High-Strength Double-Network Conductive Hydrogels Based on Polyvinyl Alcohol and Polymerizable Deep Eutectic Solvent
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Preparation of Hydrogels
3.2.1. Preparation of DES (ChCl/AA)
3.2.2. Preparation of PVA-DES Hydrogels
3.3. Construction of Strain Sensor and Its Integration as a Wearable Sensor
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Qiu, Z.; Wan, Y.; Zhou, W.; Yang, J.; Yang, J.; Huang, J.; Zhang, J.; Liu, Q.; Huang, S.; Bai, N.; et al. Ionic skin with biomimetic dielectric layer templated from calathea zebrine leaf. Adv. Funct. Mater. 2018, 28, 1802343. [Google Scholar] [CrossRef]
- Yue, O.; Wang, X.; Liu, X.; Hou, M.; Zheng, M.; Wang, Y.; Cui, B. Spider-web and ant-tentacle doubly bio-inspired multifunctional self-poweredelectronic skin with hierarchical nanostructure. Adv. Sci. 2021, 8, 2004377. [Google Scholar] [CrossRef]
- Qin, Z.; Chen, X.; Lv, Y.; Zhao, B.; Fang, X.; Pan, K. Wearable and high-performance piezoresistive sensor based on nanofiber/sodium alginate synergistically enhanced MXene composite aerogel. Chem. Eng. J. 2023, 451, 138586. [Google Scholar] [CrossRef]
- Zhai, Y.; Yu, Y.; Zhou, K.; Yun, Z.; Huang, W.; Liu, H.; Xia, Q.; Dai, K.; Zheng, G.; Liu, C.; et al. Flexible and wearable carbon black/thermoplastic polyurethane foam with a pinnate-veined aligned porous structure for multifunctional piezoresistive sensors. Chem. Eng. J. 2020, 382, 122985. [Google Scholar] [CrossRef]
- Wu, H.; Qi, H.; Wang, X.; Qiu, Y.; Shi, K.; Zhang, H.; Zhang, Z.; Zhang, W.; Tian, Y. Stretchable, sensitive, flexible strain sensor incorporated with patterned liquid metal on hydrogel for human motion monitoring and human–machine interaction. J. Mater. Chem. C 2022, 10, 8206–8217. [Google Scholar] [CrossRef]
- Wu, M.; Pan, M.; Qiao, C.; Ma, Y.; Yan, B.; Yang, W.; Peng, Q.; Han, L.; Zeng, H. Ultra stretchable, tough, elastic and transparent hydrogel skins integrated with intelligent sensing functions enabled by machine learning algorithms. Chem. Eng. J. 2022, 450, 138212. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Q.; Mugo, S.M.; An, L.; Zhang, Q.; Lu, Y. Self-healing andshape-editable wearable supercapacitors based on highly stretchable hydrogel electrolytes. Adv. Sci. 2022, 9, 2201039. [Google Scholar] [CrossRef]
- Wang, D.; Yang, F.; Cong, L.; Feng, W.; Wang, C.; Chu, F.; Nan, J.; Chen, R. Lignin-containing hydrogel matrices with enhanced adhesion and toughness for all-hydrogel supercapacitors. Chem. Eng. J. 2022, 450, 138025. [Google Scholar] [CrossRef]
- Lee, J.; Tan, M.W.M.; Parida, K.; Thangavel, G.; Park, S.A.; Park, T.; Lee, P.S. Water-processable, stretchable, self-healable, thermally stable, and transparent ionic conductors for actuators and sensors. Adv. Mater. 2020, 32, 1906679. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, Q.; Sun, S.; Zhu, W.; Wu, P. A bioinspired mineral hydrogel as a self-healable, mechanically adaptable ionic skin for highly sensitive pressure sensing. Adv. Mater. 2017, 29, 1700321. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Wang, S.; Wang, L.; Wu, M. Neuron-inspired multifunctional conductive hydrogels for flexible wearable sensors. J. Mater. Chem. C 2022, 10, 4327–4335. [Google Scholar] [CrossRef]
- Ge, G.; Lu, Y.; Qu, X.; Zhao, W.; Ren, Y.; Wang, W.; Wang, Q.; Huang, W.; Dong, X. Muscle-inspired self-healing hydrogels for strain and temperature sensor. ACS Nano 2020, 14, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Honda, W.; Harada, S.; Arie, T.; Akita, S.; Takei, K. Wearable, human-interactive, health-monitoring, wireless devices fabricated by macroscale printing techniques. Adv. Funct. Mater. 2014, 24, 3299–3304. [Google Scholar] [CrossRef]
- Wang, R.; Chi, W.; Wan, F.; Wei, J.; Ping, H.; Zou, Z.; Xie, J.; Wang, W.; Fu, Z. Nanocage ferritin reinforced polyacrylamide hydrogel for wearable flexible strain sensors. ACS Appl. Mater. Interfaces 2022, 14, 21278–21286. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zhong, Q.; Hu, Q.; Wu, N.; Li, W.; Wang, B.; Hu, B.; Zhou, J. Stretchable self-powered fiber-based strain sensor. Adv. Funct. Mater. 2015, 25, 1798–1803. [Google Scholar] [CrossRef]
- Hines, L.; Petersen, K.H.; Lum, G.Z.; Sitti, M. Soft actuators for small-scale robotics. Adv. Mater. 2017, 29, 1603483. [Google Scholar] [CrossRef]
- Nojoomi, A.; Arslan, H.; Lee, K.; Yum, K. Bioinspired 3D structures with programmable morphologies and motions. Nat. Commun. 2018, 9, 3705. [Google Scholar] [CrossRef]
- Shao, L.; Li, Y.; Ma, Z.; Bai, Y.; Wang, J.; Zeng, P.; Gong, P.; Shi, F.; Ji, Z.; Qiao, Y.; et al. Highly sensitive strain sensor based on a stretchable and conductive poly(vinyl alcohol)/phytic acid/NH2-POSS hydrogel with a 3D microporous structure. ACS Appl. Mater. Interfaces 2020, 12, 26496–26508. [Google Scholar] [CrossRef]
- Roh, E.; Hwang, B.U.; Kim, D.; Kim, B.Y.; Lee, N.E. Stretchable, transparent, ultrasensitive, and patchable strain sensor for human–machine interfaces comprising a nanohybrid of carbon nanotubes and conductive elastomers. ACS Nano 2015, 9, 6252–6261. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Moon, H.C. Ionoskins: Nonvolatile, highly transparent, ultrastretchable ionic sensory platforms for wearable electronics. Adv. Funct. Mater. 2020, 30, 1907290. [Google Scholar] [CrossRef]
- Wei, H.; Kong, D.; Li, T.; Xue, Q.; Wang, S.; Cui, D.; Huang, Y.; Wang, L.; Hu, S.; Wan, T.; et al. Solution-processable conductive composite hydrogels with multiple synergetic networks toward wearable pressure/strain sensors. ACS Sens. 2021, 6, 2938–2951. [Google Scholar] [CrossRef] [PubMed]
- Suneetha, M.; Moo, O.S.; Choi, S.M.; Zo, S.; Rao, K.M.; Han, S.S. Tissue-adhesive, stretchable, and self-healable hydrogels based on carboxymethyl cellulose-dopamine/PEDOT:PSS via mussel-inspired chemistry for bioelectronic applications. Chem. Eng. J. 2021, 426, 130847. [Google Scholar] [CrossRef]
- Wang, P.; Li, G.; Liu, J.; Hou, Z.; Meng, C.; Guo, S.; Liu, C.; Fan, S. Tailorable capacitive tactile sensor based on stretchable and dissolvable porous silver nanowire/polyvinyl alcohol nanocomposite hydrogel for wearable human motion detection. Adv. Mater. Interfaces 2021, 8, 2100998. [Google Scholar] [CrossRef]
- Fan, L.; Hu, L.; Xie, J.; He, Z.; Zheng, Y.; Wei, D.; Yao, D.; Su, F. Biosafe, self-adhesive, recyclable, tough, and conductive hydrogels for multifunctional sensors. Biomater. Sci. 2021, 9, 5884–5896. [Google Scholar] [CrossRef]
- Park, J.-E.; Kang, H.S.; Baek, J.; Park, T.H.; Oh, S.; Lee, H.; Koo, M.; Park, C. Rewritable, printable conducting liquid metal hydrogel. ACS Nano 2019, 13, 9122–9130. [Google Scholar] [CrossRef]
- Su, Y.; Zhao, J.; Zhan, W.; Yuan, H.; Wu, L.; Sui, G.; Zhang, H. A multifunctional hydrogel fabricated via ultra-fast polymerization by graphene oxide-adsorbed liquid metal nanodroplets. Chem. Eng. J. 2022, 435, 135018. [Google Scholar] [CrossRef]
- Deng, Y.; Shang, T.; Wu, Z.; Tao, Y.; Luo, C.; Liang, J.; Han, D.; Lyu, R.; Qi, C.; Lv, W.; et al. Fast gelation of Ti3C2Tx MXene initiated by metal ions. Adv. Mater. 2019, 31, 1902432. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; El-Demellawi, J.K.; Jiang, Q.; Ge, G.; Liang, H.; Lee, K.; Dong, X.; Alshareef, H.N. MXene hydrogels: Fundamentals and applications. Chem. Soc. Rev. 2020, 49, 7229–7251. [Google Scholar] [CrossRef]
- Lu, Y.; Yue, Y.; Ding, Q.; Mei, C.; Xu, X.; Wu, Q.; Xiao, H.; Han, J. Self-recovery, fatigue-resistant, and multifunctional sensor assembled by a nanocellulose/carbon nanotube nanocomplex-mediated hydrogel. ACS Appl. Mater. Interfaces 2021, 13, 50281–50297. [Google Scholar] [CrossRef]
- Crowhurst, L.; Lancaster, N.L.; Arlandis, J.M.P.; Welton, T. Manipulating solute nucleophilicity with room temperature ionic liquids. J. Am. Chem. Soc. 2004, 126, 11549–11555. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zakeeruddin, S.M.; Exnar, I.; Grätzel, M. High efficiency dye-sensitized nanocrystalline solar cells based on ionic liquid polymer gel electrolyte. Chem. Commun. 2002, 24, 2972–2973. [Google Scholar] [CrossRef]
- Kamio, E.; Minakata, M.; Iida, Y.; Yasui, T.; Matsuoka, A.; Matsuyama, H. Inorganic/organic double-network ion gel membrane with a high ionic liquid content for CO2 separation. Polym. J. 2021, 53, 137–147. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, J.; Chang, L.; Zhang, X.; Liu, H.; Jiang, L. Preparation of high-performance ionogels with excellent transparency, good mechanical strength, and high conductivity. Adv. Mater. 2017, 29, 1704253. [Google Scholar] [CrossRef] [PubMed]
- Buzzeo, M.C.; Evans, R.G.; Compton, R.G. Non-haloaluminate room-temperature ionic liquids in electrochemistry—A review. ChemPhysChem 2004, 5, 1106–1120. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Owyeung, R.E.; Sonkusale, S.R.; Panzer, M.J. Highly stretchable and nonvolatile gelatin-supported deep eutectic solvent gel electrolyte-based ionic skins for strain and pressure sensing. J. Mater. Chem. C 2019, 7, 601–608. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- del Monte, F.; Carriazo, D.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L. Deep eutectic solvents in polymerizations: A greener alternative to conventional syntheses. ChemSusChem 2014, 7, 999–1009. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Weaver, K.D.; Kim, H.J.; Sun, J.; MacFarlane, D.R.; Elliott, G.D. Cyto-toxicity and biocompatibility of a family of choline phosphate ionic liquids designed for pharmaceutical applications. Green Chem. 2010, 12, 507–513. [Google Scholar] [CrossRef]
- Ilgen, F.; Ott, D.; Kralisch, D.; Reil, C.; Palmberger, A.; König, B. Conversion of carbohydrates into 5-hydroxymethylfurfural in highly concentrated low melting mixtures. Green Chem. 2009, 11, 1948–1954. [Google Scholar] [CrossRef]
- Wang, M.; Li, R.; Chen, G.; Zhou, S.; Feng, X.; Chen, Y.; He, M.; Liu, D.; Song, T.; Qi, H. Highly stretchable, transparent, and conductive wood fabricated by in situ photopolymerization with polymerizable deep eutectic solvents. ACS Appl. Mater. Interfaces 2019, 11, 14313–14321. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, X.; Xu, M.; Zhao, L.; Huang, D.; Wei, X.; Chen, W. Carbon nanotube reinforced polyvinyl alcohol/biphasic calcium phosphate scaffold for bone tissue engineering. RSC Advances 2019, 9, 38998–39010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahab, A.H.A.; Saad, A.P.M.; Harun, M.N.; Syahrom, A.; Ramlee, M.H.; Sulong, M.A.; Kadir, M.R.A. Developing functionally graded PVA hydrogel using simple freeze-thaw method for artificial glenoid labrum. J. Mech. Behav. Biomed. Mater. 2019, 91, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.G.; Morton, L.M.; Phillips, T.J. Dressings for chronic wounds. Dermatol. Ther. 2013, 26, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, F.; Miano, F.; D’Antona, P.; Paradossi, G. Study of gelling behavior of poly(vinyl alcohol)-methacrylate for potential utilizations in tissue replacement and drug delivery. Biomacromolecules 2004, 5, 2439–2446. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, S.; Feng, W. PVA hydrogel properties for biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef]
- Abral, H.; Atmajaya, A.; Mahardika, M.; Hafizulhaq, F.; Handayani, D.; Sapuan, S.M.; Ilyas, R.A. Effect of ultrasonication duration of polyvinyl alcohol (PVA) gel on characterizations of PVA film. J. Mater. Res. Technol. 2020, 9, 2477–2486. [Google Scholar] [CrossRef]
- Ricciardi, R.; Auriemma, F.; De Rosa, C.; Lauprêtre, F. X-ray diffraction analysis of poly(vinyl alcohol) hydrogels, obtained by freezing and thawing techniques. Macromolecules 2004, 37, 1921–1927. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Pathak, S.K.; Cui, J.; Jeon, I. Hydrogels with superior mechanical properties from the synergistic effect in hydrophobic–hydrophilic copolymers. Chem. Eng. J. 2019, 362, 325–338. [Google Scholar] [CrossRef]
- Tran, V.T.; Mredha, M.T.I.; Pathak, S.K.; Yoon, H.; Cui, J.; Jeon, I. Conductive Tough Hydrogels with a Staggered Ion-Coordinating Structure for High Self-Recovery Rate. ACS Appl. Mater. Interfaces 2019, 11, 24598–24608. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Nonoyama, T.; Wada, S.; Kiyama, R.; Kitamura, N.; Mredha, M.T.I.; Zhang, X.; Kurokawa, T.; Nakajima, T.; Takagi, Y.; Yasuda, K.; et al. Double-Network Hydrogels Strongly Bondable to Bones by Spontaneous Osteogenesis Penetration. Adv. Mater. 2016, 28, 6740–6745. [Google Scholar] [CrossRef] [Green Version]
- Holloway, J.L.; Lowman, A.M.; Palmese, G.R. The role of crystallization and phase separation in the formation of physically cross-linked PVA hydrogels. Soft Matter 2013, 9, 826–833. [Google Scholar] [CrossRef]
- Mota-Morales, J.D.; Gutiérrez, M.C.; Sanchez, I.C.; Luna-Bárcenas, G.; del Monte, F. Frontal polymerizations carried out in deep-eutectic mixtures providing both the monomers and the polymerization medium. Chem. Commun. 2011, 47, 5328–5330. [Google Scholar] [CrossRef]
- Li, R.; Chen, G.; He, M.; Tian, J.; Su, B. Patternable transparent and conductive elastomers towards flexible tactile/strain sensors. J. Mater. Chem. C 2017, 5, 8475–8481. [Google Scholar] [CrossRef]
- Wang, X.; Chen, G.; Cai, L.; Li, R.A.; He, M. Wearable transparent conductive fibers with harsh environment tolerance. ACS Appl. Mater. Interfaces 2021, 13, 8952–8959. [Google Scholar] [CrossRef]
- Fang, X.; Li, Y.; Li, X.; Liu, W.; Yu, X.; Yan, F.; Sun, J. Dynamic hydrophobic domains enable the fabrication of mechanically robust and highly elastic poly(vinyl alcohol)-based hydrogels with excellent self-healing ability. ACS Mater. Lett. 2020, 2, 764–770. [Google Scholar] [CrossRef]
- Lu, L.; Sun, H.; Peng, F.; Jiang, Z. Novel graphite-filled PVA/CS hybrid membrane for pervaporation of benzene/cyclohexane mixtures. J. Membr. Sci. 2006, 281, 245–252. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Martínez, G.; Gómez, M.A. Synthesis of poly(vinyl alcohol)/reduced graphite oxide nanocomposites with improved thermal and electrical properties. J. Mater. Chem. 2009, 19, 5027–5032. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Li, B.; Zhang, P.; Kan, L.; Wang, G.; Wei, H.; Zhang, X.; Ma, N. One-step preparation of a highly stretchable, conductive, and transparent poly(vinyl alcohol)–phytic acid hydrogel for casual writing circuits. ACS Appl. Mater. Interfaces 2019, 11, 32441–32448. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-N.; Peng, L.; Liu, T.; Wang, Y.; Shi, S.; Wang, H. Poly(vinyl alcohol)–Tannic Acid Hydrogels with Excellent Mechanical Properties and Shape Memory Behaviors. ACS Appl. Mater. Interfaces 2016, 8, 27199–27206. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Peng, X.; Chen, Y.-N.; Bai, Q.-W.; Shang, C.; Zhang, L.; Wang, H. Hydrogen-Bonded Polymer-Small Molecule Complexes with Tunable Mechanical Properties. Macromol. Rapid Commun. 2018, 39, 1800050. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Wan, P.; Wen, J.; Gong, M.; Wu, X.; Wang, Y.; Shi, R.; Zhang, L. Wearable, healable, and adhesive epidermal sensors assembled from mussel-inspired conductive hybrid hydrogel framework. Adv. Funct. Mater. 2017, 27, 1703852. [Google Scholar] [CrossRef]
- Xu, X.; Jerca, V.V.; Hoogenboom, R. Bioinspired double network hydrogels: From covalent double network hydrogels via hybrid double network hydrogels to physical double network hydrogels. Mater. Horiz. 2021, 8, 1173–1188. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, B.; Mao, Z.; Sui, X.; Feng, X. Nonvolatile, stretchable and adhesive ionogel fiber sensor designed for extreme environments. Chem. Eng. J. 2022, 433, 133500. [Google Scholar] [CrossRef]
- Huang, H.; Han, L.; Li, J.; Fu, X.; Wang, Y.; Yang, Z.; Xu, X.; Pan, L.; Xu, M. Super-stretchable, elastic and recoverable ionic conductive hydrogel for wireless wearable, stretchable sensor. J. Mater. Chem. A 2020, 8, 10291–10300. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, S.; Qian, L.; Wei, N.; Nica, V.; Coseri, S.; Han, F. Super stretchable, self-healing, adhesive ionic conductive hydrogels based on tailor-made ionic liquid for high-performance strain sensors. Adv. Funct. Mater. 2022, 32, 2204565. [Google Scholar] [CrossRef]
- Xu, J.; Wang, G.; Wu, Y.; Ren, X.; Gao, G. Ultrastretchable wearable strain and pressure sensors based on adhesive, tough, and self-healing hydrogels for human motion monitoring. ACS Appl. Mater. Interfaces 2019, 11, 25613–25623. [Google Scholar] [CrossRef]
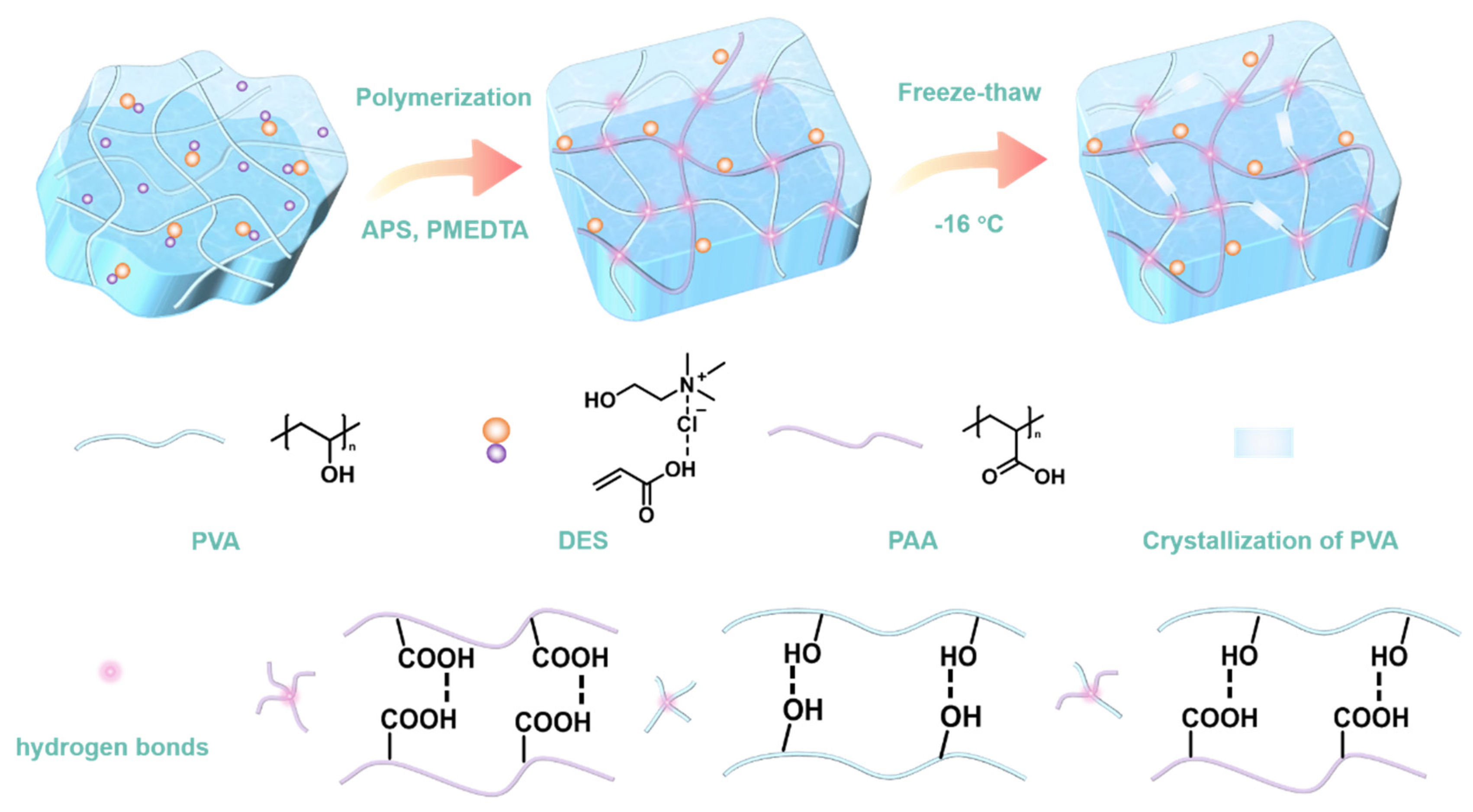
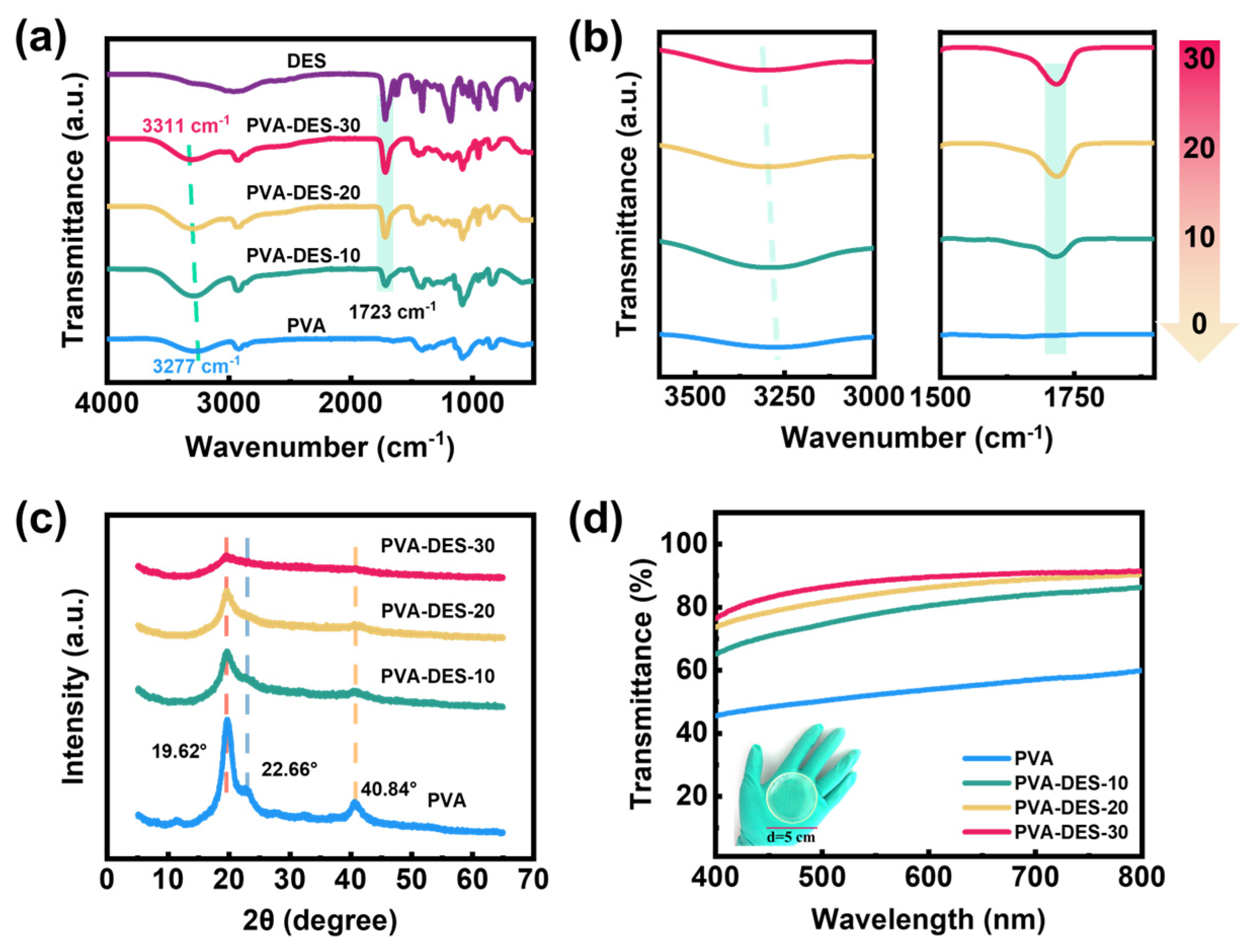
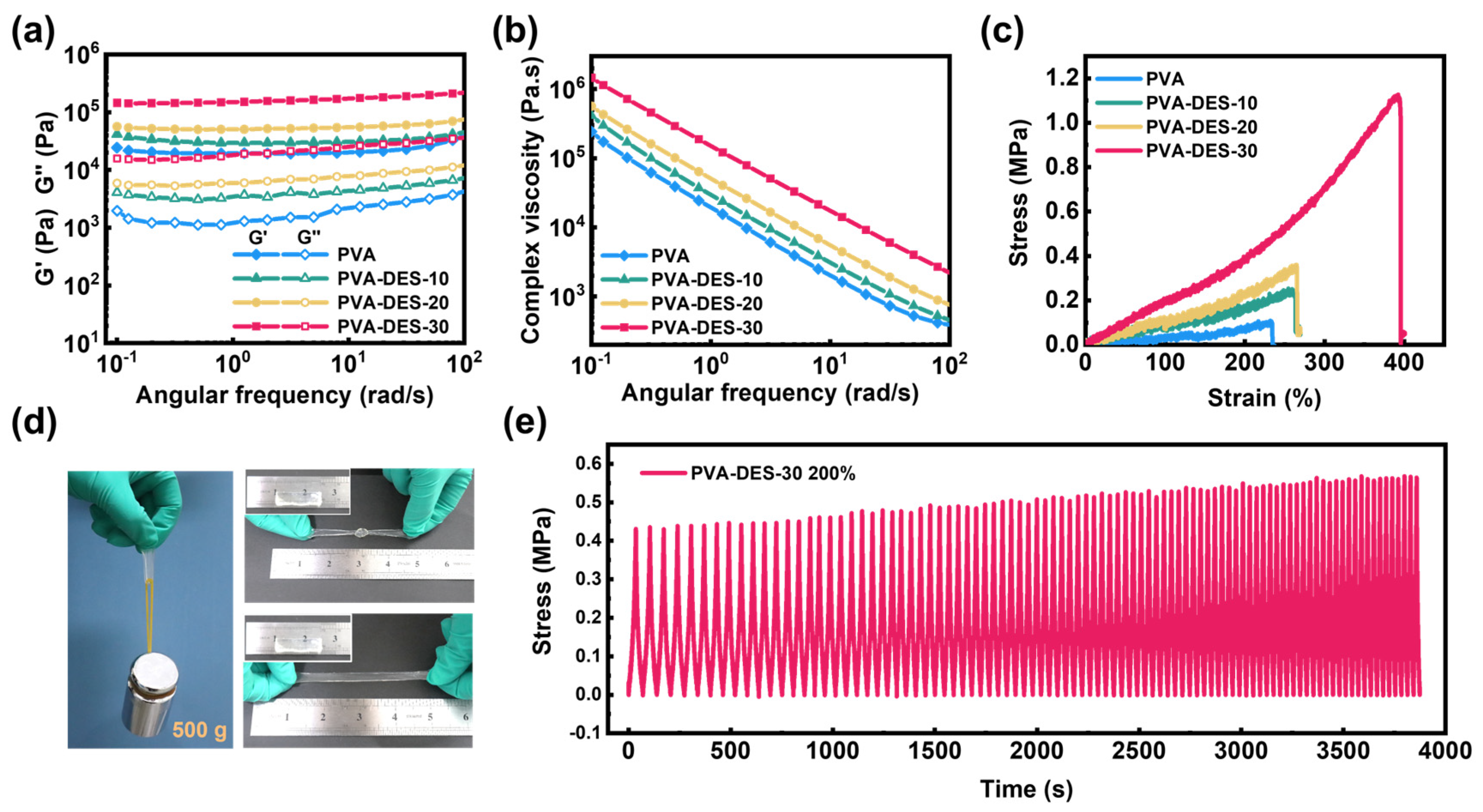
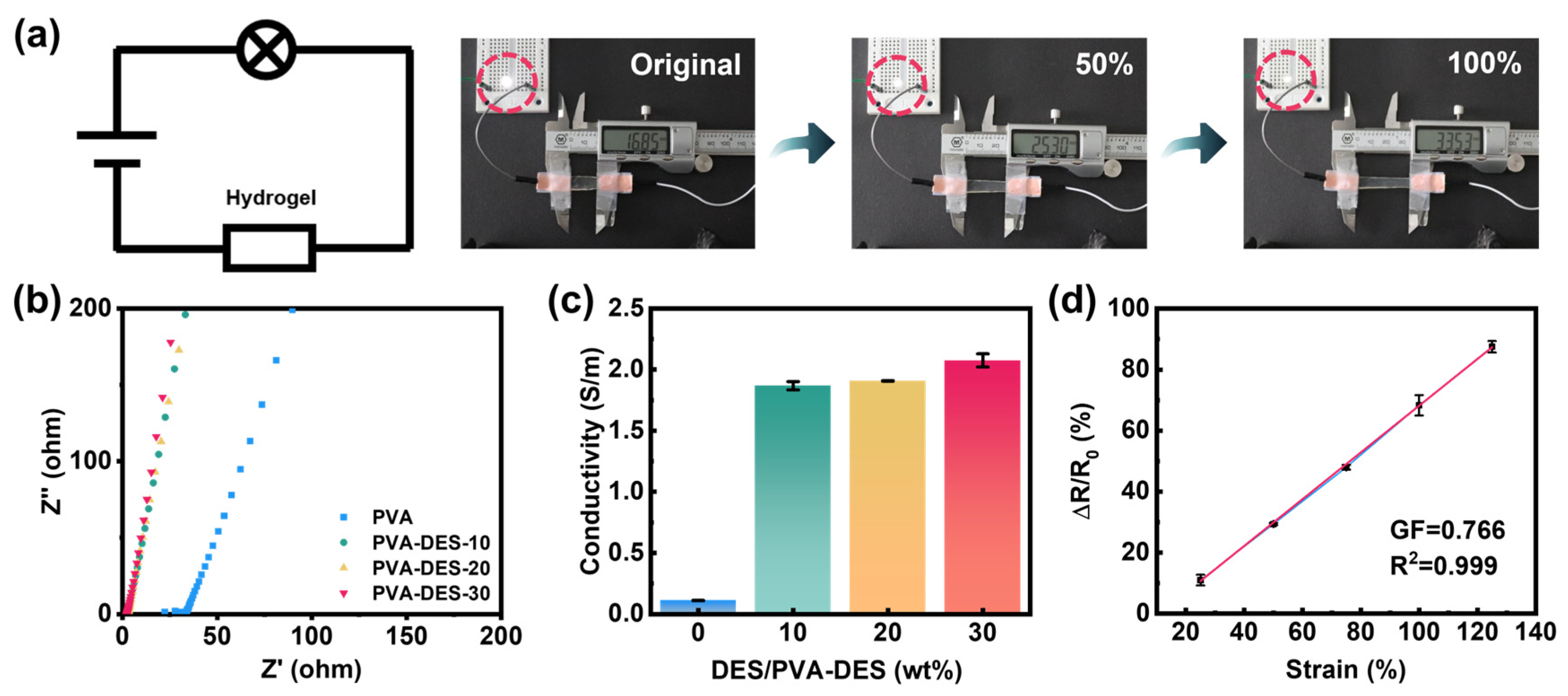

| Hydrogels | PVA (g) | DI (g) | DES (g) | APS (g) | PMDETA (µL) | Content of DI (wt%) |
|---|---|---|---|---|---|---|
| PVA | 1.5 | 10.5 | 0 | 0 | 0 | 87.5 |
| PVA-DES-10 | 1.5 | 10.5 | 1.33 | 0.014 | 5.6 | 78.7 |
| PVA-DES-20 | 1.5 | 10.5 | 3.00 | 0.031 | 12.4 | 69.8 |
| PVA-DES-30 | 1.5 | 10.5 | 5.14 | 0.052 | 21.2 | 61.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Jiang, L.; Zhang, H.; Li, Q.; Ma, N.; Zhang, X.; Ma, L. High-Strength Double-Network Conductive Hydrogels Based on Polyvinyl Alcohol and Polymerizable Deep Eutectic Solvent. Molecules 2023, 28, 4690. https://doi.org/10.3390/molecules28124690
Zhang Y, Jiang L, Zhang H, Li Q, Ma N, Zhang X, Ma L. High-Strength Double-Network Conductive Hydrogels Based on Polyvinyl Alcohol and Polymerizable Deep Eutectic Solvent. Molecules. 2023; 28(12):4690. https://doi.org/10.3390/molecules28124690
Chicago/Turabian StyleZhang, Yihan, Lei Jiang, Haibing Zhang, Qingyin Li, Ning Ma, Xinyue Zhang, and Li Ma. 2023. "High-Strength Double-Network Conductive Hydrogels Based on Polyvinyl Alcohol and Polymerizable Deep Eutectic Solvent" Molecules 28, no. 12: 4690. https://doi.org/10.3390/molecules28124690






