Effects of Different Cooking Methods on Phenol Content and Antioxidant Activity in Sprouted Peanut
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of Different Cooking Methods on Total Phenol Content in Peanut Sprout
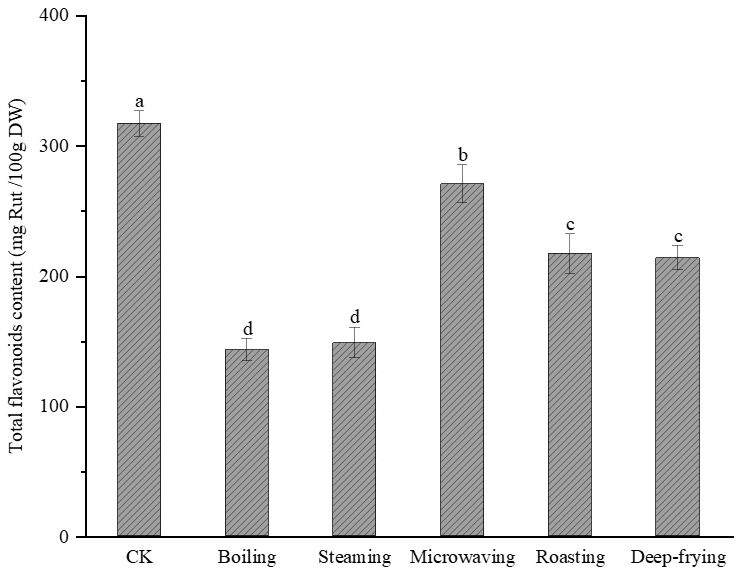
2.2. Effects of Different Cooking Methods on Total Flavonoid Content in Peanut Sprout
2.3. Effects of Different Cooking Methods on Antioxidant Activity in Peanut Sprout
2.4. Effects of Different Cooking Methods on Monomer Phenolic Compounds in Peanut Sprout
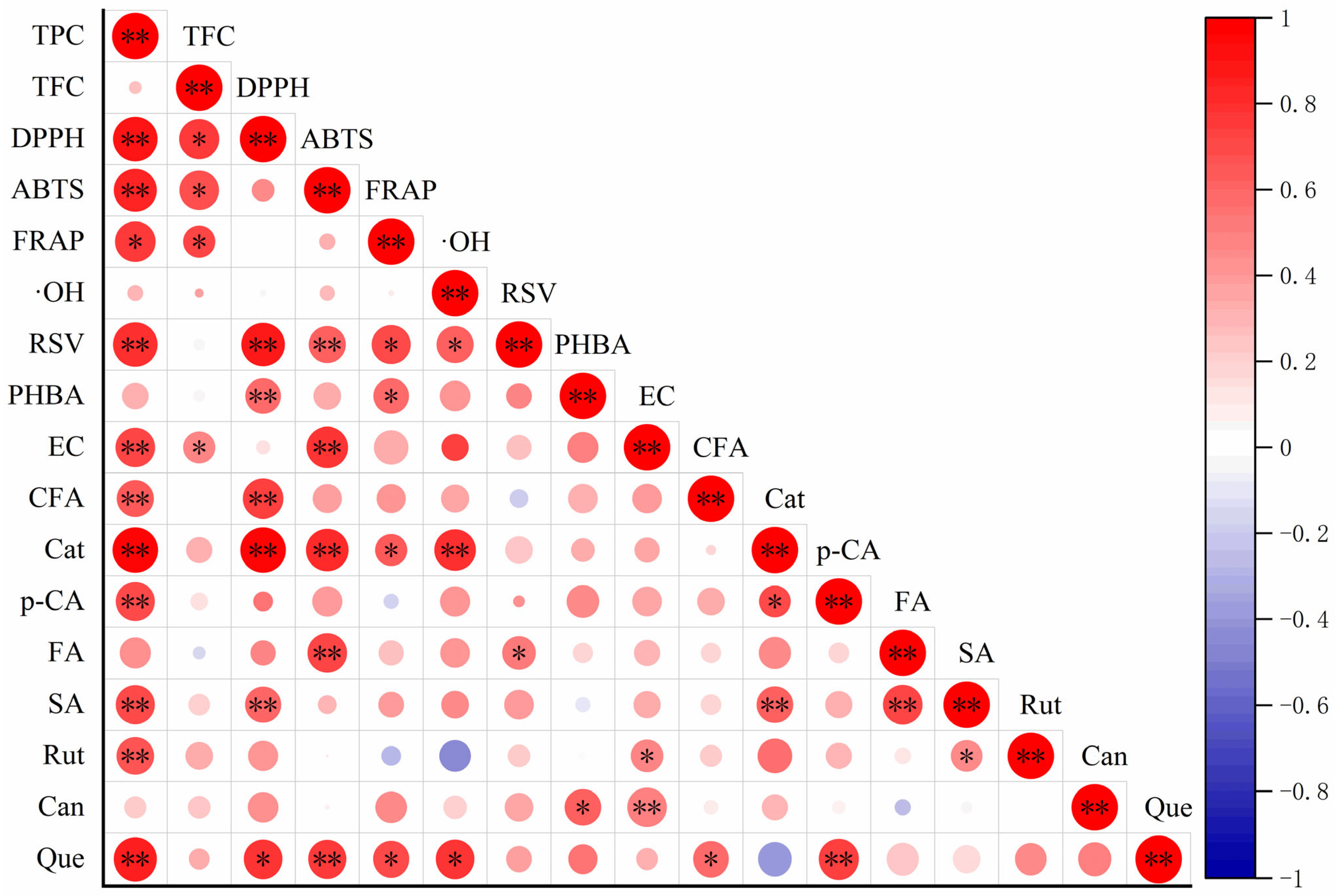
2.5. Correlation Analysis of Phenolic Compounds and Antioxidant Activities of Peanut Sprout
2.6. Principal Component Analysis of Cooking Methods, Phenolic Compounds, and Antioxidant Activities
3. Materials and Methods
3.1. Peanut Seeds and Chemicals
3.2. Peanut Germination
3.3. Preparation of Peanut Sprout Products by Different Cooking Methods
3.4. Preparation of Peanut Sprout Extract
3.5. Determination of Total Phenol Content
3.6. Determination of Total Flavonoid Content
3.7. Determination of Antioxidant Activity
3.7.1. Determination of DPPH Scavenging Capacity
3.7.2. Determination of ABTS Scavenging Capacity
3.7.3. Determination of FRAP
3.7.4. Determination of Hydroxyl Free Radical Scavenging Capacity
3.8. Determination of Phenolic Composition
3.9. Data Analysis
4. Conclusions
5. Limitations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Isanga, J.; Zhang, G. –N. Biologically active components and nutraceuticals in peanuts and related products: Review. Food Rev. Int. 2007, 23, 123–140. [Google Scholar] [CrossRef]
- Toomer, O. Nutritional chemistry of the peanut (Arachis hypogaea). Crit. Rev. Food Sci. Nutr. 2018, 58, 3042–3053. [Google Scholar] [CrossRef]
- Yu, M.; Liu, H.; Yang, Y.; Shi, A.; Liu, L.; Hu, H.; Wang, Q. Optimisation for resveratrol accumulation during peanut germination with phenylalanine feeding & ultrasound-treatment using response surface methodology. Int. J. Food Sci. Technol. 2016, 51, 938–945. [Google Scholar]
- Weng, B.B.-C.; Liu, Y.-C.; White, B.L.; Chang, J.-C.; Davis, J.P.; Hsiao, S.-H.; Chiou, R.Y.-Y. Allergenicity reduction of the bio–elicited peanut sprout powder (BPSP) and toxicological acceptance of BPSP–supplemented diets assessed with ICR mice. J. Food Sci. Technol. 2022, 59, 4583–4593. [Google Scholar] [CrossRef]
- Rao, H.; Chen, C.; Tian, Y.; Gao, Y.; Tao, S.; Xue, W. Germination results in reduced allergenicity of peanut by degradation of allergens and resveratrol enrichment. Innov. Food Sci. Emerg. 2018, 50, 188–195. [Google Scholar] [CrossRef]
- Yu, M.; Liu, H.-Z.; Yang, Y.; Shi, A.-M.; Liu, L.; Hu, H.; Wang, Q.; Yu, H.-W.; Wang, X.-H. Optimising germinated conditions to enhance yield of resveratrol content in peanut sprout using response surface methodology. Int. J. Food Sci. Technol. 2016, 51, 1754–1761. [Google Scholar]
- King, R.E.; Bomser, J.A.; Min, D.B. Bioactivity of resveratrol. Compr. Rev. Food Sci. Food Saf. 2006, 5, 65–70. [Google Scholar] [CrossRef]
- Kim, S.S.; Seo, J.Y.; Kim, B.R.; Kim, H.J.; Lee, H.Y.; Kim, J.S. Anti–obesity activity of peanut sprout extract. Food Sci. Biotechnol. 2014, 23, 601–607. [Google Scholar] [CrossRef]
- Ha, A.W.; Kim, W.K.; Kim, J.H.; Kang, N.E. The supplementation effects of peanut sprout on reduction of abdominal fat and health indices in overweight and obese women. Nutr. Res. Pract. 2015, 9, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Vong, C.I.; Rathinasabapathy, T.; Moncada, M.; Komarnytsky, S. All polyphenols are not created equal: Exploring the diversity of phenolic metabolites. J. Agric. Food Chem. 2022, 70, 2077–2091. [Google Scholar] [CrossRef]
- Özcan, M.M.; Al Juhaimi, F.; Ahmed, I.A.M.; Uslu, N.; Babiker, E.E.; Ghafoor, K. Effect of microwave and oven drying processes on antioxidant activity, total phenol and phenolic compounds of kiwi and pepino fruits. J. Food Sci. Technol. 2020, 57, 233–242. [Google Scholar] [CrossRef]
- Yu, M.; Liu, H.; Shi, A.; Liu, L.; Wang, Q. Preparation of resveratrol–enriched and poor allergic protein peanut sprout from ultrasound treated peanut seeds. Ultrason. Sonochem. 2016, 28, 334–340. [Google Scholar]
- Limmongkon, A.; Nopprang, P.; Chaikeandee, P.; Somboon, T.; Wongshaya, P.; Pilaisangsuree, V. LC–MS/MS profiles and interrelationships between the anti–inflammatory activity, total phenolic content and antioxidant potential of Kalasin 2 cultivar peanut sprout crude extract. Food Chem. 2017, 239, 569–578. [Google Scholar] [CrossRef]
- Ravichandran, K.; Ahmed, A.R.; Knorr, D.; Smetanska, I. The effect of different processing methods on phenolic acid content and antioxidant activity of red beet. Food Res. Int. 2012, 48, 16–20. [Google Scholar] [CrossRef]
- Vadivel, V.; Stuetz, W.; Scherbaum, V.; Biesalski, H.K. Total free phenolic content and health relevant functionality of Indian wild legume grains: Effect of indigenous processing methods. J. Food Compos. Anal. 2011, 24, 935–943. [Google Scholar]
- de Queiroz, Y.S.; Antunes, P.B.; Vicente, S.J.V.; Sampaio, G.R.; Shibao, J.; Bastos, D.H.M.; da, S. Torres, E.A.F. Bioactive compounds, in vitro antioxidant capacity and Maillard reaction products of raw, boiled and fried garlic (Allium sativum L.). Int. J. Food Sci. Technol. 2014, 49, 1308–1314. [Google Scholar] [CrossRef]
- Perla, V.; Holm, D.G.; Jayanty, S.S. Effects of cooking methods on polyphenols, pigments and antioxidant activity in potato tubers. LWT–Food Sci. Technol. 2012, 45, 161–171. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Tang, Y.; Chen, P.X.; Liu, R.; Ramdath, D.; Liu, Q.; Hernandez, M.; Tsao, R. Effect of domestic cooking on carotenoids, tocopherols, fatty acids, phenolics, and antioxidant activities of lentils (Lens culinaris). J. Agric. Food Chem. 2014, 62, 12585–12594. [Google Scholar] [CrossRef] [PubMed]
- Hefnawy, T. Effect of processing methods on nutritional composition and anti–nutritional factors in lentils (Lens culinaris). Ann. Agr. Sci. 2011, 56, 57–61. [Google Scholar] [CrossRef]
- del Pilar Ramírez–Anaya, J.; Samaniego–Sánchez, C.; Castañeda–Saucedo, M.C.; Villalón–Mir, M.; de la Serrana, H.L.G. Phenols and the antioxidant capacity of Mediterranean vegetables prepared with extra virgin olive oil using different domestic cooking techniques. Food Chem. 2015, 188, 430–438. [Google Scholar] [CrossRef]
- Friedman, M. Food browning and its prevention: An overview. J. Agric. Food Chem. 1996, 44, 631–653. [Google Scholar] [CrossRef]
- Giallourou, N.; Oruna–Concha, M.J.; Harbourne, N. Effects of domestic processing methods on the phytochemical content of watercress (Nasturtium officinale). Food Chem. 2016, 212, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, B.; Xia, Y.; Li, H.; Shi, X.; Wang, J.; Deng, Z. Bioaccessibility and transformation pathways of phenolic compounds in processed mulberry (Morus alba L.) leaves after in vitro gastrointestinal digestion and faecal fermentation. J. Funct. Foods 2019, 60, 103406. [Google Scholar] [CrossRef]
- Li, Q.; Shi, X.; Zhao, Q.; Cui, Y.; Ouyang, J.; Xu, F. Effect of cooking methods on nutritional quality and volatile compounds of Chinese chestnut (Castanea mollissima Blume). Food Chem. 2016, 201, 80–86. [Google Scholar] [CrossRef]
- Li, B.; Yang, W.; Nie, Y.; Kang, F.; Goff, H.D.; Cui, S.W. Effect of steam explosion on dietary fiber, polysaccharide, protein and physicochemical properties of okara. Food Hydrocolloid. 2019, 94, 48–56. [Google Scholar] [CrossRef]
- Ma, H.; Xu, X.; Wang, S.; Wang, J.; Peng, W. Effects of microwave irradiation on the expression of key flavonoid biosynthetic enzyme genes and the accumulation of flavonoid products in Fagopyrum tataricum sprouts. J. Cereal Sci. 2021, 101, 103275. [Google Scholar] [CrossRef]
- Taskın, B.; Aksoylu Özbek, Z. Optimisation of microwave effect on bioactives contents and colour attributes of aqueous green tea extracts by central composite design. J. Food Meas. Charact. 2020, 14, 2240–2252. [Google Scholar] [CrossRef]
- Boukhanouf, S.; Louaileche, H.; Perrin, D. Phytochemical content and in vitro antioxidant activity of faba bean (Vicia faba L.) as affected by maturity stage and cooking practice. Int. Food Res. J. 2016, 23, 954–961. [Google Scholar]
- Liu, K.; Ning, M. Antioxidant activity stability and digestibility of protein from Se–enriched germinated brown rice. LWT–Food Sci. Technol. 2021, 142, 111032. [Google Scholar] [CrossRef]
- Ti, H.; Li, Q.; Zhang, R.; Zhang, M.; Deng, Y.; Wei, Z.; Chi, J.; Zhang, Y. Free and bound phenolic profiles and antioxidant activity of milled fractions of different indica rice varieties cultivated in southern China. Food Chem. 2014, 159, 166–174. [Google Scholar] [CrossRef]
- Ismail, A.; Marjan, Z.M.; Foong, C.W. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Choo, T.M.; Dhillon, S.; Rabalski, I. Free and bound phenolic acids and total phenolics in black, blue, and yellow barley and their contribution to free radical scavenging capacity. Cereal Chem. 2012, 89, 198–204. [Google Scholar] [CrossRef]
- Techo, J.; Soponronnarit, S.; Devahastin, S.; Wattanasiritham, L.S.; Thuwapanichayanan, R.; Prachayawarakorn, S. Effects of heating method and temperature in combination with hypoxic treatment on γ-aminobutyric acid, phenolics content and antioxidant activity of germinated rice. J. Food Sci. Technol. 2019, 54, 1330–1341. [Google Scholar] [CrossRef]
- De Santiago, E.; Domínguez–Fernández, M.; Cid, C.; De Peña, M.P. Impact of cooking process on nutritional composition and antioxidants of cactus cladodes (Opuntia ficus–indica). Food Chem. 2018, 240, 1055–1062. [Google Scholar] [CrossRef]
- Xu, F.; Zheng, Y.; Yang, Z.; Cao, S.; Shao, X.; Wang, H. Domestic cooking methods affect the nutritional quality of red cabbage. Food Chem. 2014, 161, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Chukwumah, Y.; Walker, L.; Vogler, B.; Verghese, M. In vitro absorption of dietary trans–resveratrol from boiled and roasted peanuts in Caco–2 cells. J. Agric. Food Chem. 2011, 59, 12323–12329. [Google Scholar] [CrossRef] [PubMed]
- Raigar, R.K.; Upadhyay, R.; Mishra, H.N. Optimization of microwave roasting of peanuts and evaluation of its physicochemical and sensory attributes. J. Food Sci. Technol. 2017, 54, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.D.; Ziegler, V.; Bubolz, V.K.; Da Silva, J.; Cardozo, M.M.C.; Elias, M.C.; De Oliveira, M. Effects of the roasting process over the content of secondary metabolites from peanut grains (Arachis hypogaea L.) with different colorations of testa. J. Food Qual. 2016, 39, 685–694. [Google Scholar] [CrossRef]
- Srichamnong, W.; Thiyajai, P.; Charoenkiatkul, S. Conventional steaming retains tocols and γ–oryzanol better than boiling and frying in the jasmine rice variety Khao dok mali 105. Food Chem. 2016, 191, 113–119. [Google Scholar] [CrossRef]
- Kim, S.-L.; Kim, S.-K.; Park, C.-H. Introduction and nutritional evaluation of buckwheat sprouts as a new vegetable. Food Res. Int. 2004, 37, 319–327. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wen, W.; Zhang, R.; Wei, Z.; Deng, Y.; Xiao, J.; Zhang, M. Complex enzyme hydrolysis releases antioxidative phenolics from rice bran. Food Chem. 2017, 214, 1–8. [Google Scholar] [CrossRef] [PubMed]




| CK (μg/g DW) | Boiling (μg/g DW) | Steaming (μg/g DW) | Microwave Heating (μg/g DW) | Roasting (μg/g DW) | Deep-Frying (μg/g DW) | |
|---|---|---|---|---|---|---|
| Resveratrol | 25.83 ± 1.89 a | 14.26 ± 1.37 b | 9.95 ± 1.88 c | 24.68 ± 0.42 a | 11.16 ± 3.38 c | 9.13 ± 1.36 c |
| p–Hydroxybenzoic acid | 595.73 ± 28.90 a | 470.05 ± 63.82 b | 305.06 ± 10.41 d | 478.34 ± 33.81 b | 432.77 ± 6.73 bc | 403.26 ± 25.88 c |
| Epicatechin | 168.64 ± 2.42 ab | 134.57 ± 12.23 cd | 107.37 ± 16.00 d | 173.73 ± 26.54 a | 149.73 ± 10.73 abc | 139.84 ± 15.38 bc |
| Caffeic acid | 1.60 ± 0.20 a | 0.72 ± 0.08 b | 0.35 ± 0.04 c | 0.45 ± 0.09 c | 0.43 ± 0.02 c | 0.65 ± 0.11 b |
| Catechin | 70.97 ± 2.60 a | 1.10 ± 1.05 f | 4.44 ± 1.89 e | 34.27 ± 2.37 b | 23.31 ± 0.89 c | 16.15 ± 0.83 d |
| p–Coumaric acid | 144.90 ± 8.33 a | 97.54 ± 3.56 c | 82.01 ± 5.87 d | 111.06 ± 3.20 b | 76.56 ± 1.11 d | 107.60 ± 11.28 bc |
| Ferulic acid | 27.17 ± 3.67 a | 18.59 ± 2.28 b | 18.55 ± 2.15 b | 27.14 ± 1.24 a | 21.45 ± 1.29 b | 8.89 ± 1.54 c |
| Sinapic acid | 4.24 ± 0.12 a | 3.28 ± 0.16 c | 4.03 ± 0.10 b | 4.18 ± 0.36 a | 3.83 ± 0.08 b | 3.75 ± 0.41 b |
| Rutin | 67.33 ± 1.28 a | 52.39 ± 1.17 c | 51.36 ± 1.04 c | 62.80 ± 1.58 b | 61.83 ± 2.41 b | 62.15 ± 0.59 b |
| Cinnamic acid | 90.26 ± 5.57 b | 95.49 ± 2.76 b | 68.86 ± 3.67 c | 102.20 ± 5.14 a | 100.25 ± 2.61 a | 69.69 ± 0.85 c |
| Quercetin | 6.20 ± 0.52 a | 1.71 ± 0.17 d | 1.66 ± 0.03 d | 5.70 ± 0.10 b | 2.68 ± 0.04 c | 1.87 ± 0.13 d |
| Phenolic Compound | Equation | R2 | Retention Time/min |
|---|---|---|---|
| Resveratrol | y = 44.725x + 14.001 | 0.9996 | 15.302 |
| p–Hydroxybenzoic acid | y = 18.062x + 5.9625 | 0.9996 | 23.787 |
| Epicatechin | y = 3.7768x + 4.2018 | 0.9998 | 25.711 |
| Caffeic acid | y = 726.29x − 1.5116 | 0.9996 | 32.984 |
| Catechin | y = 49.1x + 34.222 | 0.9995 | 39.128 |
| p–Coumaric acid | y = 50.49x − 36.573 | 0.9996 | 45.698 |
| Ferulic acid | y = 41.891x + 33.402 | 0.9999 | 50.978 |
| Sinapic acid | y = 692.7x − 20.507 | 0.9996 | 52.904 |
| Rutin | y = 103.12x − 15.667 | 0.9999 | 56.009 |
| Cinnamic acid | y = 60.505x − 13.032 | 0.9999 | 57.819 |
| Quercetin | y = 61.857x − 1.9518 | 0.9997 | 61.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Qu, H.; Xie, M.; Shi, T.; Shi, P.; Yu, M. Effects of Different Cooking Methods on Phenol Content and Antioxidant Activity in Sprouted Peanut. Molecules 2023, 28, 4684. https://doi.org/10.3390/molecules28124684
Zhang L, Qu H, Xie M, Shi T, Shi P, Yu M. Effects of Different Cooking Methods on Phenol Content and Antioxidant Activity in Sprouted Peanut. Molecules. 2023; 28(12):4684. https://doi.org/10.3390/molecules28124684
Chicago/Turabian StyleZhang, Liangchen, Haolin Qu, Mengxi Xie, Taiyuan Shi, Puxiang Shi, and Miao Yu. 2023. "Effects of Different Cooking Methods on Phenol Content and Antioxidant Activity in Sprouted Peanut" Molecules 28, no. 12: 4684. https://doi.org/10.3390/molecules28124684
APA StyleZhang, L., Qu, H., Xie, M., Shi, T., Shi, P., & Yu, M. (2023). Effects of Different Cooking Methods on Phenol Content and Antioxidant Activity in Sprouted Peanut. Molecules, 28(12), 4684. https://doi.org/10.3390/molecules28124684







