In Situ Encapsulation of Graphene Quantum Dots in Highly Stable Porphyrin Metal-Organic Frameworks for Efficient Photocatalytic CO2 Reduction
Abstract
:1. Introduction
2. Results and Discussion
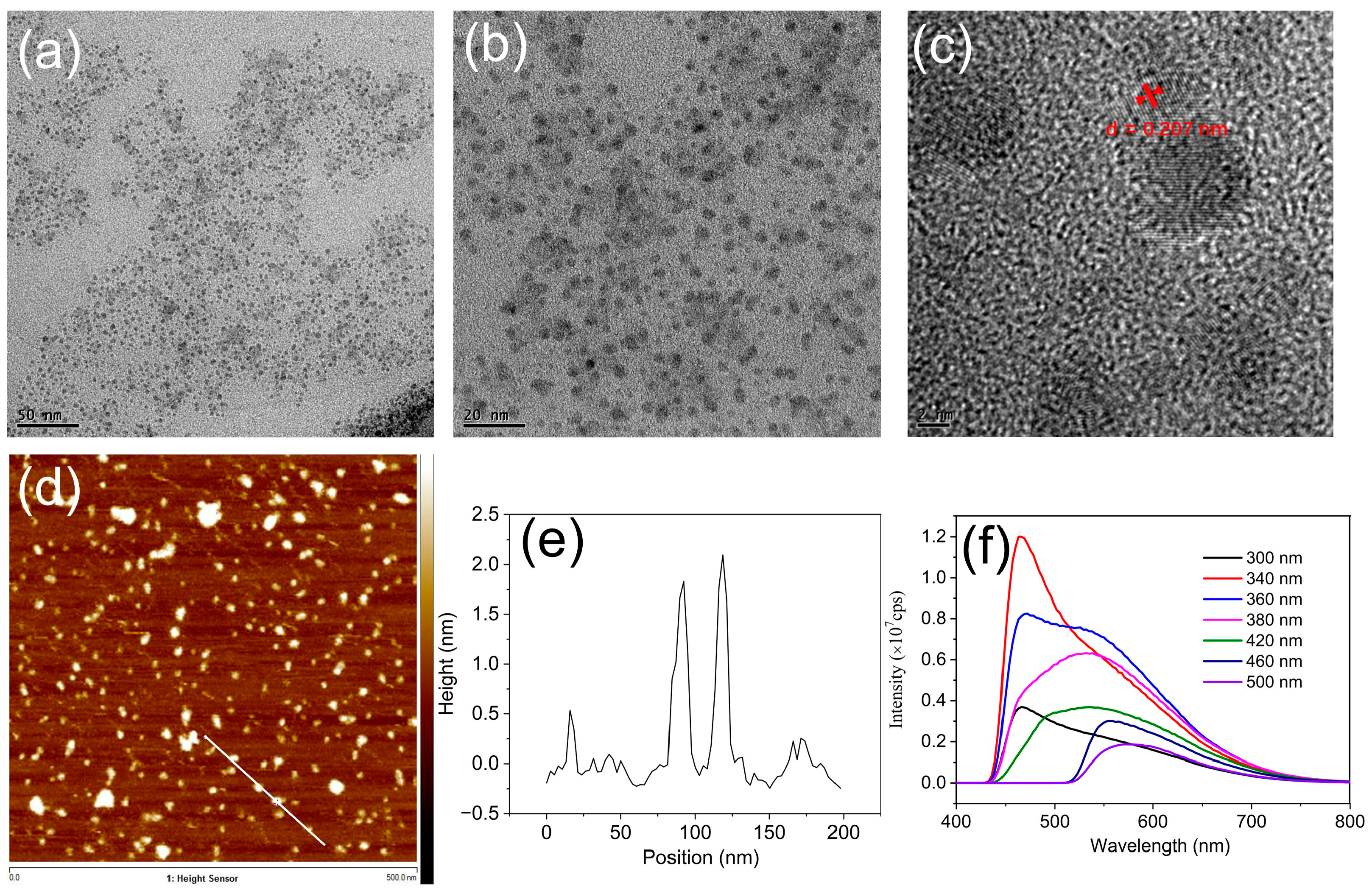
2.1. Synthesis and Characterization of GQDs
2.2. Synthesis and Characterization of PCN-222 and GQDs@PCN-222
2.3. Photocatalytic Properties
3. Materials and Methods
3.1. Chemical Regents
3.2. The Synthesis of Graphene Quantum Dots (GQDs)
3.3. Preparation of GQDs Dispersion
3.4. Synthesis of PCN-222
3.5. Synthesis of GQDs@PCN-222
3.6. Characterizations
3.7. Photoelectrochemical Measurements
3.8. Photocatalytic Reduction of CO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dahl, T.W.; Harding, M.A.R.; Brugger, J.; Feulner, G.; Norrman, K.; Lomax, B.H.; Junium, C.K. Low atmospheric CO2 levels before the rise of forested ecosystems. Nat. Commun. 2022, 13, 7616. [Google Scholar] [CrossRef]
- Xu, A.; Hung, S.-F.; Cao, A.; Wang, Z.; Karmodak, N.; Huang, J.E.; Yan, Y.; Sedighian Rasouli, A.; Ozden, A.; Wu, F.-Y.; et al. Copper/alkaline earth metal oxide interfaces for electrochemical CO2-to-alcohol conversion by selective hydrogenation. Nat. Catal. 2022, 5, 1081–1088. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Rahaman, M.; Andrei, V.; Miller, M.; Rodríguez-Jiménez, S.; Lam, E.; Pornrungroj, C.; Reisner, E. Photoelectrochemical CO2-to-fuel conversion with simultaneous plastic reforming. Nat. Synth. 2023, 2, 182–192. [Google Scholar] [CrossRef]
- Ye, R.; Wang, X.; Wang, G.; Chen, Y.; Lai, Q.; Zhang, R. Editorial: Heterogeneous catalysts for C1 molecules conversion. Front. Chem. 2022, 10, 1121871. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, Y.; Wang, Q.; Wang, X.; Zuo, S.; Zhang, H.; Ren, X.; He, Y.; Ichihara, F.; Ye, J. Synergy between Confined Cobalt Centers and Oxygen Defects on α-Fe2O3 Platelets for Efficient Photocatalytic CO2 Reduction. Sol. RRL 2022, 6, 2100833. [Google Scholar] [CrossRef]
- Xu, R.; Si, D.H.; Zhao, S.S.; Wu, Q.J.; Wang, X.S.; Liu, T.F.; Zhao, H.; Cao, R.; Huang, Y.B. Tandem Photocatalysis of CO2 to C2H4 via a Synergistic Rhenium-(I) Bipyridine/Copper-Porphyrinic Triazine Framework. J. Am. Chem. Soc. 2023, 145, 8261–8270. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Wu, Y.; Hu, Z.; Wang, S.; Jiao, X.; Zhu, J.; Sun, Y.; Xie, Y. Progress and perspective for conversion of plastic wastes into valuable chemicals. Chem. Soc. Rev. 2023, 52, 8–29. [Google Scholar] [CrossRef]
- Xiong, Q.; Chen, Y.; Xu, T.; Zhu, Z.; Chen, W.; Lu, W. Highly efficient purification of emerging pollutants and bacteria in natural water by g-C3N4-sheltered fibers containing TiO2. Appl. Surf. Sci. 2021, 559, 149839. [Google Scholar] [CrossRef]
- Wang, T.; Dai, Z.; Kang, J.; Fu, F.; Zhang, T.; Wang, S. A TiO2 nanocomposite hydrogel for hydroponic plants in efficient water improvement. Mater. Chem. Phys. 2018, 215, 242–250. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, Y.; Zhu, Y.; Fu, Y. Improved visible-light photocatalytic activity of sodium tantalum oxide via biomass-derived silk fibroin doping. Text. Res. J. 2018, 89, 1332–1339. [Google Scholar] [CrossRef]
- Jiang, T.; Jiang, G.; Li, L.; Chen, H.; Zhou, H.; Yao, J.; Kong, X.; Chen, W. N-Doped carbon hybrid conjugates as vectors for photocatalytic CS2 production. Mater. Res. Express 2015, 2, 045603. [Google Scholar] [CrossRef]
- Liu, B.; Xu, Y.; Cui, J.; Wang, S.; Wang, T. Carbon nanotubes-dispersed TiO2 nanoparticles with their enhanced photocatalytic activity. Mater. Res. Bull. 2014, 59, 278–282. [Google Scholar] [CrossRef]
- Liu, C.; Qian, X.; Wei, Q.; Chen, Z.; Chen, J.; Wang, W.; Chen, X.; Gao, J.; Liu, Y.; Xie, L. Construction of hydrostable cesium lead bromide-titania for visible-light degradation of tetracycline hydrochloride in water. J. Clean. Prod. 2022, 365, 132830. [Google Scholar] [CrossRef]
- Gao, J.; Qian, X.; Wei, Q.; Chen, Z.; Liu, C.; Wang, W.; Chen, J.; Chen, X.; Liu, Y.; Wei, G. Construction of core-shell cesium lead bromide-silica by precipitation coating method with applications in aqueous photocatalysis. J. Colloid Interface Sci. 2022, 623, 974–984. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Zhao, W.; Liu, C.; Qian, X.; Zhang, M.; Wei, G.; Khan, E.; Hau Ng, Y.; Sik Ok, Y. Recent advances in photodegradation of antibiotic residues in water. Chem. Eng. J. 2021, 405, 126806. [Google Scholar] [CrossRef]
- Chandel, M.; Thakur, M.; Sharma, A.; Pathania, D.; Kumar, A.; Singh, L. Chlorophyll sensitized (BiO)2CO3/ CdWO4/rGO nano-hybrid assembly for solar assisted photo-degradation of chlorzoxazone. Chemosphere 2022, 305, 135472. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.; Thakur, M.; Pathania, D.; Sharma, A. Fabrication of high visible light active LaFeO3/Cl-g-C3N4/RGO heterojunction for solar assisted photo-degradation of aceclofenac. J. Environ. Chem. Eng. 2022, 10, 108098. [Google Scholar] [CrossRef]
- Li, D.; Kassymova, M.; Cai, X.; Zang, S.-Q.; Jiang, H.-L. Photocatalytic CO2 reduction over metal-organic framework-based materials. Coord. Chem. Rev. 2020, 412, 213262. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.S.; Liu, T.F.; Ye, J. Titanium-based MOF materials: From crystal engineering to photocatalysis. Small Methods 2020, 4, 2000486. [Google Scholar] [CrossRef]
- Wang, X.S.; Li, L.; Li, D.; Ye, J. Recent progress on exploring stable metal–organic frameworks for photocatalytic solar fuel production. Sol. RRL 2020, 4, 1900547. [Google Scholar] [CrossRef]
- Lei, Q.; Yuan, H.; Du, J.; Ming, M.; Yang, S.; Chen, Y.; Lei, J.; Han, Z. Photocatalytic CO2 reduction with aminoanthraquinone organic dyes. Nat. Commun. 2023, 14, 1087. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, C.; Yang, Z.; Wei, J. Encapsulated CdSe/CdS nanorods in double-shelled porous nanocomposites for efficient photocatalytic CO2 reduction. Nat. Commun. 2022, 13, 6466. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Kan, L.; Zhang, L.; Huang, Q.; Yan, Y.; Chen, Y.; Liu, J.; Li, S.L.; Lan, Y.Q. Linking oxidative and reductive clusters to prepare crystalline porous catalysts for photocatalytic CO2 reduction with H2O. Nat. Commun. 2022, 13, 4681. [Google Scholar] [CrossRef]
- Lv, J.; Xie, J.; Mohamed, A.G.A.; Zhang, X.; Feng, Y.; Jiao, L.; Zhou, E.; Yuan, D.; Wang, Y. Solar utilization beyond photosynthesis. Nat. Rev. Chem. 2022, 7, 91–105. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, H.; Zhu, X.W.; Lu, W.; Li, D. Metal-organic frameworks as photoluminescent biosensing platforms: Mechanisms and applications. Chem. Soc. Rev. 2021, 50, 4484–4513. [Google Scholar] [CrossRef]
- Li, L.; Fang, Z.-B.; Deng, W.; Yi, J.-D.; Wang, R.; Liu, T.-F. Precise Construction of Stable Bimetallic Metal–Organic Frameworks with Single-Site Ti(IV) Incorporation in Nodes for Efficient Photocatalytic Oxygen Evolution. CCS Chem. 2022, 4, 2782–2792. [Google Scholar] [CrossRef]
- Datta, S.J.; Mayoral, A.; Murthy Srivatsa Bettahalli, N.; Bhatt, P.M.; Karunakaran, M.; Carja, I.D.; Fan, D.; Graziane, M.M.P.; Semino, R.; Maurin, G.; et al. Rational design of mixed-matrix metal-organic framework membranes for molecular separations. Science 2022, 376, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.; Meng, L.; Imaz, I.; Maspoch, D. Self-assembly of colloidal metal-organic framework (MOF) particles. Chem. Soc. Rev. 2023, 52, 2528–2543. [Google Scholar] [CrossRef]
- Hu, N.; Cai, Y.; Li, L.; Wang, X.; Gao, J. Amino-functionalized titanium based metal-organic framework for photocatalytic hydrogen production. Molecules 2022, 27, 4241. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yu, Q.; Chen, J.; Khan, U.; Wang, X.; Gao, J. Achievements and perspectives in metal–organic framework-based materials for photocatalytic nitrogen reduction. Catalysts 2022, 12, 1005. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Ferrer, B.; Garcia, H. Metal-Organic Frameworks as Photocatalysts for Solar-Driven Overall Water Splitting. Chem. Rev. 2023, 123, 445–490. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, J.; Wang, L.; Sun, P.; Wang, P.; Yao, Z.; Yang, Y. Coupling bimetallic NiMn-MOF nanosheets on NiCo2O4 nanowire arrays with boosted electrochemical performance for hybrid supercapacitor. Mater. Res. Bull. 2022, 149, 111707. [Google Scholar] [CrossRef]
- Yu, Z.; Kang, S.; Wang, J.; Tai, M.; Wang, X.; Ding, Y.; Jin, D.; Wang, L. Highly improved photoluminescence properties of novel ternary Eu(cpioa)phen metal–organic frameworks. Funct. Mater. Lett. 2022, 15, 2251026. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, J.; Huang, J.; Wang, L.; Wang, P.; Cai, C.; Lu, M.; Yao, Z.; Yang, Y. Bimetallic MOF-derived (CuCo)Se nanoparticles embedded in nitrogen-doped carbon framework with boosted electrochemical performance for hybrid supercapacitor. Mater. Res. Bull. 2021, 137, 111196. [Google Scholar] [CrossRef]
- Lei, Z.; Tang, Q.; Ju, Y.; Lin, Y.; Bai, X.; Luo, H.; Tong, Z. Block copolymer@ZIF-8 nanocomposites as a pH-responsive multi-steps release system for controlled drug delivery. J. Biomater. Sci. Polym. Ed. 2020, 31, 695–711. [Google Scholar] [CrossRef]
- Li, G.; Cai, H.; Li, X.; Zhang, J.; Zhang, D.; Yang, Y.; Xiong, J. Construction of hierarchical NiCo2O4@Ni-MOF hybrid arrays on carbon cloth as superior battery-type electrodes for flexible solid-state hybrid supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 37675–37684. [Google Scholar] [CrossRef]
- He, H.; Wang, X.; Yu, Q.; Wu, W.; Feng, X.; Kong, D.; Ren, X.; Gao, J. In Situ Growth of Ti3C2/UiO-66-NH2 Composites for Photoreduction of Cr(VI). Catalysts 2023, 13, 876. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Chen, C.; Li, H.; Huang, Y.; Cao, R. Graphene quantum dots supported on Fe-based metal-organic frameworks for efficient photocatalytic CO2 reduction. Acta Chim. Sin. 2022, 80, 22–28. [Google Scholar] [CrossRef]
- Lin, J.B.; Nguyen, T.T.T.; Vaidhyanathan, R.; Burner, J.; Taylor, J.M.; Durekova, H.; Akhtar, F.; Mah, R.K.; Ghaffari-Nik, O.; Marx, S.; et al. A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture. Science 2021, 374, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, J.; Liu, J.J.; Dong, L.Z.; Xin, Z.F.; Teng, Y.L.; Lan, Y.Q. Adenine Components in Biomimetic Metal-Organic Frameworks for Efficient CO2 Photoconversion. Angew. Chem. Int. Ed. 2019, 58, 5226–5231. [Google Scholar] [CrossRef]
- Chen, E.X.; Qiu, M.; Zhang, Y.F.; He, L.; Sun, Y.Y.; Zheng, H.L.; Wu, X.; Zhang, J.; Lin, Q. Energy Band Alignment and Redox-Active Sites in Metalloporphyrin-Spaced Metal-Catechol Frameworks for Enhanced CO2 Photoreduction. Angew. Chem. Int. Ed. 2022, 61, e202111622. [Google Scholar]
- Jiang, Z.; Xu, X.; Ma, Y.; Cho, H.S.; Ding, D.; Wang, C.; Wu, J.; Oleynikov, P.; Jia, M.; Cheng, J.; et al. Filling Metal-Organic Framework Mesopores with TiO2 for CO2 Photoreduction. Nature 2020, 586, 549–554. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Y.-Z.; Li, X.; Wei, Z.; Xu, Y.-W.; Zhang, L.; Su, C.-Y. A porous rhodium(III)-porphyrin metal-organic framework as an efficient and selective photocatalyst for CO2 reduction. Appl. Catal. B Environ. 2018, 231, 173–181. [Google Scholar] [CrossRef]
- Yan, Z.H.; Du, M.H.; Liu, J.; Jin, S.; Wang, C.; Zhuang, G.L.; Kong, X.J.; Long, L.S.; Zheng, L.S. Photo-generated dinuclear {Eu(II)}2 active sites for selective CO2 reduction in a photosensitizing metal-organic framework. Nat. Commun. 2018, 9, 3353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.Z.; Zhang, L.; Liu, J.; Huang, Q.; Lu, M.; Ji, W.X.; Lan, Y.Q. Stable Heterometallic Cluster-Based Organic Framework Catalysts for Artificial Photosynthesis. Angew. Chem. Int. Ed. 2020, 59, 2659–2663. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, Y.; Zhang, X.; Weinert, M.; Song, X.; Ai, J.; Han, L.; Fei, H. N-Heterocyclic Carbene-Stabilized Ultrasmall Gold Nanoclusters in a Metal-Organic Framework for Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 17388–17393. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chem. Int. Ed. 2012, 51, 3364–3367. [Google Scholar] [CrossRef]
- Xu, H.Q.; Hu, J.; Wang, D.; Li, Z.; Zhang, Q.; Luo, Y.; Yu, S.H.; Jiang, H.L. Visible-Light Photoreduction of CO2 in a Metal-Organic Framework: Boosting Electron-Hole Separation via Electron Trap States. J. Am. Chem. Soc. 2015, 137, 13440–13443. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Zhang, D.; Li, G.; Li, H. Microwave irradiation induced UIO-66-NH2 anchored on graphene with high activity for photocatalytic reduction of CO2. Appl. Catal. B Environ. 2018, 228, 47–53. [Google Scholar] [CrossRef]
- Choi, K.M.; Kim, D.; Rungtaweevoranit, B.; Trickett, C.A.; Barmanbek, J.T.; Alshammari, A.S.; Yang, P.; Yaghi, O.M. Plasmon-Enhanced Photocatalytic CO2 Conversion within Metal-Organic Frameworks under Visible Light. J. Am. Chem. Soc. 2017, 139, 356–362. [Google Scholar] [CrossRef]
- Shi, L.; Wang, T.; Zhang, H.; Chang, K.; Ye, J. Electrostatic Self-Assembly of Nanosized Carbon Nitride Nanosheet onto a Zirconium Metal-Organic Framework for Enhanced Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2015, 25, 5360–5367. [Google Scholar] [CrossRef]
- Sun, D.; Liu, W.; Fu, Y.; Fang, Z.; Sun, F.; Fu, X.; Zhang, Y.; Li, Z. Noble metals can have different effects on photocatalysis over metal-organic frameworks (MOFs): A case study on M/NH2-MIL-125(Ti) (M=Pt and Au). Chem. Eur. J. 2014, 20, 4780–4788. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, X.; Feng, J.; Chen, Y.; Yang, X.; Gao, S.; Cao, R. Synthesis and characterization of Zn2GeO4/Mg-MOF-74 composites with enhanced photocatalytic activity for CO2 reduction. Catal. Sci. Technol. 2018, 8, 1288–1295. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Chen, N.; Qu, L. Graphene quantum dots: An emerging material for energy-related applications and beyond. Energy Environ. Sci. 2012, 5, 8869. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef]
- Ming, H.; Ma, Z.; Liu, Y.; Pan, K.; Yu, H.; Wang, F.; Kang, Z. Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property. Dalton Trans. 2012, 41, 9526–9531. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Gu, Z.G.; Li, D.J.; Zheng, C.; Kang, Y.; Woll, C.; Zhang, J. MOF-Templated Synthesis of Ultrasmall Photoluminescent Carbon-Nanodot Arrays for Optical Applications. Angew. Chem. Int. Ed. 2017, 56, 6853–6858. [Google Scholar] [CrossRef]
- Wang, X.-S.; Chen, C.-H.; Ichihara, F.; Oshikiri, M.; Liang, J.; Li, L.; Li, Y.; Song, H.; Wang, S.; Zhang, T.; et al. Integration of adsorption and photosensitivity capabilities into a cationic multivariate metal-organic framework for enhanced visible-light photoreduction reaction. Appl. Catal. B Environ. 2019, 253, 323–330. [Google Scholar] [CrossRef]
- Huang, H.; Wang, X.-S.; Philo, D.; Ichihara, F.; Song, H.; Li, Y.; Li, D.; Qiu, T.; Wang, S.; Ye, J. Toward visible-light-assisted photocatalytic nitrogen fixation: A titanium metal organic framework with functionalized ligands. Appl. Catal. B Environ. 2020, 267, 118686. [Google Scholar] [CrossRef]
- Chen, E.X.; Qiu, M.; Zhang, Y.F.; Zhu, Y.S.; Liu, L.Y.; Sun, Y.Y.; Bu, X.; Zhang, J.; Lin, Q. Acid and Base Resistant Zirconium Polyphenolate-Metalloporphyrin Scaffolds for Efficient CO2 Photoreduction. Adv. Mater. 2018, 30, 1704388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, J.; Dong, J.; Liu, G.; Shi, L.; An, P.; Zhao, G.; Kong, J.; Wang, X.; Meng, X.; et al. Efficient Visible-Light-Driven Carbon Dioxide Reduction by a Single-Atom Implanted Metal-Organic Framework. Angew. Chem. Int. Ed. 2016, 55, 14310–14314. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, J.; Feng, L.; Wang, Q.; Guan, W.; Dong, L.Z.; Zhang, L.; Yan, L.K.; Lan, Y.Q.; Zhou, H.C. Multielectron transportation of polyoxometalate-grafted metalloporphyrin coordination frameworks for selective CO2-to-CH4 photoconversion. Natl. Sci. Rev. 2020, 7, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Fang, Z.; Li, L.; Yang, H.; Liu, T.F. Creating Chemisorption Sites for Enhanced CO2 Photoreduction Activity through Alkylamine Modification of MIL-101-Cr. ACS Appl. Mater. Interfaces 2019, 11, 27017–27023. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.-C.; Liao, J.-F.; Dong, Y.-J.; Xu, Y.-F.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. Core@Shell CsPbBr3@Zeolitic Imidazolate Framework Nanocomposite for Efficient Photocatalytic CO2 Reduction. ACS Energy Lett. 2018, 3, 2656–2662. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Zhu, L.; Li, Y.; Yan, Z.; Shen, Z.; Cao, X. Facile microwave assisted synthesis of N-rich carbon quantum dots/dual-phase TiO2 heterostructured nanocomposites with high activity in CO2 photoreduction. Appl. Catal. B Environ. 2018, 231, 269–276. [Google Scholar] [CrossRef]
- Li, H.; Deng, Y.; Liu, Y.; Zeng, X.; Wiley, D.; Huang, J. Carbon quantum dots and carbon layer double protected cuprous oxide for efficient visible light CO2 reduction. Chem. Commun. 2019, 55, 4419–4422. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Wang, X.; Wu, W.; Feng, X.; Kong, D.; Khan, U.; Ren, X.; Li, L. In Situ Encapsulation of Graphene Quantum Dots in Highly Stable Porphyrin Metal-Organic Frameworks for Efficient Photocatalytic CO2 Reduction. Molecules 2023, 28, 4703. https://doi.org/10.3390/molecules28124703
Yu Q, Wang X, Wu W, Feng X, Kong D, Khan U, Ren X, Li L. In Situ Encapsulation of Graphene Quantum Dots in Highly Stable Porphyrin Metal-Organic Frameworks for Efficient Photocatalytic CO2 Reduction. Molecules. 2023; 28(12):4703. https://doi.org/10.3390/molecules28124703
Chicago/Turabian StyleYu, Qin, Xusheng Wang, Wenbin Wu, Xinya Feng, Deyu Kong, Usman Khan, Xiaohui Ren, and Lan Li. 2023. "In Situ Encapsulation of Graphene Quantum Dots in Highly Stable Porphyrin Metal-Organic Frameworks for Efficient Photocatalytic CO2 Reduction" Molecules 28, no. 12: 4703. https://doi.org/10.3390/molecules28124703




