Effect of Light Conditions on Polyphenol Production in Transformed Shoot Culture of Salvia bulleyana Diels
Abstract
:1. Introduction
2. Results
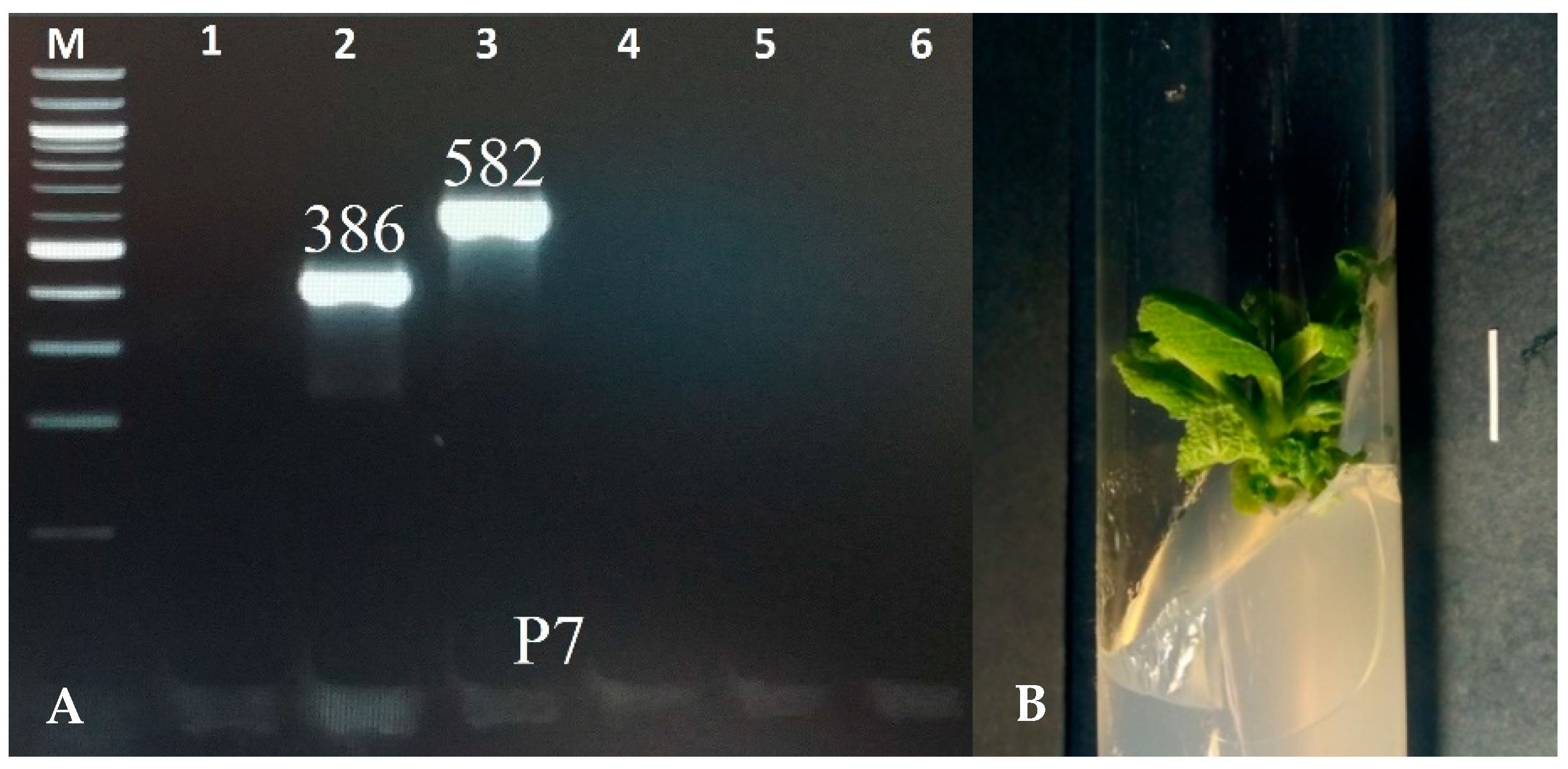
2.1. Molecular Analysis
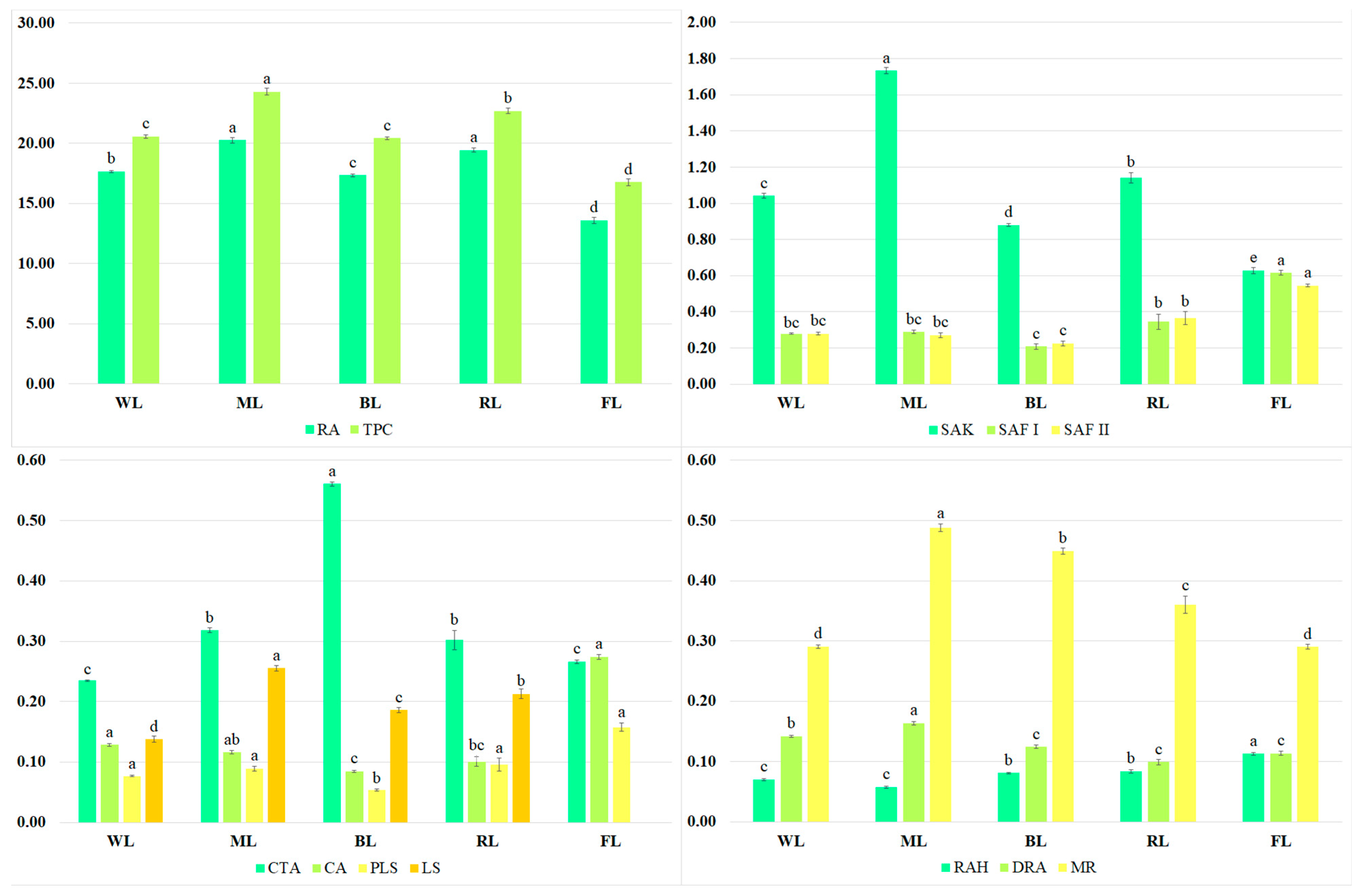
2.2. Secondary Metabolite Content
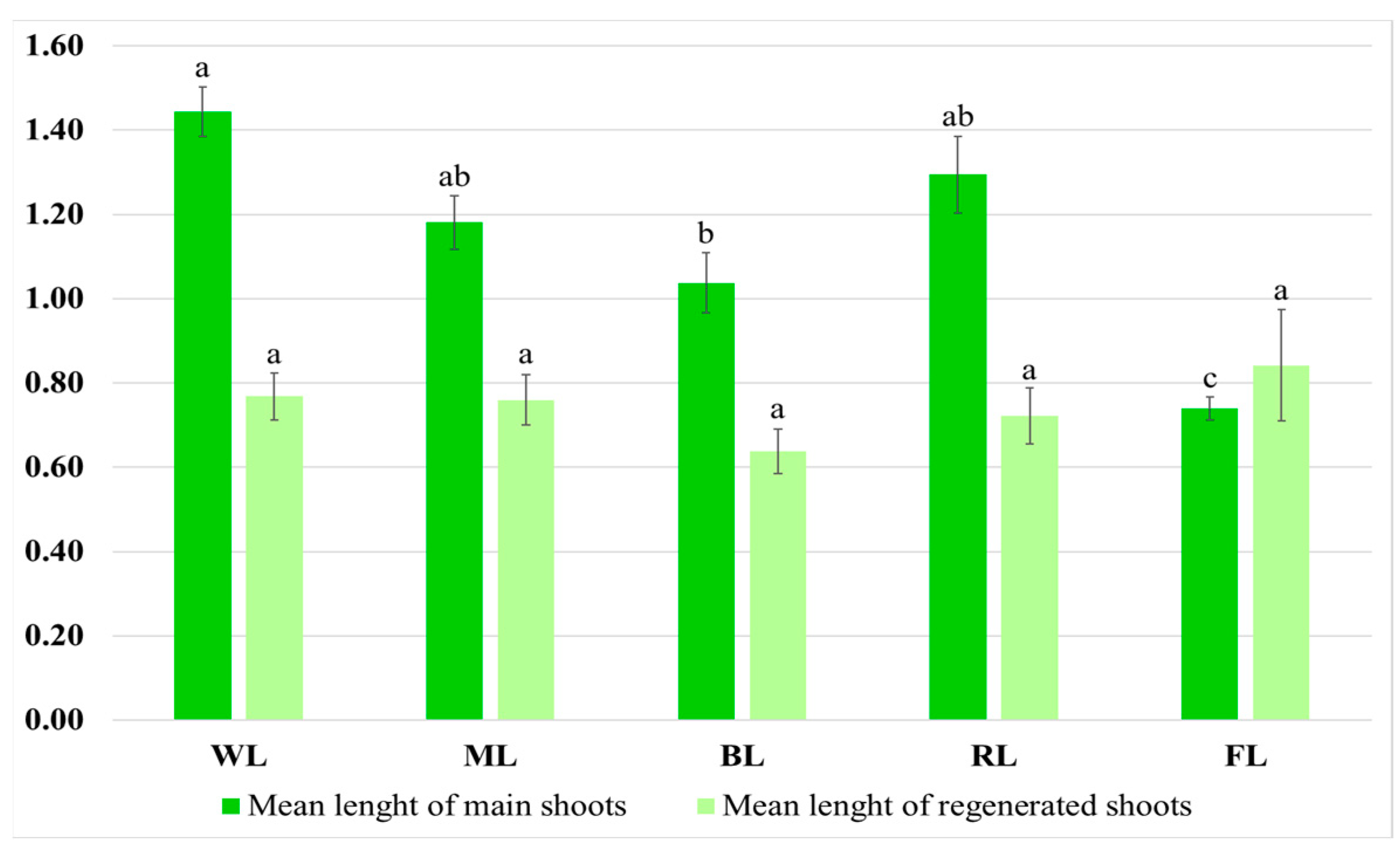
2.3. Growth and Morphological Characterization of the Culture
2.4. Activities of Antioxidant Enzymes in Culture
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Polymerase Chain Reaction Analysis
4.3. Phytochemical Analysis
4.4. Culture Growth, Morphology, and Physiology under Different Light Conditions
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Agrawal, S.; Rami, E. A review: Agrobacterium-mediated gene transformation to increase plant productivity. J. Phytopharm. 2022, 11, 111–117. [Google Scholar] [CrossRef]
- Giri, A.; Narasu, M.L. Transgenic hairy roots: Recent trends and applications. Biotechnol. Adv. 2000, 18, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk, I.; Królicka, A.; Wysokińska, H. Establishment of Salvia officinalis L. hairy root cultures for the production of rosmarinic acid. Z. Für Nat. C J. Biosci. 2006, 61, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Kentsop, R.A.D.; Iobbi, V.; Donadio, G.; Ruffoni, B.; De Tommasi, N.; Bisio, A. Abietane diterpenoids from the hairy roots of Salvia corrugata. Molecules 2021, 26, 5144. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.; Georgiev, V.; Ivanov, I.; Badjakov, I.; Pavlov, A. Two-phase temporary immersion system for Agrobacterium rhizogenes genetic transformation of sage (Salvia tomentosa Mill.). Biotechnol. Lett. 2011, 33, 1873–1878. [Google Scholar] [CrossRef]
- Norouzi, R.; Babalar, M.; Mirmasoumi, M. Investigation of hairy root induction in some Salvia L. species. Nova Biol. Reper. 2017, 4, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Zhi, B.H.; Alfermann, A.W. Diterpenoid production in hairy root cultures of Salvia miltiorrhiza. Phytochemistry 1993, 32, 699–703. [Google Scholar] [CrossRef]
- Wang, Q.J.; Zheng, L.P.; Yuan, H.Y.; Wang, J.W. Propagation of Salvia miltiorrhiza from hairy root explants via somatic embryogenesis and tanshinone content in obtained plants. Ind. Crops Prod. 2013, 50, 648–653. [Google Scholar] [CrossRef]
- Bercetche, J.; Chriqui, D.; Adam, S.; David, C. Morphogenetic and cellular reorientations induced by Agrobacterium rhizogenes (strains 1855, 2659 and 8196) on carrot, pea and tobacco. Plant Sci. 1987, 52, 195–210. [Google Scholar] [CrossRef]
- Chaudhuri, K.N.; Ghosh, B.; Tepfer, D.; Jha, S. Spontaneous plant regeneration in transformed roots and calli from Tylophora indica: Changes in morphological phenotype and tylophorine accumulation associated with transformation by Agrobacterium rhizogenes. Plant Cell Rep. 2006, 25, 1059–1066. [Google Scholar] [CrossRef]
- Gangopadhyay, M.; Chakraborty, D.; Bhattacharyya, S.; Bhattacharya, S. Regeneration of transformed plants from hairy roots of Plumbago indica. Plant Cell Tissue Organ Cult. 2010, 102, 109–114. [Google Scholar] [CrossRef]
- Xu, J.; Wei, K.; Zhang, G.; Lei, L.; Yang, D.; Wang, W.; Han, Q.; Xia, Y.; Bi, Y.; Yang, M.; et al. Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: A review. J. Ethnopharmacol. 2018, 225, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Kasimu, R.; Tanaka, K.; Tezuka, Y.; Gong, Z.-N.; Li, J.-X.; Basnet, P.; Namba, T.; Kadota, S. Comparative study of seventeen Salvia plants: Aldose reductase inhibitory activity of water and MeOH extracts and liquid chromatography-mass spectrometry (LC-MS) analysis of water extracts. Chem. Pharm. Bull. 1998, 46, 500–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.-H.; Chen, J.-M.; Peng, Y.; Wu, Q.; Xiao, P.-G. Investigation of Danshen and related medicinal plants in China. J. Ethnopharmacol. 2008, 120, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk-Karolak, I.; Krzemińska, M.; Kiss, A.K.; Olszewska, M.A.; Owczarek, A. Phytochemical profile and antioxidant activity of aerial and underground parts of Salvia bulleyana Diels. plants. Metabolites 2020, 10, 497. [Google Scholar] [CrossRef]
- Krzemińska, M.; Owczarek, A.; Olszewska, M.A.; Grzegorczyk-Karolak, I. In vitro strategy for the enhancement of the production of bioactive polyphenols in transformed roots of Salvia bulleyana. Int. J. Mol. Sci. 2022, 23, 7771. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Owczarek, A.; Kiss, A.K.; Grąbkowska, R.; Olszewska, M.A.; Grzegorczyk-Karolak, I. Establishment of hairy root cultures of Salvia bulleyana Diels for production of polyphenolic compounds. J. Biotechnol. 2020, 318, 10–19. [Google Scholar] [CrossRef]
- Casanova, E.; Trillas, M.I.; Moysset, L.; Vainstein, A. Influence of rol genes in floriculture. Biotechnol. Adv. 2005, 23, 3–39. [Google Scholar] [CrossRef]
- Piątczak, E.; Kuźma, Ł.; Skała, E.; Żebrowska, M.; Balcerczak, E.; Wysokińska, H. Iridoid and phenylethanoid glycoside production and phenotypical changes in plants regenerated from hairy roots of Rehmannia glutinosa Libosch. Plant Cell Tissue Organ Cult. 2015, 122, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Alvarenga, I.C.A.; Pacheco, F.V.; Silva, S.T.; Bertolucci, S.K.V.; Pinto, J.E.B.P. In vitro culture of Achillea millefolium L.: Quality and intensity of light on growth and production of volatiles. Plant Cell Tissue Organ Cult. 2015, 122, 299–308. [Google Scholar] [CrossRef]
- Kawka, B.; Kwiecień, I.; Ekiert, H. Influence of culture medium composition and light conditions on the accumulation of bioactive compounds in shoot cultures of Scutellaria lateriflora L. (American skullcap) grown in vitro. Appl. Biochem. Biotechnol. 2017, 183, 1414–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.T.; Yeo, S.K.; Sathasivam, R.; Park, J.S.; Kim, J.K.; Park, S.U. Influence of light-emitting diodes on phenylpropanoid biosynthetic gene expression and phenylpropanoid accumulation in Agastache rugosa. Appl. Biol. Chem. 2020, 63, 25. [Google Scholar] [CrossRef]
- Pedroso, R.C.N.; Branquinho, N.A.A.; Hara, A.C.B.A.M.; Costa, A.C.; Silva, F.G.; Pimenta, L.P.; Silva, M.L.A.; Cunha, W.R.; Pauletti, P.M.; Januario, A.H. Impact of light quality on flavonoid production and growth of Hyptis marrubioides seedlings cultivated in vitro. Rev. Bras. Farmacogn. 2017, 27, 466–470. [Google Scholar] [CrossRef]
- Pedroso, R.C.N.; Pimenta, L.P.; Tozatti, M.G.; Branquinho, N.A.A.; Hara, A.C.B.A.M.; Silva, F.H.L.; Costa, A.C.; Silva, F.G.; Miranda, C.E.S.; Silva, M.L.A.; et al. Effects of light quality and chemical elicitors on the growth parameters and rosmarinic acid content of in vitro cultures of Hyptis pectinata (L.) Poit. J. Braz. Chem. Soc. 2019, 30, 2430–2437. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Hnatuszko-Konka, K.; Krzemińska, M.; Olszewska, M.A.; Owczarek, A. Cytokinin-based tissue cultures for stable medicinal plant production: Regeneration and phytochemical profiling of Salvia bulleyana shoots. Biomolecules 2021, 11, 1513. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk-Karolak, I.; Krzemińska, M.; Kiss, A.K.; Owczarek-Januszkiewicz, A.; Olszewska, M.A. Role of phytohormones in biomass and polyphenol accumulation in Salvia bulleyana in vitro culture. Biomolecules 2023, 13, 227. [Google Scholar] [CrossRef]
- Gunjan, S.K.; Lutz, J.; Bushong, A.; Rogers, D.T.; Littleton, J. Hairy root cultures and plant regeneration in Solidago nemoralis transformed with Agrobacterium rhizogenes. Am. J. Plant Sci. 2013, 4, 1675–1678. [Google Scholar] [CrossRef] [Green Version]
- Tusevski, O.; Vinterhalter, B.; Krstić Milošević, D.; Soković, M.; Ćirić, A.; Vinterhalter, D.; Zdravković Korać, S.; Petreska Stanoeva, J.; Stefova, M.; Gadzovska Simic, S. Production of phenolic compounds, antioxidant and antimicrobial activities in hairy root and shoot cultures of Hypericum perforatum L. Plant Cell Tissue Organ Cult. 2017, 128, 589–605. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Skała, E.; Kuźma, Ł.; Kiss, A.K.; Grzegorczyk-Karolak, I. The effect of purine-type cytokinin on the proliferation and production of phenolic compounds in transformed shoots of Dracocephalum forrestii. J. Biotechnol. 2019, 306, 125–133. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.-J.; Paek, K.-Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Morrow, R.C. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef] [Green Version]
- Kong, Y.; Schiestel, K.; Zheng, Y. Pure blue light effects on growth and morphology are slightly changed by adding low-level UVA or far-red light: A comparison with red light in four microgreen species. Environ. Exp. Bot. 2019, 157, 58–68. [Google Scholar] [CrossRef]
- Makowski, W.; Tokarz, B.; Banasiuk, R.; Królicka, A.; Dziurka, M.; Wojciechowska, R.; Tokarz, K.M. Is a blue–red light a good elicitor of phenolic compounds in the family Droseraceae? A comparative study. J. Photochem. Photobiol. B Biol. 2019, 201, 111679. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Koycheva, I.K.; Balcheva-Sivenova, Z.P.; Vasileva, S.M.; Georgiev, M.I. Rosmarinic acid—From bench to valuable applications in food industry. Trends Food Sci. Technol. 2021, 117, 182–193. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic acid: New aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Gam, D.T.; Khoi, P.H.; Ngoc, P.B.; Linh, L.K.; Hung, N.K.; Anh, P.T.L.; Thu, N.T.; Hien, N.T.; Khanh, T.D.; Ha, C.H.; et al. LED lights promote growth and flavonoid accumulation of Anoectochilus roxburghii and are linked to the enhanced expression of several related genes. Plants 2020, 9, 1344. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Hnatuszko-Konka, K.; Lebelt, L.; Grzegorczyk-Karolak, I. The protective function and modification of secondary metabolite accumulation in response to light stress in Dracocephalum forrestii shoots. Int. J. Mol. Sci. 2021, 22, 7965. [Google Scholar] [CrossRef]
- Chen, C.C.; Agrawal, D.C.; Lee, M.-R.; Lee, R.-J.; Kuo, C.-L.; Wu, C.-R.; Tsay, H.-S.; Chang, H.-C. Influence of LED light spectra on in vitro somatic embryogenesis and LC–MS analysis of chlorogenic acid and rutin in Peucedanum japonicum Thunb: A medicinal herb. Bot. Stud. 2016, 57, 9. [Google Scholar] [CrossRef] [Green Version]
- Szopa, A.; Ekiert, H.; Szewczyk, A.; Fugas, E. Production of bioactive phenolic acids and furanocoumarins in in vitro cultures of Ruta graveolens L. and Ruta graveolens ssp. divaricata (Tenore) Gams. under different light conditions. Plant Cell Tissue Organ Cult. 2012, 110, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Shiga, T.; Shoji, K.; Shimada, H.; Hashida, S.; Goto, F.; Yoshihara, T. Effect of light quality on rosmarinic acid content and antioxidant activity of sweet basil, Ocimum basilicum L. Plant Biotechnol. 2009, 26, 255–259. [Google Scholar] [CrossRef] [Green Version]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H.; Jeong, B.R. Blue LED light enhances growth, phytochemical contents and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic. Environ. Biotechnol. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, S.S.; Akbar, F.; Kanwal, F. Correlation of different spectral lights with biomass accumulation and production of antioxidant secondary metabolites in callus cultures of medicinally important Prunella vulgaris L. J. Photochem. Photobiol. B Biol. 2016, 159, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Starzec, A.; Ekiert, H. The importance of monochromatic lights in the production of phenolic acids and flavonoids in shoot cultures of Aronia melanocarpa, Aronia arbutifolia and Aronia × prunifolia. J. Photochem. Photobiol. B Biol. 2018, 179, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ouzounis, T.; Parjikolaei, B.R.; Fretté, X.; Rosenqvist, E.; Ottosen, C.-O. Predawn and high intensity application of supplemental blue light decreases the quantum yield of PSII and enhances the amount of phenolic acids, flavonoids, and pigments in Lactuca sativa. Front. Plant Sci. 2015, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Dewir, Y.H.; Chakrabarty, D.; Ali, M.B.; Hahn, E.J.; Paek, K.Y. Lipid peroxidation and antioxidant enzyme activities of Euphorbia millii hyperhydric shoots. Environ. Exp. Bot. 2006, 58, 93–99. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Stress-triggered redox signalling: What’s in pROSpect? Plant Cell Environ. 2016, 39, 951–964. [Google Scholar] [CrossRef] [Green Version]
- Ye, S.; Shao, Q.; Xu, M.; Li, S.; Wu, M.; Tan, X.; Su, L. Effects of light quality on morphology, enzyme activities, and bioactive compound contents in Anoectochilus roxburghii. Front. Plant Sci. 2017, 8, 857. [Google Scholar] [CrossRef] [Green Version]
- Mengxi, L.; Zhigang, X.; Yang, Y.; Yijie, F. Effects of different spectral lights on Oncidium PLBs induction, proliferation, and plant regeneration. Plant Cell Tissue Organ Cult. 2011, 106, 1–10. [Google Scholar] [CrossRef]
- Mehrotra, S.; Goel, M.K.; Rahman, L.U.; Kukreja, A.K. Molecular and chemical characterization of plants regenerated from Ri-mediated hairy root cultures of Rauwolfia serpentina. Plant Cell Tissue Organ Cult. 2013, 114, 31–38. [Google Scholar] [CrossRef]
- Casanova, E.; Zuker, A.; Trillas, M.I.; Moysset, L.; Vainstein, A. The rolC gene in carnation exhibits cytokinin- and auxin-like activities. Sci. Hortic. 2003, 97, 321–331. [Google Scholar] [CrossRef]
- Dehio, C.; Grossmann, K.; Schell, J.; Schmülling, T. Phenotype and hormonal status of transgenic tobacco plants overexpressing the rolA gene of Agrobacterium rhizogenes T-DNA. Plant Mol. Biol. 1993, 23, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Tsuro, M.; Tsukimori, A.; Shizukawa, Y.; Takemoto, T.; Inaba, K.; Shiozaki, S. Morphological and physiological changes in transgenic Chrysanthemum morifolium Ramat. “Ogura-nishiki” with rolC. J. Jpn. Soc. Hortic. Sci. 2006, 75, 312–317. [Google Scholar] [CrossRef] [Green Version]
- Boase, M.R.; Winefield, C.S.; Lill, T.A.; Bendall, M.J. Transgenic regal pelargoniums that express the rolC gene from Agrobacterium rhizogenes exhibit a dwarf floral and vegetative phenotype. In Vitro Cell. Dev. Biol. Plant 2004, 40, 46–50. [Google Scholar] [CrossRef]
- Mitiouchkina, T.Y.; Dolgov, S.V. Modification of chrysanthemum plant and flower architecture by rolC gene from Agrobacterium rhizogenes introduction. Acta Hortic. 2000, 508, 163–172. [Google Scholar] [CrossRef]
- Kim, S.J.; Hahn, E.J.; Heo, J.W.; Paek, K.Y. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of Chrysanthemum plantlets in vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Kuźma, Ł.; Grzegorczyk-Karolak, I. The effect of different light treatments on morphogenesis, phenolic compound accumulation and antioxidant potential of Dracocephalum forrestii transformed shoots cultured in vitro. J. Photochem. Photobiol. B Biol. 2021, 224, 112329. [Google Scholar] [CrossRef]
- Zhao, J.; Park, Y.G.; Jeong, B.R. Light quality affects growth and physiology of Carpesium triste Maxim. cultured in vitro. Agriculture 2020, 10, 258. [Google Scholar] [CrossRef]
- Long, J.H.; Pu, M.; Huang, Z.W.; Li, J.P.; Xu, Z.G. Research progress of spectral regulation of plant growth and development. China Illum. Eng. J. 2018, 29, 8–16. [Google Scholar]
- Dagron, S. Die International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). In Handbuch Ethik und Recht der Forschung am Menschen; Springer: Berlin/Heidelberg, Germany, 2014; pp. 541–545. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Light Condition | Fresh Weight (g) | Dry Weight (g) | Proliferation Factor | Ratio of Number of Obtained Shoots to Buds |
|---|---|---|---|---|
| WL | 1.22 ± 0.104 a | 0.145 ± 0.010 a | 3.04 ± 0.272 a | 20:80 |
| ML | 1.09 ± 0.107 ab | 0.134 ± 0.011 ab | 2.72 ± 0.257 ab | 26:74 |
| BL | 0.545 ± 0.093 c | 0.070 ± 0.010 c | 1.88 ± 0.254 b | 19:81 |
| RL | 0.796 ± 0.105 bc | 0.100 ± 0.012 bc | 2.00 ± 0.267 ab | 32:68 |
| FL | 0.895 ± 0.048 b | 0.109 ± 0.006 b | 2.91 ± 0.228 a | 5:95 |
| Light Condition | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Carotenoids |
|---|---|---|---|---|
| WL | 0.473 ± 0.002 b | 0.253 ± 0.003 b | 0.723 ± 0.006 c | 0.132 ± 0.002 b |
| ML | 0.413 ± 0.004 c | 0.211 ± 0.004 c | 0.624 ± 0.005 d | 0.093 ± 0.002 c |
| BL | 0.551 ± 0.005 a | 0.250 ± 0.007 b | 0.807 ± 0.010 b | 0.232 ± 0.005 a |
| RL | 0.526 ± 0.004 a | 0.604 ± 0.001 a | 1.13 ± 0.010 a | 0.231 ± 0.040 a |
| FL | 0.422 ± 0.010 c | 0.109 ± 0.002 d | 0.531 ± 0.002 e | 0.108 ± 0.001 c |
| Light Condition | Enzyme Activity (U/mg of Protein) | ||
|---|---|---|---|
| POD | SOD | CAT | |
| WL | 22.26 ± 0.410 b | 3.13 ± 0.440 c | 2.44 ± 0.030 c |
| ML | 12.72 ± 0.270 d | 3.02 ± 0.400 c | 7.80 ± 0.040 b |
| BL | 28.56 ± 0.680 a | 7.15 ± 0.400 a | 8.60 ± 0.020 a |
| RL | 14.68 ± 0.340 c | 4.94 ± 0.350 b | 2.34 ± 0.010 d |
| FL | 1.49 ± 0.010 e | 0.688 ± 0.230 d | 0.224 ± 0.010 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzemińska, M.; Hnatuszko-Konka, K.; Weremczuk-Jeżyna, I.; Owczarek-Januszkiewicz, A.; Ejsmont, W.; Olszewska, M.A.; Grzegorczyk-Karolak, I. Effect of Light Conditions on Polyphenol Production in Transformed Shoot Culture of Salvia bulleyana Diels. Molecules 2023, 28, 4603. https://doi.org/10.3390/molecules28124603
Krzemińska M, Hnatuszko-Konka K, Weremczuk-Jeżyna I, Owczarek-Januszkiewicz A, Ejsmont W, Olszewska MA, Grzegorczyk-Karolak I. Effect of Light Conditions on Polyphenol Production in Transformed Shoot Culture of Salvia bulleyana Diels. Molecules. 2023; 28(12):4603. https://doi.org/10.3390/molecules28124603
Chicago/Turabian StyleKrzemińska, Marta, Katarzyna Hnatuszko-Konka, Izabela Weremczuk-Jeżyna, Aleksandra Owczarek-Januszkiewicz, Wiktoria Ejsmont, Monika A. Olszewska, and Izabela Grzegorczyk-Karolak. 2023. "Effect of Light Conditions on Polyphenol Production in Transformed Shoot Culture of Salvia bulleyana Diels" Molecules 28, no. 12: 4603. https://doi.org/10.3390/molecules28124603






