Cytotoxic Compounds from Belamcanda chinensis (L.) DC Induced Apoptosis in Triple-Negative Breast Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
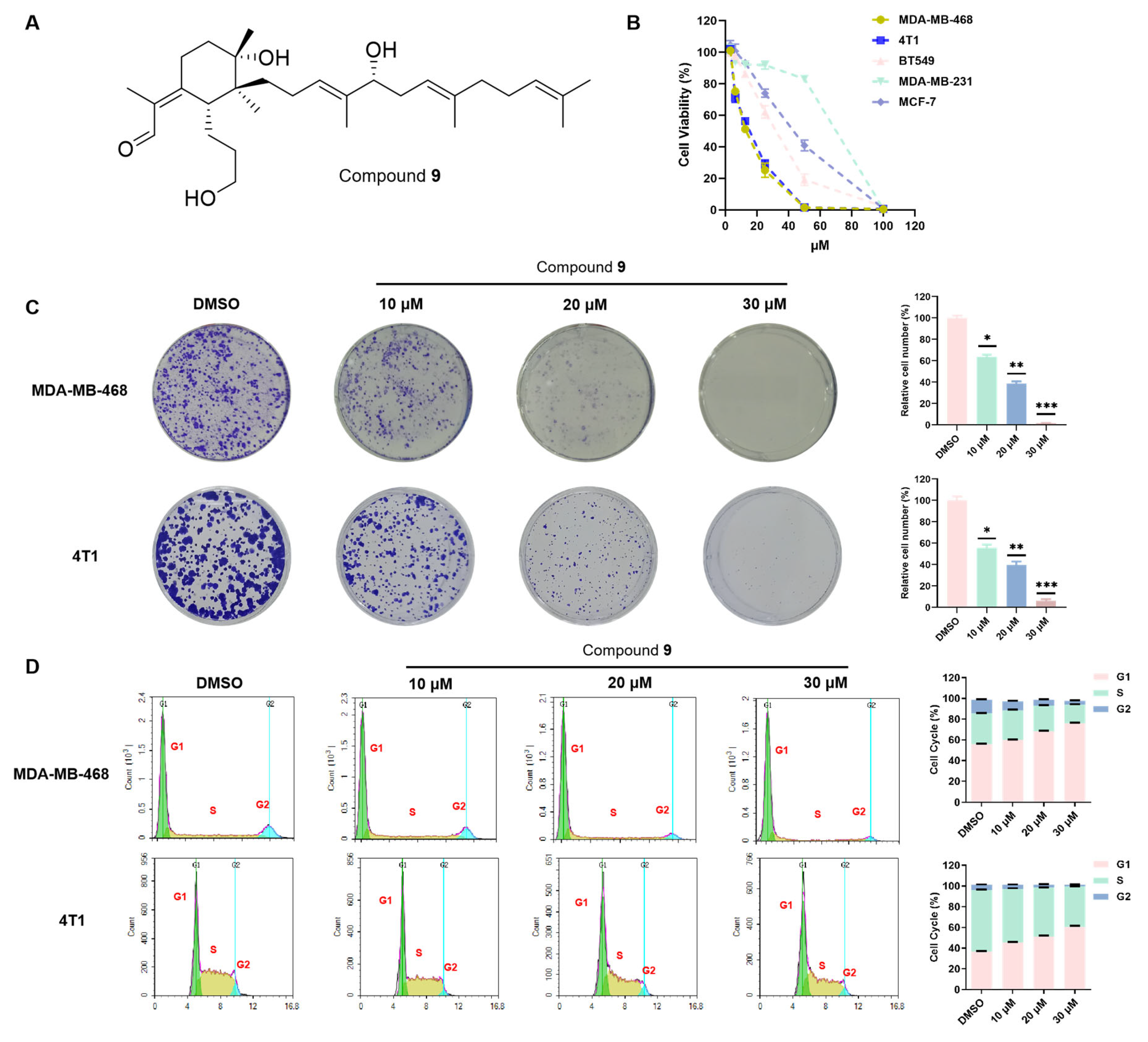
2.1. Identification of Compounds 1–10
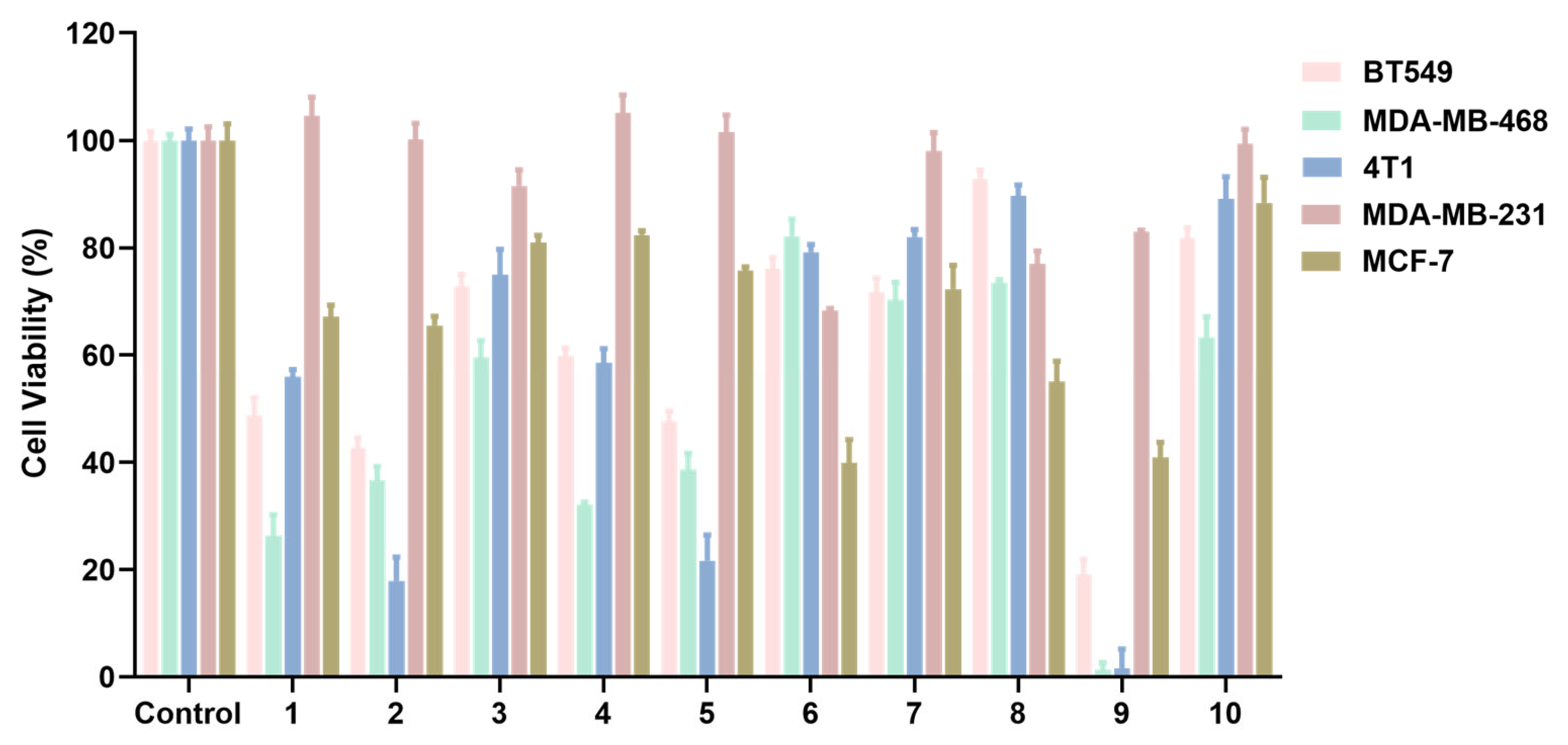
2.2. Effects of Compounds 1–10 on the Proliferation of Breast Cancer Cells
2.3. Compound 9 Inhibited TNBC Cell Proliferation
2.4. Compound 9 Suppressed Metastasis in the TNBC Cells
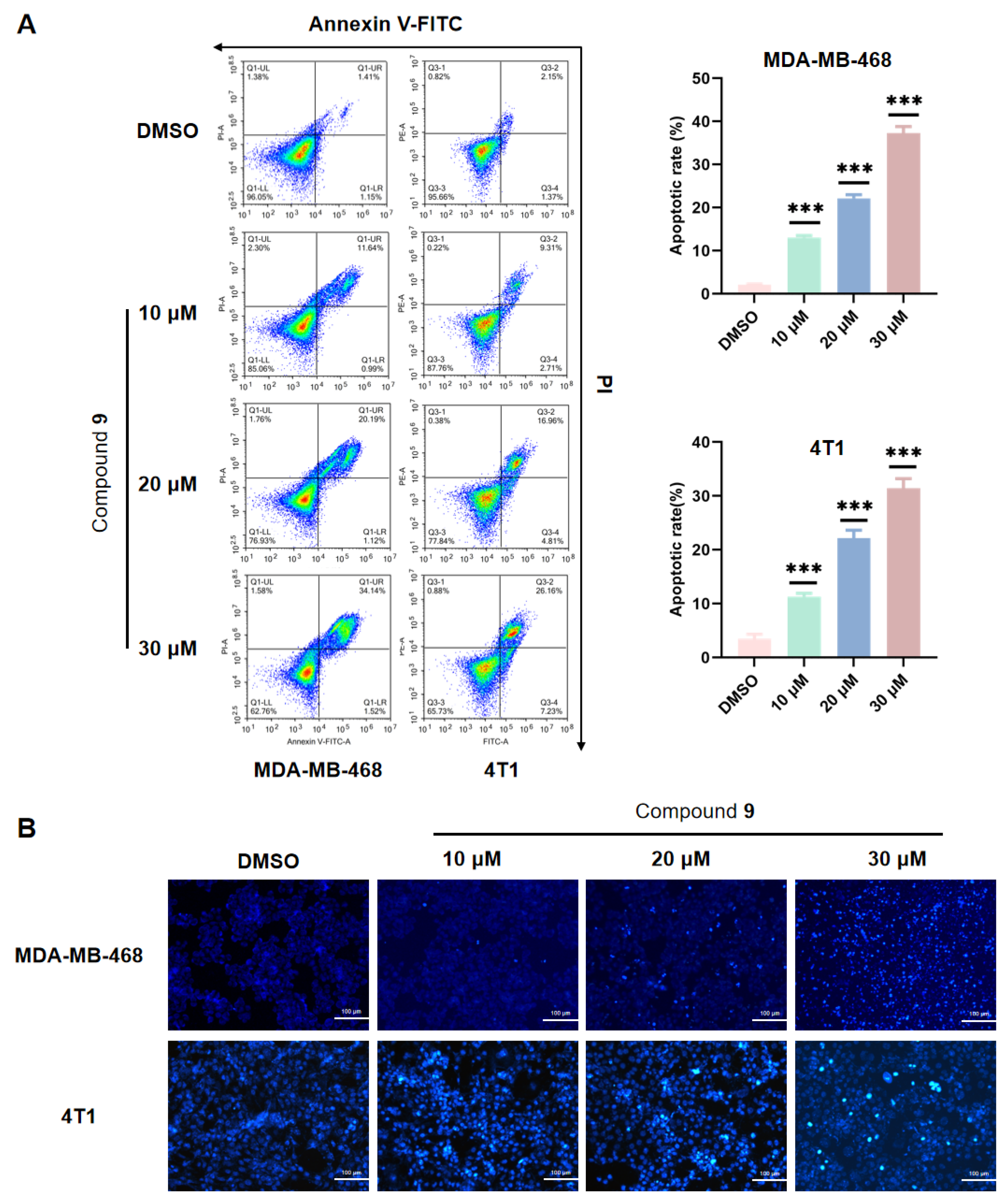
2.5. Effect of Compound 9 on TNBC Cell Apoptosis
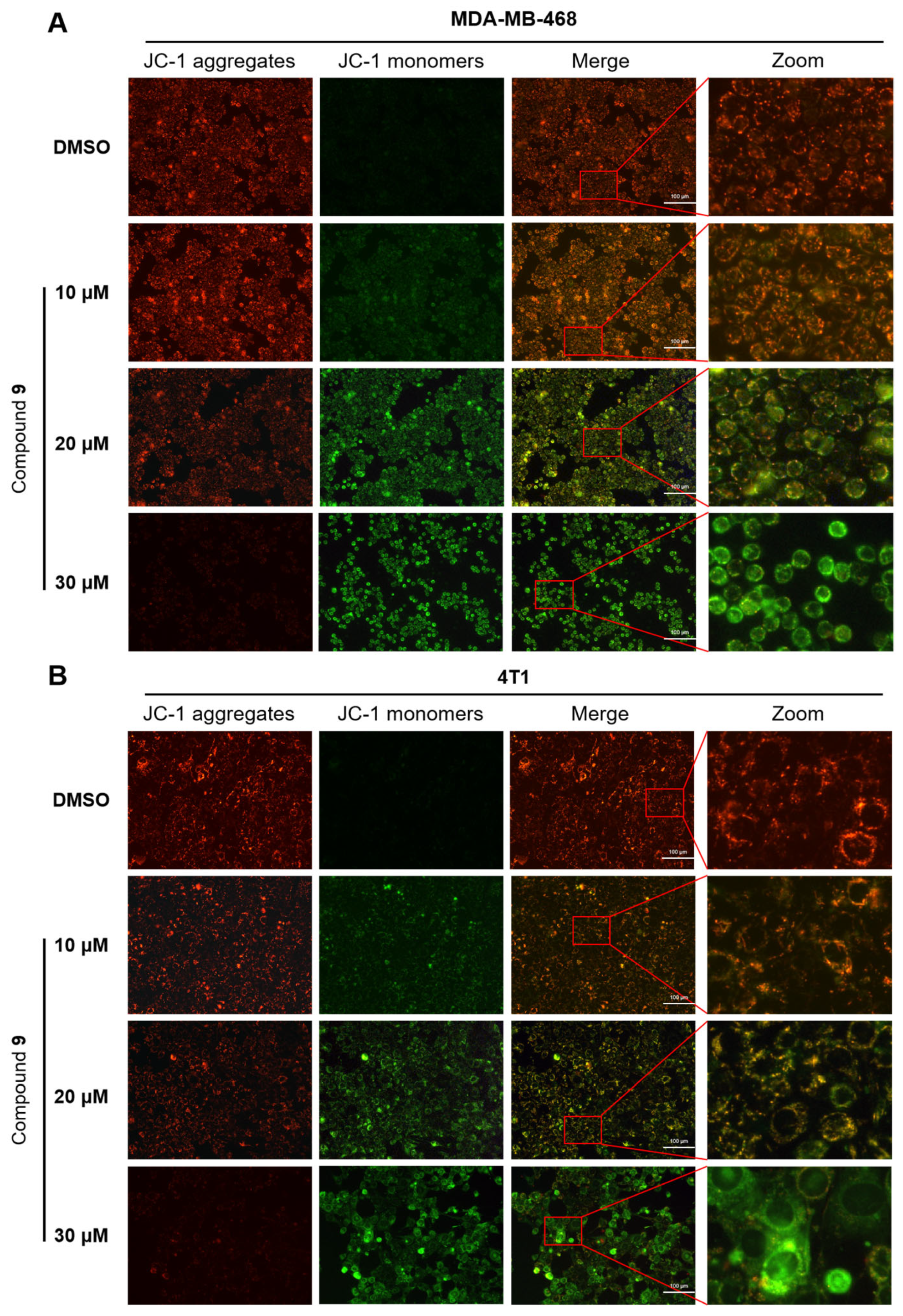
2.6. Effect of Compound 9 on Mitochondrial Membrane Potential Level
2.7. Effect of Compound 9 on ROS Level
3. Materials and Methods
3.1. Separation and Purification
3.2. Cell Culture
3.3. Cell Viability Assay
3.4. Colony Formation
3.5. Cell Cycle Analysis
3.6. Wound-Healing Assay
3.7. Transwell Assay
3.8. Annexin V-FITC/PI Double Staining Assay
3.9. Hoechst 33342 Staining
3.10. JC-1 Staining
3.11. Measurement of Intracellular ROS Levels
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Woźniak, D.; Matkowski, A. Belamcandae chinensis rhizome—A review of phytochemistry and bioactivity. Fitoterapia 2015, 107, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, X. Drug characteristics, regularity and application of Shennong’s Classic of Materia Medica in the treatment of cough with dyspnea. Chin. Med. Her. 2021, 18, 4. [Google Scholar]
- Mavrodiev, E.V.; Martínez-Azorín, M.; Dranishnikov, P. At least 23 genera instead of one: The case of Iris L. s.l. (iridaceae). PLoS ONE 2014, 9, e106459. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Chen, T. Chinese Medical Herbology and Pharmacology; Art of Medicine Press: Industry, CA, USA, 2006. [Google Scholar]
- Hempen, C.H.; Fischer, T. A Materia Medica for Chinese Medicine: Plants, Minerals, and Animal Products, Churchill Livingstone; Elsevier Health Sciences: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Xiong, H.; Gao, M.; Yang, Y.F. Research status and thinking of the active components for heat-clearing and detoxification in Belamcandae Rhizoma and Iridis tectori Rhizoma. Chin. J. Hosp. Pharm. 2023, 43, 576–583. [Google Scholar]
- Jin, L.; Chen, H.S.; Jin, Y.S.; Liang, S.; Xiang, Z.B.; Lu, J. Chemical constituents from Belamcanda chinensis. J. Asian Nat. Prod. Res. 2008, 10, 89–94. [Google Scholar] [CrossRef]
- Peng, C.; Liang, Y.; Wang, X. Preparative Isolation and Purification of Flavonoids from the Chinese Medicinal Herb Belamcanda by High-Speed Countercurrent Chromatography. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 2451–2461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, K.; Xu, J.; Yang, D.; Zhang, C.; Wang, Z.; Li, M. Belamcanda chinensis (L.) DC-An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2016, 186, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, C.M.; Dai, R.J.; Li, L.; Yu, Y.H.; Li, Y.; Meng, W.W.; Zhang, L.; Zhang, Y.; Deng, Y.L. Combination of HPLC chromatogram and hypoglycemic effect identifies isoflavones as the principal active fraction of Belamcanda chinensis leaf extract in diabetes treatment. J. Chromatogr. B 2011, 879, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T.; Mizuno, T. Flavonoids and Xanthones from the Genus Iris: Phytochemistry, Relationships with Flower Colors and Taxonomy, and Activities and Function. Nat. Prod. Commun. 2020, 15, 1934578X20937151. [Google Scholar]
- Mykchailenko, O.O.; Kovalyov, M.V. Phenolic compounds of the genus Iris plants (Iridaceae). Ceska. Slov. Farm. 2016, 65, 70–77. [Google Scholar] [PubMed]
- Zhou, L.X.; Lin, M. A new stilbene dimer-shegansu B from Belamcanda chinensis. J. Asian Nat. Prod. Res. 2000, 2, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Hoshino, Y.; Suzuki, S.; Hano, Y.; Nomura, T. Iridals from Iris tectorum and Belamcanda chinensis. Phytochemistry 2000, 53, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Onoue, S.; Miyake, Y.; Yoshida, T. Iridal-type triterpenoids with ichthyotoxic activity from Belamcanda Chinensis. J. Nat. Prod. 1999, 62, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ni, G.; Liu, Y.; Mai, Z.; Wang, R.; Yu, D. Iridal-Type Triterpenoids with a Cyclopentane Unit from the Rhizomes of Belamcanda chinensis. J. Nat. Prod. 2019, 82, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ni, G.; Liu, Y.; Wang, R.; Yu, D. New iridal-type triterpenoid analogues with 6/5/6-fused carbon skeleton from the rhizomes of Belamcanda chinensis. Fitoterapia 2022, 157, 105040. [Google Scholar] [CrossRef]
- Li, J.; Ni, G.; Liu, Y.; Mai, Z.; Yu, D. Seconoriridone A: A C16-seco-noriridal derivative with a 5/5/7 tricyclic skeleton from Belamcanda chinensis. Tetrahedron. Lett. 2019, 60, 900–905. [Google Scholar] [CrossRef]
- Li, J.; Ni, G.; Li, L.; Liu, Y.; Mai, Z.; Wang, R.; Yu, D. New iridal-type triterpenoid derivatives with cytotoxic activities from Belamcanda chinensis. Bioorg. Chem. 2019, 83, 20–28. [Google Scholar] [CrossRef]
- Song, Y.Y.; Jiang, J.B.; Yan, Y.M.; Cheng, Y.X. Isolation and identification of belamcandaoids A-N from Belamcanda chinensis seeds and their inhibition on extracellular matrix in TGF-beta1 induced kidney proximal tubular cells. Bioorg. Chem. 2021, 114, 105067. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wang, Q.; Qi, L.W.; Qin, X.Y.; Qin, M.J. Characterization and determination of the major constituents in Belamcandae Rhizoma by HPLC-DAD-ESI-MS(n). J. Pharm. Biomed. Anal. 2011, 56, 304–314. [Google Scholar] [CrossRef]
- Yamaki, K.; Kim, D.H.; Ryu, N.; Kim, Y.P.; Shin, K.H.; Ohuchi, K. Effects of naturally occurring isoflavones on prostaglandin E2 production. Planta Med. 2002, 68, 97–100. [Google Scholar] [CrossRef]
- Jung, S.H.; Lee, Y.S.; Lee, S.; Lim, S.S.; Kim, Y.S.; Ohuchi, K.; Shin, K.H. Anti-angiogenic and anti-tumor activities of isoflavonoids from the rhizomes of Belamcanda chinensis. Planta Med. 2003, 69, 617–622. [Google Scholar]
- Guha, S.; Ghosal, S.; Chattopadhyay, U. Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a naturally occurring glucosylxanthone. Chemotherapy 1996, 42, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Noh, E.J.; Cha, K.H.; Kim, Y.S.; Lim, S.S.; Shin, K.H.; Jung, S.H. Inhibitory effects of Irigenin from the rhizomes of Belamcanda chinensis on nitric oxide and prostaglandin E2 production in murine macrophage RAW 264.7 cells. Life Sci. 2006, 78, 2336–2342. [Google Scholar] [CrossRef]
- Pan, C.H.; Kim, E.S.; Jung, S.H.; Nho, C.W.; Lee, J.K. Tectorigenin inhibits IFN-gamma/LPS-induced inflammatory responses in murine macrophage RAW 264.7 cells. Arch. Pharm. Res. 2008, 31, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Leiro, J.; Arranz, J.A.; Yáñez, M.; Ubeira, F.M.; Sanmartín, M.L.; Orallo, F. Expression profiles of genes involved in the mouse nuclear factor-kappa B signal transduction pathway are modulated by mangiferin. Int. Immunopharmacol. 2004, 4, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Garrido, G.; González, D.; Lemus, Y.; Delporte, C.; Delgado, R. Protective effects of a standard extract of Mangifera indica L. (VIMANG) against mouse ear edemas and its inhibition of eicosanoid production in J774 murine macrophages. Phytomedicine 2006, 13, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, H.S.; Candelario-Jalil, E.; de Oliveira, A.C.; Olajide, O.A.; Martínez-Sánchez, G.; Fiebich, B.L. Mangiferin inhibits cyclooxygenase-2 expression and prostaglandin E2 production in activated rat microglial cells. Arch. Biochem. Biophys. 2008, 477, 253–258. [Google Scholar] [CrossRef]
- Wang, Q.L.; Lin, M.; Liu, G.T. Antioxidative activity of natural isorhapontigenin. Jpn. J. Pharmacol. 2001, 87, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Kim, S.Y.; Kim, H.S.; Kim, D.H.; Hyun, J.W. Cytoprotective effect of tectorigenin, a metabolite formed by transformation of tectoridin by intestinal microflora, on oxidative stress induced by hydrogen peroxide. Eur. J. Pharmacol. 2005, 519, 16–23. [Google Scholar] [CrossRef]
- Wozniak, D.; Janda, B.; Kapusta, I.; Oleszek, W.; Matkowski, A. Antimutagenic and anti-oxidant activities of isoflavonoids from Belamcanda chinensis (L.) DC. Mutat. Res. 2010, 696, 148–153. [Google Scholar] [CrossRef]
- Woźniak, D.; Kuś, P.; Goralska, E.; Matkowski, A. Antimutagenic and antioxidant properties of natural products from the Chinese herbal medicine—Belamcandae rhizome. Acta Biochim. Pol. 2011, 58, 23–26. [Google Scholar]
- Lautraite, S.; Musonda, A.C.; Doehmer, J.; Edwards, G.O.; Chipman, J.K. Flavonoids inhibit genetic toxicity produced by carcinogens in cells expressing CYP1A2 and CYP1A1. Mutagenesis 2002, 17, 45–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakura, A.; Shimada, H.; Nakajima, M.; Sui, H.; Kitamoto, S.; Suzuki, S.; Satoh, T. Salmonella/human S9 mutagenicity test: A collaborative study with 58 compounds. Mutagenesis 2005, 20, 217–228. [Google Scholar] [CrossRef]
- Matsushima, T.; Araki, A.; Yagame, O.; Muramatsu, M.; Koyama, K.; Ohsawa, K.; Natori, S.; Tomimori, H. Mutagenicities of xanthone derivatives in Salmonella typhimurium TA100, TA98, TA97, and TA2637. Mutat. Res. 1985, 150, 141–146. [Google Scholar] [CrossRef]
- De-Eknamkul, W.; Umehara, K.; Monthakantirat, O.; Toth, R.; Frecer, V.; Knapic, L.; Braiuca, P.; Noguchi, H.; Miertus, S. QSAR study of natural estrogen-like isoflavonoids and diphenolics from Thai medicinal plants. J. Mol. Graph. Model. 2011, 29, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Morito, K.; Aomori, T.; Hirose, T.; Kinjo, J.; Hasegawa, J.; Ogawa, S.; Inoue, S.; Muramatsu, M.; Masamune, Y. Interaction of phytoestrogens with estrogen receptors alpha and beta (II). Biol. Pharm. Bull. 2002, 25, 48–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Li, Y.; Chen, Y.; Lao, X.; Sheng, L.; Dai, R.; Meng, W.; Deng, Y. Hypoglycemic effect of Belamcanda chinensis leaf extract in normal and STZ-induced diabetic rats and its potential active faction. Phytomedicine 2011, 18, 292–297. [Google Scholar] [CrossRef]
- Wu, C.; Shen, J.; He, P.; Chen, Y.; Li, L.; Zhang, L. The α-glucosidase inhibiting isoflavones isolated from Belamcanda chinensis leaf extract, Rec. Nat. Prod. 2012, 6, 110–120. [Google Scholar]
- Jung, S.H.; Lee, Y.S.; Lee, S.; Lim, S.S.; Kim, Y.S.; Shin, K.H. Isoflavonoids from the rhizomes of Belamcanda chinensis and their effects on aldose reductase and sorbitol accumulation in streptozotocin induced diabetic rat tissues. Arch. Pharm. Res. 2002, 25, 306–312. [Google Scholar] [CrossRef]
- Muruganandan, S.; Srinivasan, K.; Gupta, S.; Gupta, P.K.; Lal, J. Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J. Ethnopharmacol. 2005, 97, 497–501. [Google Scholar] [CrossRef]
- Oh, K.B.; Kang, H.; Matsuoka, H. Detection of antifungal activity in Belamcanda Chinensis by a single-cell bioassay method and isolation of its active compound, tectorigenin. Biosci. Biotechnol. Biochem. 2001, 65, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.L. Effects of Tectorigenin on proliferation of cervical cancer Caski cells. Med. J. Chin. Peop. Heal. 2014, 26, 6–8. [Google Scholar]
- Guo, Y.; Chen, Y.H.; Cheng, Z.H.; Ou-Yang, H.N.; Luo, C.; Guo, Z.L. Tectorigenin inhibits osteosarcoma cell migration through downregulation of matrix metalloproteinases in vitro. Anticancer. Drugs. 2016, 27, 540–546. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.F.; Liu, L.; Chen, Y.X.; Liang, J.Q.; Liu, Y.Y.; Te, B.Q. Study on the inhibitory effect of Belamcanda Chinensis extract on the malignant behavior of lung cancer cells. Forum. Tradit. Chin. Med. 2018, 33, 57–59. [Google Scholar]
- Xiong, F.L. Effects of tectoridin, a blackberry lily-Extract of chinese traditional medicine on the suppression of the proliferation and invasion in human colon Cancer Cells. Hubei Univ. Chin. Med. 2016, 11–49. [Google Scholar]
- Morrissey, C.; Bektic, J.; Spengler, B.; Galvin, D.; Christoffel, V.; Klocker, H.; Fitzpatrick, J.M.; Watson, R.W. Phytoestrogens derived from Belamcanda Chinensis have an antiproliferative effect on prostate cancer cells in vitro. J. Urol. 2004, 172, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- Hasibeder, A.; Venkataramani, V.; Thelen, P.; Radzun, H.J.; Schweyer, S. Phytoestrogens regulate the proliferation and expression of stem cell factors in cell lines of malignant testicular germ cell tumors. Int. J. Oncol. 2013, 43, 1385–1394. [Google Scholar] [CrossRef] [Green Version]
- Bezabih, M.; Famuyiwa, S.O.; Abegaz, B.M. HPLC analysis and NMR identification of homoisoflavonoids and stilbenoids from the inter-bulb surfaces of Scilla nervosa. Nat. Prod. Commun. 2009, 4, 1367–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.L.; Zhu, W.; Wang, D.M.; Chen, W.L.; Hu, M.M.; Wang, M.; Xu, X.J.; Lu, C.J. Inhibitory Effects of Chemical Compounds Isolated from the Rhizome of Smilax glabra on Nitric Oxide and Tumor Necrosis Factor-α Production in Lipopolysaccharide-Induced RAW264.7 Cell. Evid. Based Complement Altern. Med. 2015, 2015, 602425. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Jin, Y.S.; Chen, H.S. Phenolic constituents of Belamcanda chinensis. Chem. Nat. Comp. 2007, 43, 700–701. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, C.; Jin, Q.; Lee, M.S.; Kim, Y.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Chemical constituents from Belamcanda chinensis and their inhibitory effects on nitric oxide production in RAW 264.7 macrophage cells. Arch. Pharm. Res. 2015, 38, 991–997. [Google Scholar] [CrossRef]
- Dong, C.X.; Ke-Si, W.U.; Shi, S.P.; Tu, P.F. Flavanoids from Clematis hexapetala. J. Chi. Pharm. Sci. 2006, 15, 15–20. [Google Scholar]
- Mohamed, G.A.; Ibrahim, S.; Ross, S.A. New ceramides and isoflavone from the Egyptian Iris germanica L. rhizomes. Phytochem. Lett. 2013, 6, 340–344. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kang, M.W.; Roh, J.H.; Choi, S.U.; Zee, O.P. Cytotoxic phenolic compounds from Chionanthus retusus. Arch. Pharm. Res. 2009, 32, 1681–1687. [Google Scholar] [CrossRef]
- Shang, M.Y.; Tezuka, Y.; Cai, S.Q. Studies on triterpenoids from common Fenugreek (Trigonella foenum-graecum). Chin. Med. Herb. 1998, 29, 655–657. [Google Scholar]
- Fang, R.; Houghton, P.J.; Luo, C.; Hylands, P.J. Isolation and structure determination of triterpenes from Iris tectorum. Phytochemistry 2007, 68, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Tomihari, T.; Haga, K.; Kaneko, R. Iristectorenes A and C-G, monocyclic triterpene esters from Iris tectorum. Phytochemistry 1994, 36, 425–431. [Google Scholar] [CrossRef]
- Swanton, C. Cell-cycle targeted therapies. Lancet Oncol. 2004, 5, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Townson, J.L.; Naumov, G.N.; Chambers, A.F. The role of apoptosis in tumor progression and metastasis. Curr. Mol. Med. 2003, 3, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Stowe, D.F.; Camara, A.K. Mitochondrial reactive oxygen species production in excitable cells: Modulators of mitochondrial and cell function. Antioxid. Redox Signal. 2009, 11, 1373–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.-P.; Yi, P.; Shi, Q.-Q.; Yu, R.-R.; Wang, J.-H.; Li, C.-Y.; Wu, H.-Q. Cytotoxic Compounds from Belamcanda chinensis (L.) DC Induced Apoptosis in Triple-Negative Breast Cancer Cells. Molecules 2023, 28, 4715. https://doi.org/10.3390/molecules28124715
Guo Y-P, Yi P, Shi Q-Q, Yu R-R, Wang J-H, Li C-Y, Wu H-Q. Cytotoxic Compounds from Belamcanda chinensis (L.) DC Induced Apoptosis in Triple-Negative Breast Cancer Cells. Molecules. 2023; 28(12):4715. https://doi.org/10.3390/molecules28124715
Chicago/Turabian StyleGuo, Ya-Ping, Peng Yi, Qi-Qi Shi, Rui-Rui Yu, Jin-Hui Wang, Chen-Yang Li, and Hai-Qiang Wu. 2023. "Cytotoxic Compounds from Belamcanda chinensis (L.) DC Induced Apoptosis in Triple-Negative Breast Cancer Cells" Molecules 28, no. 12: 4715. https://doi.org/10.3390/molecules28124715




