Assessment of the Effects of Structural Modification of Gastrodia elata Polysaccharide on Anti-Breast Cancer Activity Using Asymmetrical Flow Field-Flow Fractionation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Modification of GEP
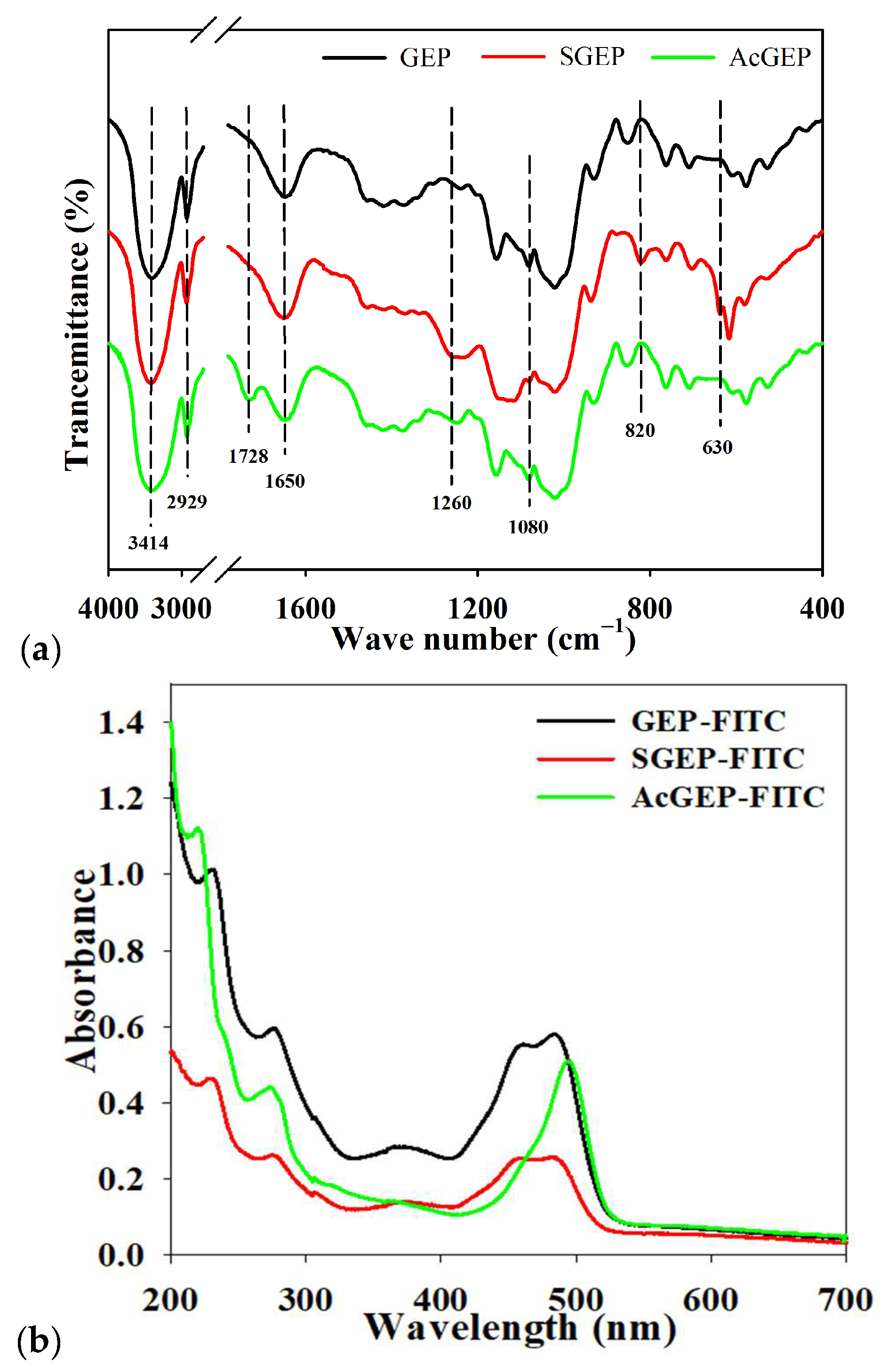
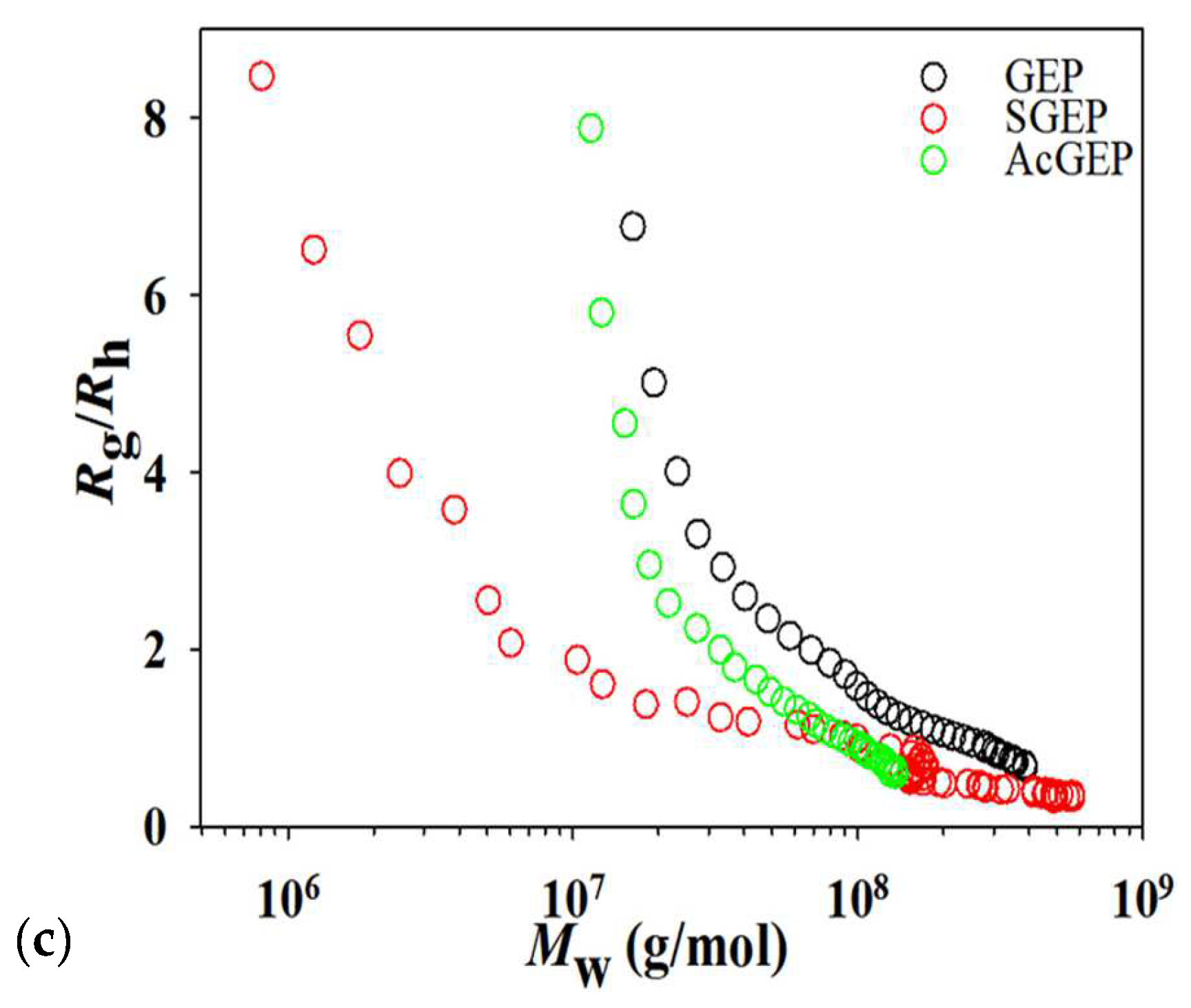
2.2. AF4 Analysis of GEP and Its Derivatives
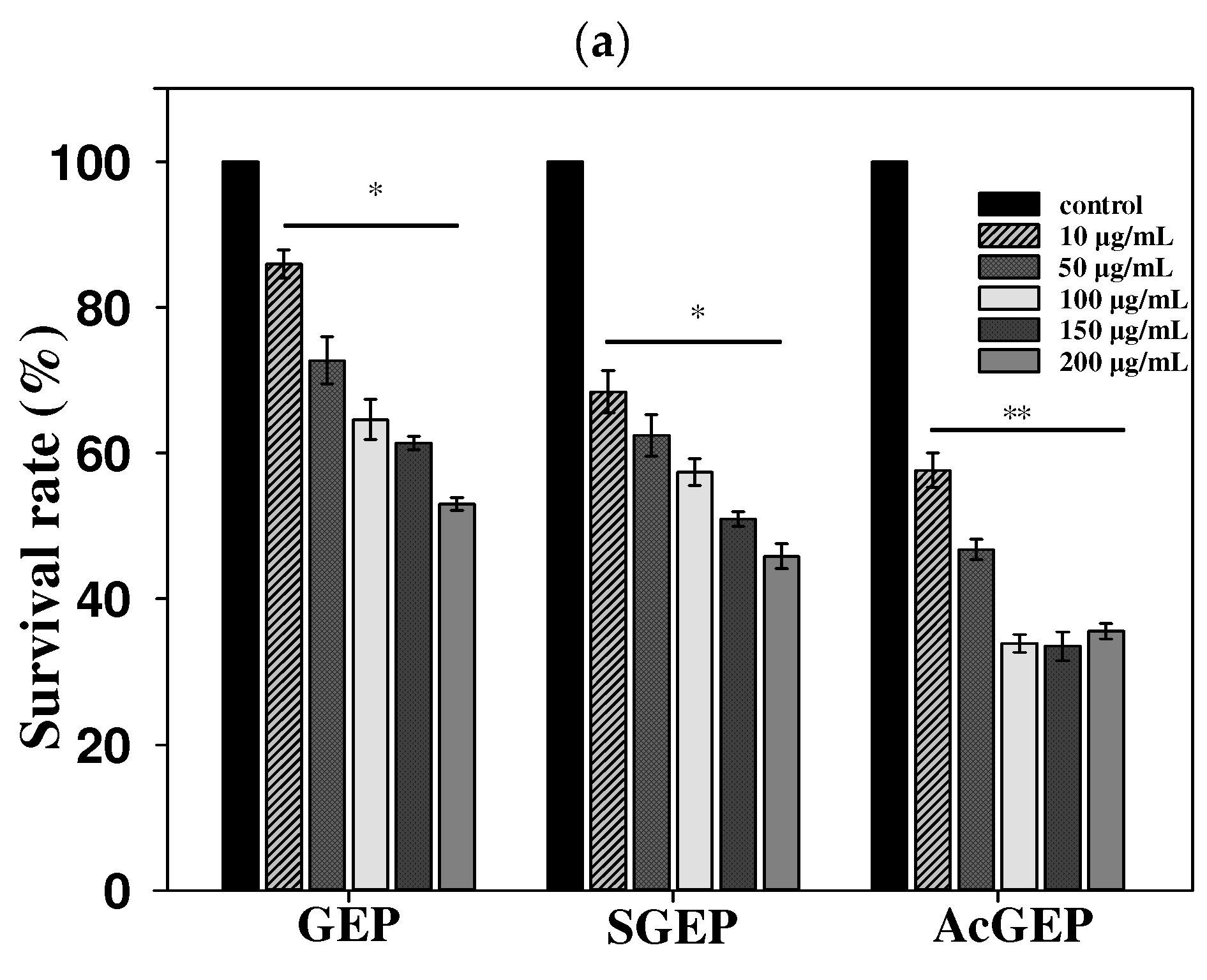
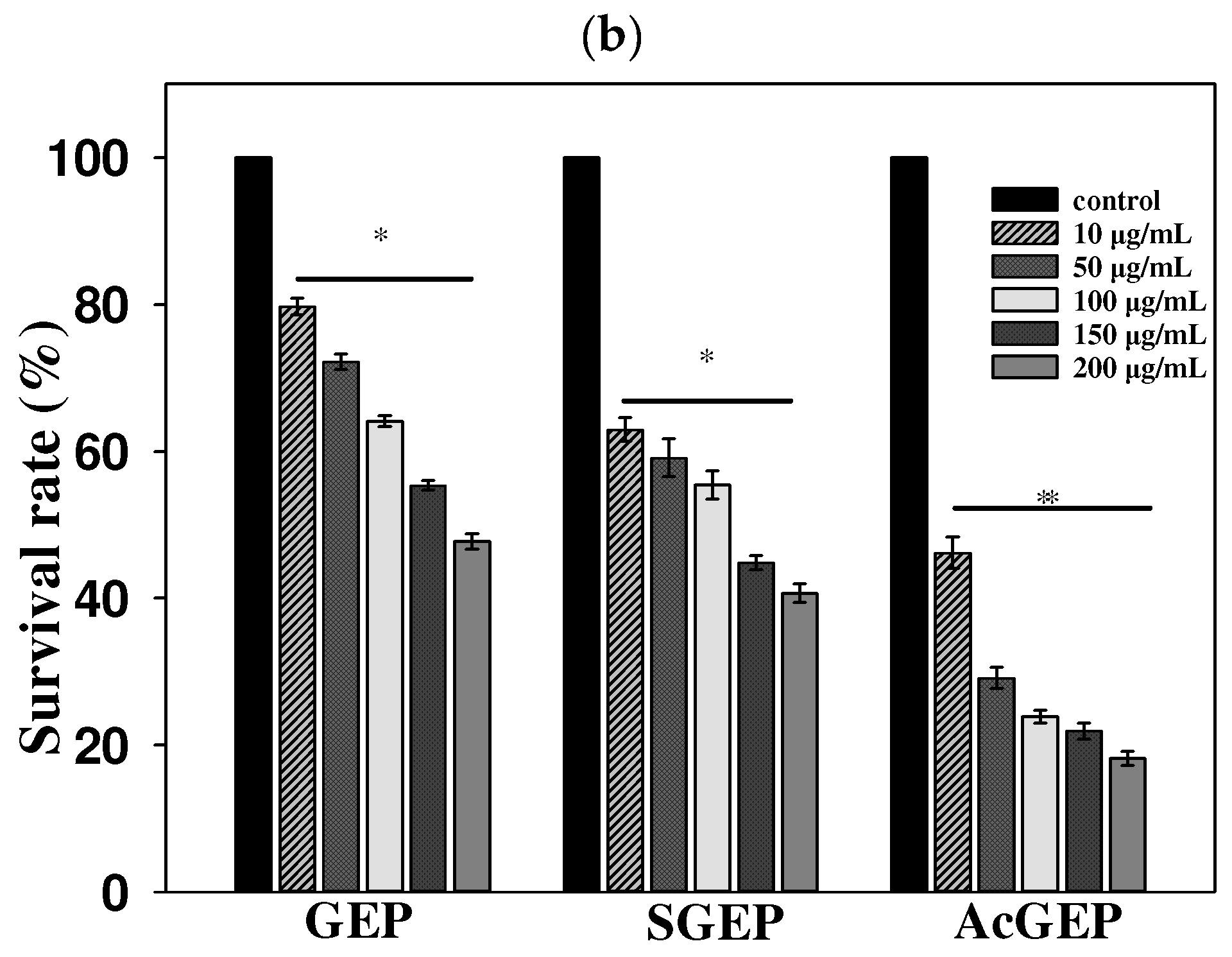
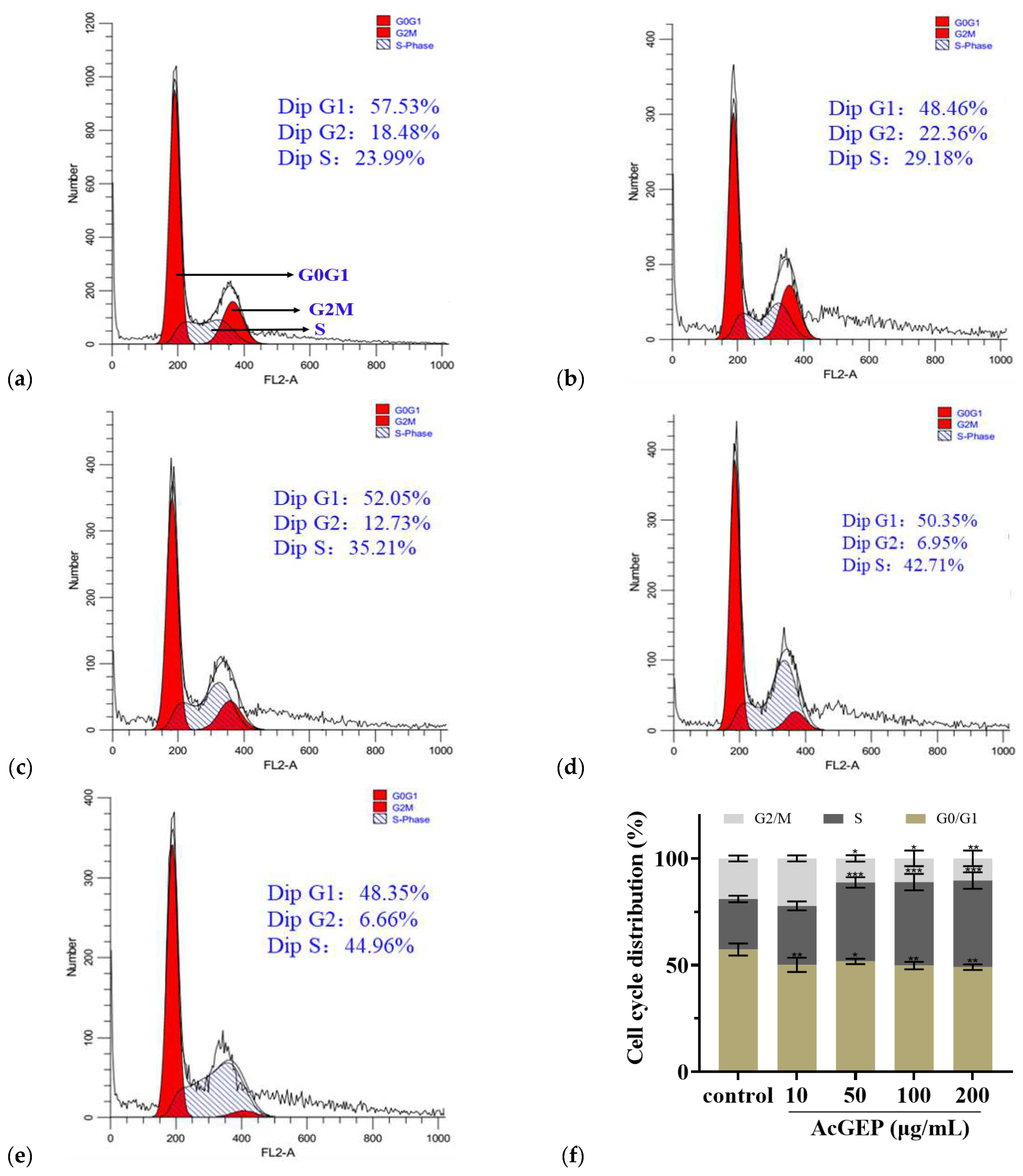
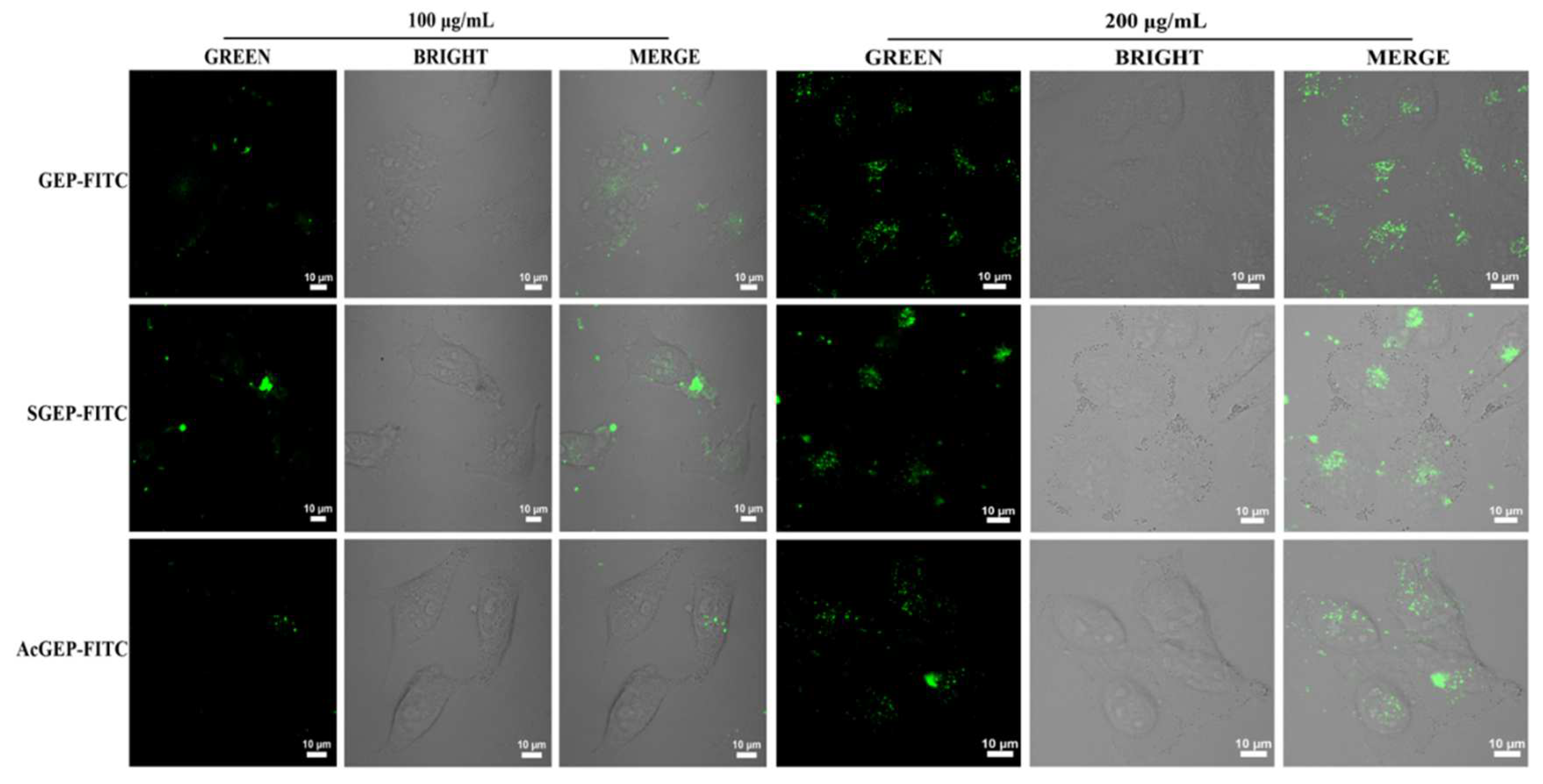
2.3. Anti-Breast Cancer Activity of GEP and Its Derivatives
3. Materials and Methods
3.1. Materials
3.2. Extraction of Polysaccharide from Gastrodia elata
3.3. Preparation of Sulfated Gastrodia elata Polysaccharide
3.4. Preparation of Acetylated Gastrodia elata Polysaccharide
3.5. Determination of Solubility of GEP and Its Derivatives
3.6. Fourier Transformed Infrared (FTIR) Spectroscopy
3.7. AF4 Analysis of GEP and Its Derivatives
3.8. Anti-Breast Cancer Activity of GEP and Its Derivatives
3.8.1. Cell Culture and MTT Assay
3.8.2. Cell Apoptosis Assay
3.8.3. Cell Cycle Analysis
3.8.4. Uptake of Fluorescent-Labeled GEP and Its Derivative by MCF-7 Cells
3.9. Data Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, X.; Cao, D.; Zhou, L.; Jin, H.; Dong, Q.; Yao, J.; Ding, K. Structure of a polysaccharide from Gastrodia elata Bl., and oligosaccharides prepared thereof with anti-pancreatic cancer cell growth activities. Carbohydr. Polym. 2011, 86, 1300–1305. [Google Scholar] [CrossRef]
- Liu, J.; Mori, A. Antioxidant and free radical scavenging activities of Gastrodia elata Bl. and Uncaria rhynchophylla (Miq.) Jacks. Neuropharmacology 1992, 31, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Qian, L.; Gong, C.; Shen, X. Immune-enhancing activity of polysaccharides from Gastrodia elate. J. Food Process. Preserv. 2017, 41, e13016. [Google Scholar] [CrossRef]
- Zhou, B.; Tan, J.; Zhang, C.; Wu, Y. Neuroprotective effect of polysaccharides from Gastrodia elata blume against corticosterone-induced apoptosis in PC12 cells via inhibition of the endoplasmic reticulum stress-mediated pathway. Mol. Med. Rep. 2018, 17, 1182–1190. [Google Scholar] [CrossRef] [Green Version]
- Li, H.B.; Wu, F.; Miao, H.C.; Xiong, K.R. Effects of polysaccharide of Gastrodia elata blume and electro-acupuncture on expressions of brain-derived neurotrophic factor and stem cell factor protein in caudate putamen of focal cerebral ischemia rats. Med. Sci. Monit. Basic Res. 2016, 22, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Yan, Y.; Hou, C.; Shi, M.; Liu, Y. Structural characterization of a galacturonic acid-rich polysaccharide from Ziziphus Jujuba cv. Muzao. Int. J. Biol. Macromol. 2020, 147, 844–852. [Google Scholar] [CrossRef]
- Li, J.; Hu, X.; Li, X.; Ma, Z. Effects of acetylation on the emulsifying properties of Artemisia sphaerocephala Krasch. polysaccharide. Carbohydr. Polym. 2016, 144, 531–540. [Google Scholar] [CrossRef]
- Li, Q.M.; Wang, J.F.; Zha, X.Q.; Pan, L.H.; Zhang, H.L.; Luo, J.P. Structural characterization and immunomodulatory activity of a new polysaccharide from jellyfish. Carbohydr. Polym. 2017, 159, 188–194. [Google Scholar] [CrossRef]
- Xie, L.; Shen, M.; Hong, Y.; Ye, H.; Huang, L.; Xie, J. Chemical modifications of polysaccharides and their anti-tumor activities. Carbohydr. Polym. 2020, 229, 115436. [Google Scholar] [CrossRef]
- Zhong, K.; Zhang, Q.; Tong, L.; Liu, L.; Zhou, X.; Zhou, S. Molecular weight degradation and rheological properties of schizophyllan under ultrasonic treatment. Ultrason. Sonochem. 2015, 23, 75–80. [Google Scholar] [CrossRef]
- Zhang, D.N.; Guo, X.Y.; Yang, Q.H.; Chen, Z.G.; Tao, L.J. An efficient enzymatic modification of cordycepin in ionic liquids under ultrasonic irradiation. Ultrason. Sonochem. 2014, 21, 1682–1687. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Huang, G.; Huang, H. Preparation, analysis, antioxidant activities in vivo of phosphorylated polysaccharide from Momordica charantia. Carbohydr. Polym. 2021, 252, 117179. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Chen, X.; Sun, P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr. Polym. 2014, 105, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Wang, Y.; Nie, S.; Li, C.; Xie, M. Acetylation and carboxymethylation of the polysaccharide from Ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2014, 156, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Wang, J.; Liu, Z.; Zhao, S. Antitumor activity of a sulfated polysaccharide from Enteromorpha intestinalis targeted against hepatoma through mitochondrial pathway. Tumour Biol. 2014, 35, 1641–1647. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Yu, Y.; Cheung, P.C.K. Enhancement of antitumor activities in sulfated and carboxymethylated polysaccharides of Ganoderma lucidum. J. Agric. Food Chem. 2009, 57, 10565–10572. [Google Scholar] [CrossRef] [PubMed]
- Otte, T.; Pasch, H.; Macko, T.; Brüll, R.; Stadler, F.J.; Kaschta, J.; Becker, F.; Buback, M. Characterization of branched ultrahigh molar mass polymers by asymmetrical flow field-flow fractionation and size exclusion chromatography. J. Chromatogr. A 2011, 1218, 4257–4267. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, W.; Dou, Y.; Liu, H.; Chen, X.; Shi, J.; Dou, H. Towards a better understanding of the relationships between the structure and antitumor activity of Gastrodia elata polysaccharides by asymmetrical flow field-flow fractionation. Food Res. Int. 2021, 149, 110673. [Google Scholar] [CrossRef]
- Wei, D.; Wei, Y.; Cheng, W.; Zhang, L. Sulfated modification, characterization and antitumor activities of Radix hedysari polysaccharide. Int. J. Biol. Macromol. 2012, 51, 471–476. [Google Scholar] [CrossRef]
- Cardozo, F.T.G.S.; Camelini, C.M.; Cordeiro, M.N.S.; Mascarello, A.; Malagoli, B.G.; Larsen, I.V.; Rossi, M.J.; Nunes, R.J.; Braga, F.C.; Brandt, C.R.; et al. Characterization and cytotoxic activity of sulfated derivatives of polysaccharides from Agaricus brasiliensis. Int. J. Biol. Macromol. 2013, 57, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhang, M.; Zhou, Q.; Chen, J.; Zeng, F. Solution properties of antitumor sulfated derivative of alpha-(1-->3)-D-glucan from Ganoderma lucidum. Biosci. Biotechnol. Biochem. 2000, 64, 2172–2178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Wang, J.; Song, H.; Zhang, H.; Niu, X. Chemical modification and influence of function groups on the in vitro-antioxidant activities of porphyran from Porphyra haitanensis. Carbohydr. Polym. 2010, 79, 290–295. [Google Scholar] [CrossRef]
- Guo, P.; Li, Y.; An, J.; Shen, S.; Dou, H. Study on structure-function of starch by asymmetrical flow field-flow fractionation coupled with multiple detectors: A review. Carbohydr. Polym. 2019, 226, 115330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, J.; Guo, P.; Dai, S.; Zhang, X.; Meng, M.; Shen, S.; Zhang, A.; Dou, H. Study on the retrogradation behavior of starch by asymmetrical flow field-flow fractionation coupled with multiple detectors. Food Chem. 2019, 277, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, L.; Xu, X.; Lin, Y.; Cheung, P.C.K.; Kennedy, J.F. Comparison on chain stiffness of a water-insoluble (1→3)-α-d-glucan isolated from Poria cocos mycelia and its sulfated derivative. Carbohydr. Polym. 2005, 59, 257–263. [Google Scholar] [CrossRef]
- Liu, C.Y.; Hu, D.J.; Zhu, H.; Zhang, Y.Y.; Qin, J.; Wang, F.; Zhang, Z.D.; Lv, G.P. Preparation, characterization and immunoregulatory activity of derivatives of polysaccharide from Atractylodes lancea (Thunb.) DC. Int. J. Biol. Macromol. 2022, 216, 225–234. [Google Scholar] [CrossRef]
- Li, Z.; Wei, Y.; Wang, Y.; Zhang, R.; Zhang, C.; Wang, C.; Yan, X. Preparation of highly substituted sulfated alfalfa polysaccharides and evaluation of their biological activity. Foods 2022, 11, 737. [Google Scholar] [CrossRef]
- Li, P.; Li, C.; Xue, Y.; Zhang, Y.; Liu, H.; Zhao, X.; Yu, G.; Guan, H. Preparation, characterization and pharmacokinetics of fluorescence labeled propylene glycol alginate sodium sulfate. J. Ocean Univ. China 2014, 13, 683–690. [Google Scholar] [CrossRef]
- Beena Rai, K.; Krishna Sharma, K.; Pattabiraman, T.N. A short-duration colorimetric method based on phenol-sulfuric acid reaction for the estimation of glucosylhemoglobin. Biochem. Med. 1984, 31, 65–72. [Google Scholar]
- Jiang, J.; Meng, F.Y.; He, Z.; Ning, Y.L.; Li, X.H.; Song, H.; Wang, J.; Zhou, R. Sulfated modification of longan polysaccharide and its immunomodulatory and antitumor activity in vitro. Int. J. Biol. Macromol. 2014, 67, 323–329. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, J.; Xue, B.; Li, X.; Gan, J.; Bian, X.; Qin, Y.; Sun, T. Effect of sulfated modification on rheological and physiological properties of oat β-glucan oligosaccharides prepared by acid or oxidative degradation. J. Cereal Sci. 2021, 99, 103209. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G.; Chen, G. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from Chinese yam. Food Chem. 2021, 361, 130089. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G. Extraction, derivatization and antioxidant activity of bitter gourd polysaccharide. Int. J. Biol. Macromol. 2019, 141, 14–20. [Google Scholar] [CrossRef]
- Chen, J.; Chen, L.; Lin, S.; Liu, C.; Cheung, P.C.K. Preparation and structural characterization of a partially depolymerized beta-glucan obtained from Poria cocos sclerotium by ultrasonic treatment. Food Hydrocoll. 2015, 46, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, M.; Zhao, J.; Zhang, X.; Hu, X.; Douglas Goff, H.; Guo, Q. Fluorescent labeling affected the structural/conformational properties of arabinoxylans. Carbohydr. Polym. 2021, 265, 118064. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, W.; Dou, Y.; Song, T.; Shen, S.; Dou, H. Applications of asymmetrical flow field-flow fractionation for separation and characterization of polysaccharides: A review. J. Chromatogr. A 2021, 1635, 461726. [Google Scholar] [CrossRef] [PubMed]


| Sample | Substitution Degree | Solubility (mg/mL) | Average Rg (nm) | Average Mw (×107 g/mol) |
|---|---|---|---|---|
| GEP | -- | 15.3 ± 1.8% | 92.4 ± 3.9% | 12.9 ± 4.4% |
| SGEP | 0.37 ± 1.39% | 35.7 ± 0.6% | 85.8 ± 6.0% | 9.3 ± 5.5% |
| AcGEP | 0.22 ± 0.82% | 24.5 ± 1.0% | 88.4 ± 2.6% | 6.4 ± 2.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Dou, Y.; Hao, T.; Wang, M.; Yang, L.; Zheng, H.; Liu, H.; Dou, H. Assessment of the Effects of Structural Modification of Gastrodia elata Polysaccharide on Anti-Breast Cancer Activity Using Asymmetrical Flow Field-Flow Fractionation. Molecules 2023, 28, 4669. https://doi.org/10.3390/molecules28124669
Liu X, Dou Y, Hao T, Wang M, Yang L, Zheng H, Liu H, Dou H. Assessment of the Effects of Structural Modification of Gastrodia elata Polysaccharide on Anti-Breast Cancer Activity Using Asymmetrical Flow Field-Flow Fractionation. Molecules. 2023; 28(12):4669. https://doi.org/10.3390/molecules28124669
Chicago/Turabian StyleLiu, Xiaoying, Yuwei Dou, Tingting Hao, Mu Wang, Liu Yang, Hailiang Zheng, Hongmei Liu, and Haiyang Dou. 2023. "Assessment of the Effects of Structural Modification of Gastrodia elata Polysaccharide on Anti-Breast Cancer Activity Using Asymmetrical Flow Field-Flow Fractionation" Molecules 28, no. 12: 4669. https://doi.org/10.3390/molecules28124669






