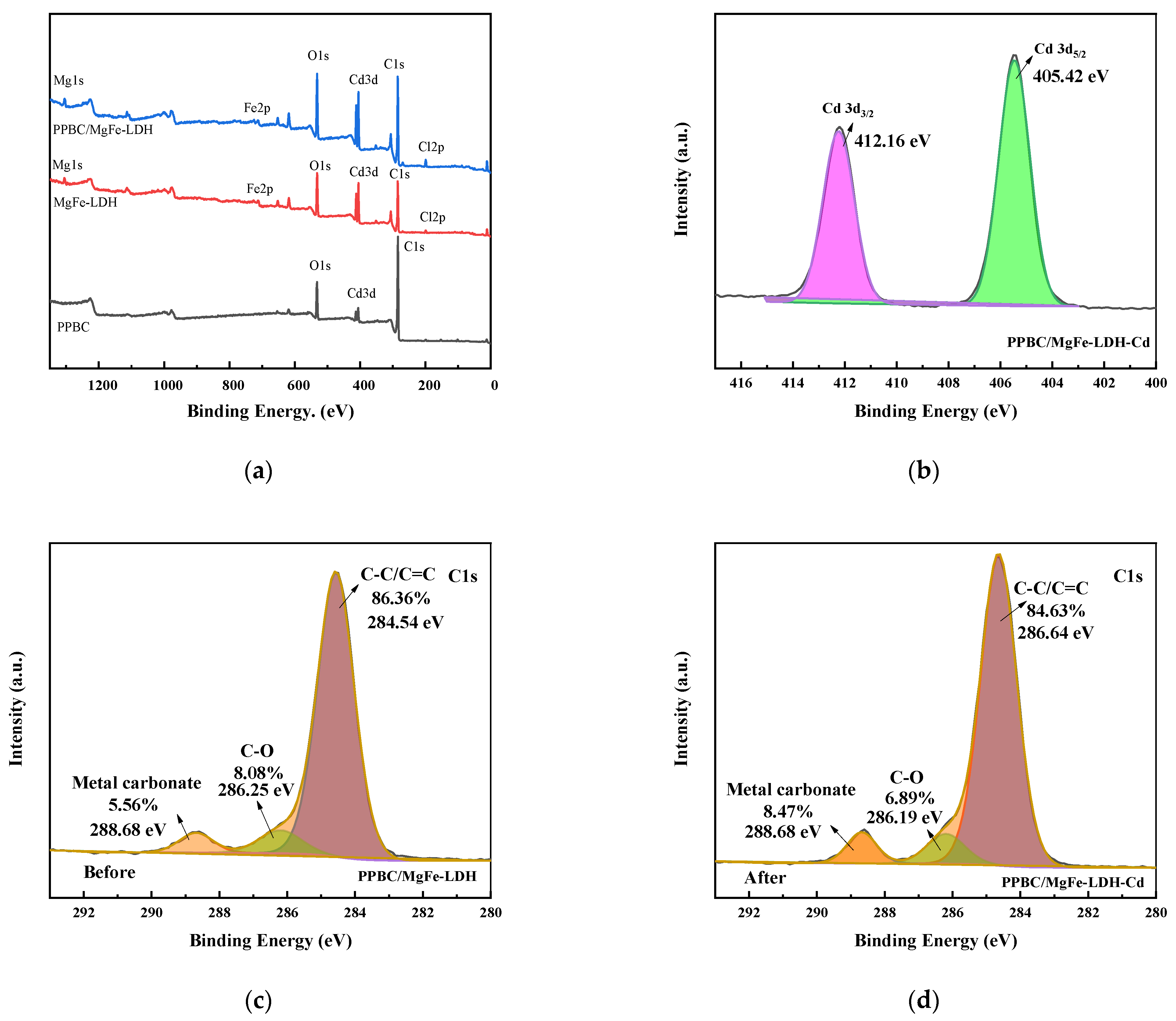
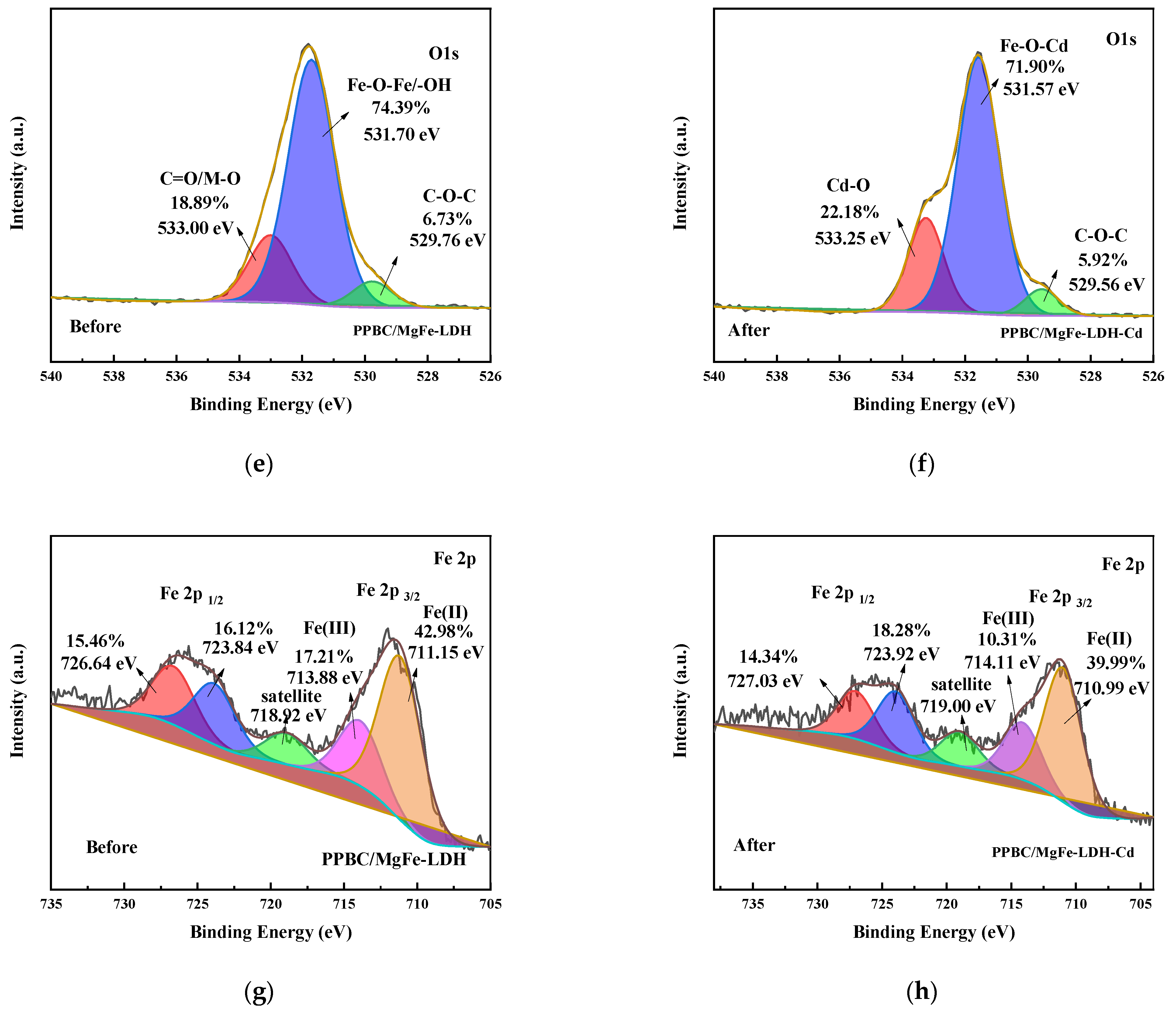

| Materials | SBET/(m2·g−1) | Vtotal/(cm3·g−1) | DBET/nm |
|---|---|---|---|
| PPBC | 230.787 | 0.139 | 2.564 |
| MgFe-LDH | 112.716 | 0.109 | 3.619 |
| PPBC/MgFe-LDH | 20.995 | 0.062 | 12.161 |
| Materials | C (%) | H (%) | O (%) | N (%) | S (%) |
|---|---|---|---|---|---|
| PPBC | 66.960 | 0.920 | 30.180 | 1.690 | 0.250 |
| MgFe-LDH | 0.740 | 3.30 | 31.440 | 1.650 | 0.290 |
| PPBC/MgFe-LDH | 25.210 | 2.390 | 71.470 | 0.420 | 0.510 |
| Adsorbent | 1st Order Kinetics | 2nd Order Kinetics | Experimental Value of Q | Intra-Particle Diffusion Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Qe (mg·g−1) | K1 (min−1) | R2 | Qe (mg·g−1) | K2 (g (mg·min) −1) | R2 | Q (mg·g−1) | K3 (mg·g−1 ·min0.5) | R2 | K3′ (mg·g−1 ·min0.5) | R2 | K3″ (mg·g−1 ·min0.5) | R2 | |
| PPBC | 89.025 | 2.596 × 10−2 | 0.751 | 90.808 | 6.885 × 10−4 | 0.898 | 92.233 | 4.282 | 0.991 | 1.033 | 0.703 | / | / |
| MgFe-LDH | 94.847 | 4.390 × 10−3 | 0.799 | 100.838 | 7.462 × 10−5 | 0.872 | 110.620 | 1.050 | 0.984 | 0.286 | 0.970 | / | / |
| PPBC/MgFe-LDH | 182.720 | 2.360 × 10−3 | 0.984 | 223.492 | 9.733 × 10−6 | 0.989 | 189.887 | 0.871 | 0.997 | 3.275 | 0.998 | 0.043 | −0.749 |
| Materials | Temperatures (°C) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|
| Qm (mg·g−1) | KL (L·mg−1) | R2 | KF (mg·g−1) (mg·L−1)–1/n | 1/n | R2 | ||
| BC | 25 | 48.498 | 71.492 | 0.851 | 49.912 | 0.094 | 0.806 |
| 35 | 68.301 | 30.645 | 0.883 | 61.408 | 0.138 | 0.880 | |
| 45 | 91.214 | 11.192 | 0.964 | 65.984 | 0.119 | 0.865 | |
| MgFe-LDH | 25 | 111.424 | 0.007 | 0.975 | 8.966 | 0.369 | 0.951 |
| 35 | 246.171 | 0.103 | 0.940 | 57.995 | 0.271 | 0.907 | |
| 45 | 349.732 | 0.111 | 0.942 | 68.309 | 0.296 | 0.715 | |
| PPBC/MgFe-LDH | 25 | 222.341 | 0.047 | 0.969 | 32.709 | 0.305 | 0.967 |
| 35 | 226.746 | 0.299 | 0.980 | 43.060 | 0.344 | 0.939 | |
| 45 | 448.960 | 0.065 | 0.993 | 50.705 | 0.418 | 0.898 | |
| Adsorbents | pH | Adsorption Capacity (mg·g−1) | References |
|---|---|---|---|
| CS/MgAl-LDH | 6 | 140.800 | [21] |
| Fe-PPBC@LDH | 4.5 | 320.650 | [43] |
| HA/MgAl-LDH | 5 | 155.280 | [44] |
| Fe3O4@NiAl-LDH@guargum bionanocomposites (GLF-BNCs) | 10 | 258 | [45] |
| LDH/MOF | 5 | 415.300 | [46] |
| PPBC/MgFe-LDH | 5.5 | 448.960 | This study |
| Materials | Temperature (K) | ΔG0 (kJ·mol−1) | ΔH0 (kJ·mol−1) | ΔS0[J·(mol·K)−1] |
|---|---|---|---|---|
| BC | 298 | −44.807 | −94.164 | −72.955 |
| 308 | −44.142 | |||
| 318 | −42.912 | |||
| MgFe-LDH | 298 | −21.936 | 109.944 | 445.930 |
| 308 | −29.557 | |||
| 318 | −30.714 | |||
| PPBC/MgFe-LDH | 298 | −26.653 | 14.207 | 141.629 |
| 308 | −32.286 | |||
| 318 | −29.300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Liu, C.; Qin, L.; Xie, M.; Xu, Z.; Yu, Y. Efficient Adsorption Capacity of MgFe-Layered Double Hydroxide Loaded on Pomelo Peel Biochar for Cd (II) from Aqueous Solutions: Adsorption Behaviour and Mechanism. Molecules 2023, 28, 4538. https://doi.org/10.3390/molecules28114538
Huang Y, Liu C, Qin L, Xie M, Xu Z, Yu Y. Efficient Adsorption Capacity of MgFe-Layered Double Hydroxide Loaded on Pomelo Peel Biochar for Cd (II) from Aqueous Solutions: Adsorption Behaviour and Mechanism. Molecules. 2023; 28(11):4538. https://doi.org/10.3390/molecules28114538
Chicago/Turabian StyleHuang, Yongxiang, Chongmin Liu, Litang Qin, Mingqi Xie, Zejing Xu, and Youkuan Yu. 2023. "Efficient Adsorption Capacity of MgFe-Layered Double Hydroxide Loaded on Pomelo Peel Biochar for Cd (II) from Aqueous Solutions: Adsorption Behaviour and Mechanism" Molecules 28, no. 11: 4538. https://doi.org/10.3390/molecules28114538
APA StyleHuang, Y., Liu, C., Qin, L., Xie, M., Xu, Z., & Yu, Y. (2023). Efficient Adsorption Capacity of MgFe-Layered Double Hydroxide Loaded on Pomelo Peel Biochar for Cd (II) from Aqueous Solutions: Adsorption Behaviour and Mechanism. Molecules, 28(11), 4538. https://doi.org/10.3390/molecules28114538






