The Effect of Terbinafine and Its Ionic Salts on Certain Fungal Plant Pathogens
Abstract
:1. Introduction
2. Results and Discussion
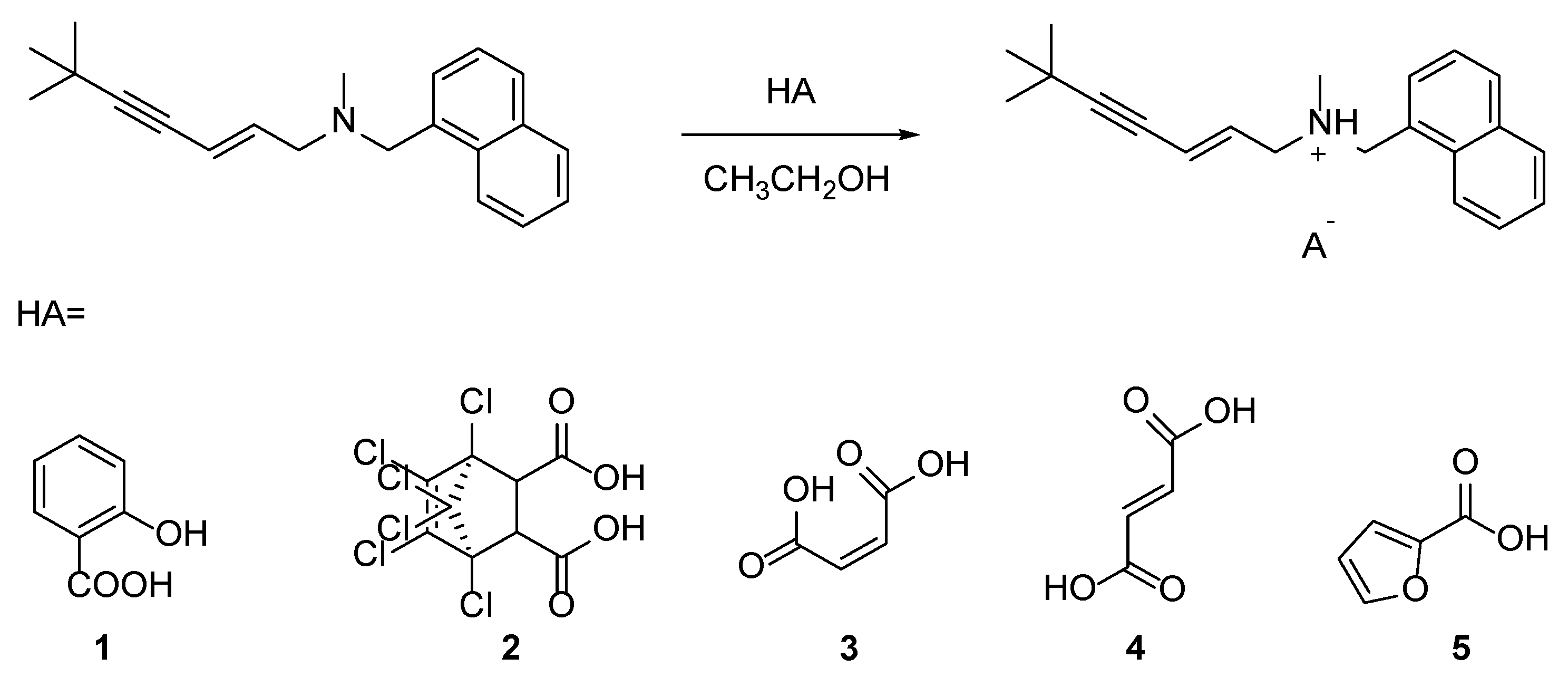
2.1. Salt Preparation
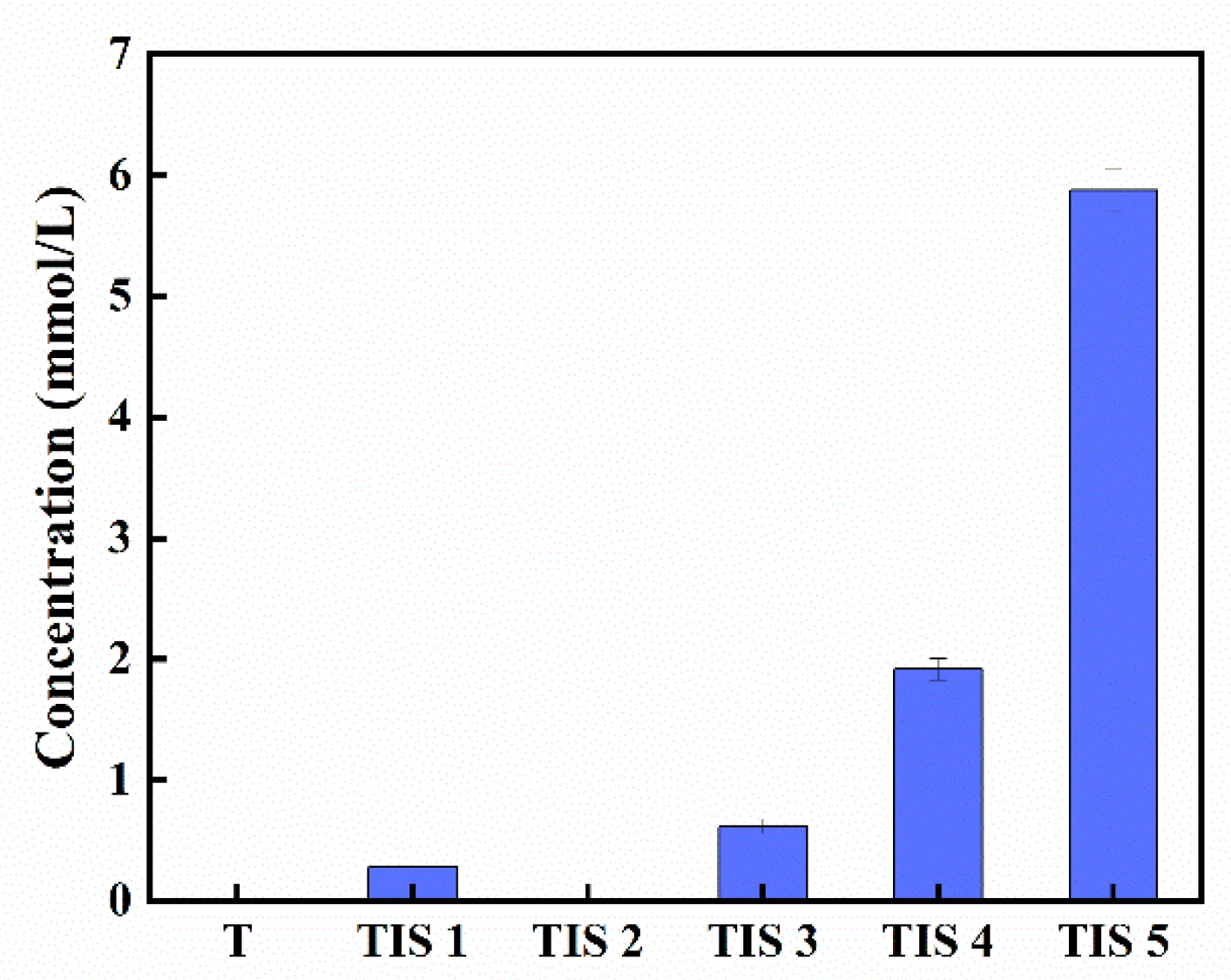
2.2. Solubilities
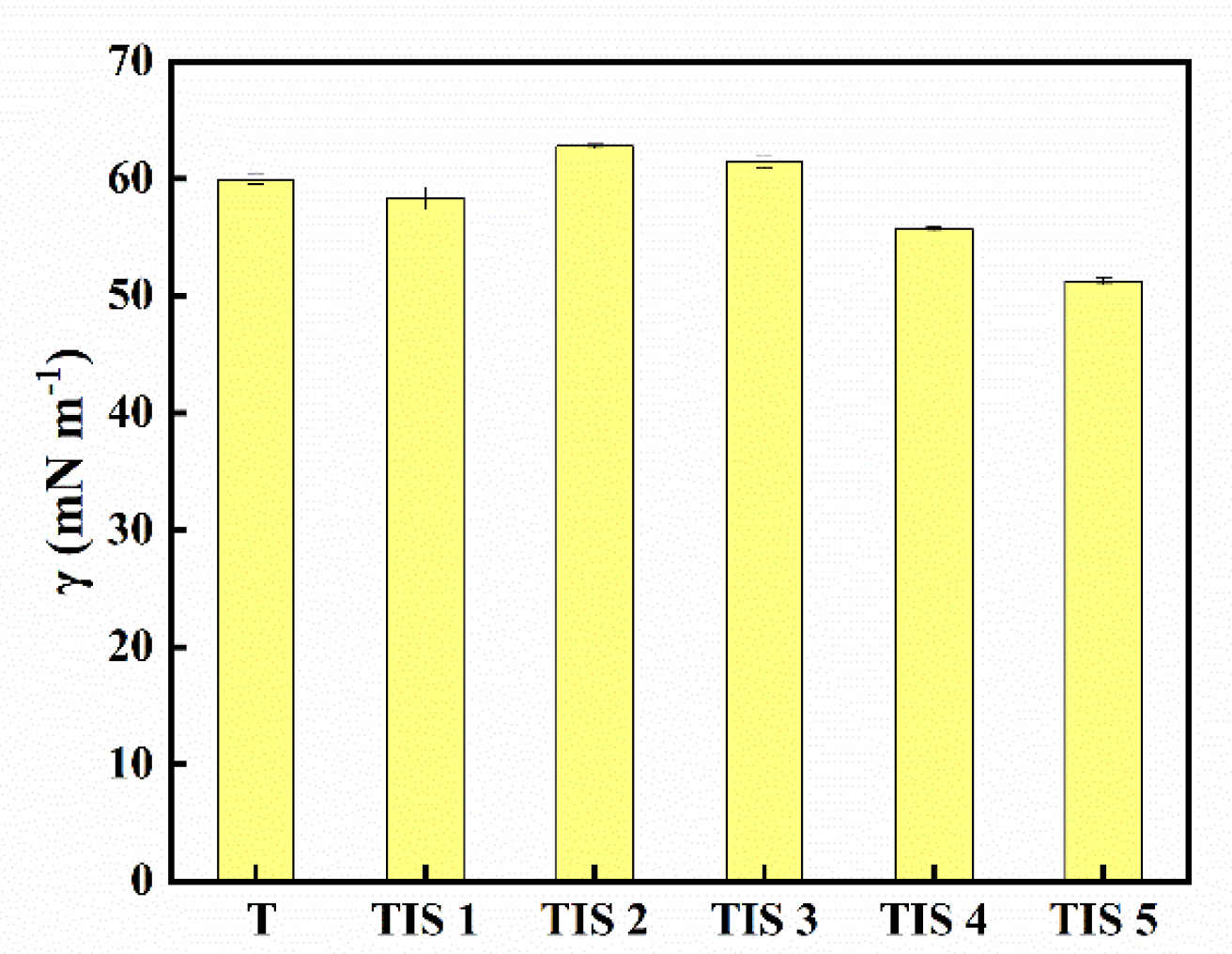
2.3. Surface Activity
2.4. In Vitro Assays of Fungicidal Activities
2.5. In Vivo Activity
3. Materials and Methods
3.1. Chemicals and Instrumentation
3.2. Preparation of Terbinafine Salts
3.3. Solubility
3.4. Surface Tension
3.5. In Vitro Assays of Fungicidal Activities
3.6. In Vivo Experiment
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Guan, A.; Liu, C.; Yang, X.; Dekeyser, M. Application of the Intermediate Derivatization Approach in Agrochemical Discovery. Chem. Rev. 2014, 114, 7079–7107. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.J.; Hitchcock, C.A.; Sibley, C.M. Current and Emerging Azole Antifungal Agents. Clin. Microbiol. Rev. 1999, 12, 40–79. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zhang, J.; Cao, Y.; Chai, X.; Zou, Y.; Wu, Q.; Zhang, D.; Jiang, Y.; Sun, Q. Synthesis, in vitro evaluation and molecular docking studies of new triazole derivatives as antifungal agents. Bioorganic Med. Chem. Lett. 2011, 21, 4471–4475. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, J.E. Pharmacology of the allylamines. J. Am. Acad. Dermatol. 1990, 23, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Ryder, N.S.; Leitner, I. Synergistic interaction of terbinafine with triazoles or amphotericin B against Aspergillus species. Med Mycol. 2001, 39, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Deng, L.; Cai, J.; Sun, R. Agricultural Antifungal Activity of Terbinafine and Terbinafine Hydrochloride. J. Trop. Ecol. 2021, 12, 448–455. [Google Scholar] [CrossRef]
- Monod, M.; Feuermann, M.; Yamada, T. Terbinafine and Itraconazole Resistance in Dermatophytes, Dermatophytes and Dermatophytoses; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [Green Version]
- Zajac, A.; Kukawka, R.; Pawlowska Zygarowicz, A.; Stolarska, O.; Smiglak, M. Ionic liquids as bioactive chemical tools for use in agriculture and the preservation of agricultural products. Green Chem. 2018, 20, 4764–4789. [Google Scholar] [CrossRef]
- Keramatnia, F.; Jouyban, A.; Valizadeh, H.; Delazar, A.; Shayanfar, A. Ketoconazole ionic liquids with citric and tartaric acid: Synthesis, characterization and solubility study. Fluid Phase Equilibria 2016, 425, 108–113. [Google Scholar] [CrossRef]
- Bica, K.; Cooke, L.R.; Nugent, P.; Rijksen, C.; Rogers, R.D. Toxic on purpose: Ionic liquid fungicides as combinatorial crop protecting agents. Green Chem. 2011, 13, 2344–2346. [Google Scholar] [CrossRef]
- Tang, R.; Tang, T.; Tang, G.; Liang, Y.; Wang, W.; Yang, J.; Niu, J.; Tang, J.; Zhou, Z.; Cao, Y. Pyrimethanil Ionic Liquids Paired with Various Natural Organic Acid Anions for Reducing Its Adverse Impacts on the Environment. J. Agric. Food Chem. 2019, 67, 11018–11024. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, T.; Zhou, Y.; Gao, H. Conversion of fungicide cyprodinil to salts with organic acids: Preparation, characterization, advantages. Pest Manag. Sci. 2022, 79, 114–124. [Google Scholar] [CrossRef]
- Settimo, L.; Bellman, K.; Knegtel, R.M.A. Comparison of the Accuracy of Experimental and Predicted pKa Values of Basic and Acidic Compounds. Pharm. Res. 2013, 31, 1082–1095. [Google Scholar] [CrossRef]
- Porretta, G.C.; Scalzo, M.; Chimenti, F.; Bolasco, A.; Biava, M.; Fischetti, M.; Riccardi, F. Research on antibacterial and antifungal agents. III. Synthesis and antimicrobial activity of 2-methyl-5-aryl-3-furoic acids and 2-methyl-3-imidazolylmethyl-5-aryl furans. Farm. Ed. Sci. 1987, 42, 629–639. [Google Scholar]
- Amborabé, B.E.; Fleurat Lessard, P.; Chollet, J.F.; Roblin, G. Antifungal effects of salicylic acid and other benzoic acid derivatives towards Eutypa lata: Structure–activity relationship. Plant Physiol. Biochem. 2002, 40, 1051–1060. [Google Scholar] [CrossRef]
- Barnes, R.H.; Karatzas, K.A.G. Investigation into the antimicrobial. activity of fumarate against Listeria monocytogenes and its mode of action under acidic conditions. Int. J. Food Microbiol. 2020, 324, 108614. [Google Scholar] [CrossRef]
- Chai, W.M.; Liu, X.; Hu, Y.H.; Feng, H.L.; Jia, Y.L.; Guo, Y.J.; Zhou, H.T.; Chen, Q.X. Antityrosinase and antimicrobial activities of furfuryl alcohol, furfural and furoic acid. Int. J. Biol. Macromol. 2013, 57, 151–155. [Google Scholar] [CrossRef]
- Hendrix, P.F.; Hamala, J.A.; Langner, C.L.; Kollig, H.P. Effects of chlorendic acid, a priority toxic substance, on laboratory aquatic ecosystems. Chemosphere 1983, 12, 1083–1099. [Google Scholar] [CrossRef]
- Kara, K.; Pirci, G.; Yılmaz, S.; Baytok, E.; Yılmaz, K. Effects of fumaric and maleic acids on the fermentation, nutrient composition, proteolysis and in vitro ruminal gas of corn silage. Grassl. Sci. 2022, 68, 362–371. [Google Scholar] [CrossRef]
- Mota, A.C.L.G.; Lima, E.d.O.; Castro, R.D.d.; Batista, A.U.D. Antifungal Activity of Maleic Acid against Candida spp. Associated with Oral Infections. Pesqui 2012, 12, 357–361. [Google Scholar] [CrossRef]
- Jadhav, N.R.; Bhosale, S.P.; Bhosale, S.S.; Mali, S.D.; Toraskar, P.B.; Kadam, T.S. Ionic liquids: Formulation avenues, drug delivery and therapeutic updates. J. Drug Deliv. Sci. Technol. 2021, 65, 102694. [Google Scholar] [CrossRef]
- Wilmarie, M.R.; Prausnitz, M.R. Enhanced Delivery to Skin Using Novel Terbinafine Ionic Liquids. Available online: https://www.aiche.org/conferences/aiche-annual-meeting/2017/proceeding/paper/542b-enhanced-delivery-skin-using-novel-terbinafine-ionic-liquids (accessed on 1 November 2017).
- Wiedmann, T.S.; Naqwi, A. Pharmaceutical salts: Theory, use in solid dosage forms and in situ preparation in an aerosol. Asian J. Pharm. Sci. 2016, 11, 722–734. [Google Scholar] [CrossRef] [Green Version]
- Pernak, J.; Syguda, A.; Janiszewska, D.; Materna, K.; Praczyk, T. Ionic liquids with herbicidal anions. Tetrahedron 2011, 67, 4838–4844. [Google Scholar] [CrossRef]
- Ryder, N. Terbinafine: Mode of action and properties of the squalene epoxidase inhibition. Br. J. Dermatol. 1992, 126, 2–7. [Google Scholar] [CrossRef]
- Fillinger, S.; Walker, A.S. Chemical Control and Resistance Management of Botrytis Diseases. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer: Berlin/Heidelberg, Germany, 2015; pp. 189–216. [Google Scholar] [CrossRef]
- Lu, T.; Zhou, Z.; Zhang, Q.; Zhang, Z.; Qian, H. Ecotoxicological Effects of Fungicides Azoxystrobin and Pyraclostrobin on Freshwater Aquatic Bacterial Communities. Bull. Environ. Contam. Toxicol. 2019, 103, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Jasrotia, R.; Langer, S.; Dhar, M. Endocrine Disrupting Chemicals in Aquatic Ecosystem: An Emerging Threat to Wildlife and Human Health. Proc. Zool. Soc. 2021, 74, 634–647. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Liang, S.; Zhou, Z.; Zhang, C.; Li, K.; Peng, X.; Qin, S.; Xing, K. Antifungal activity of volatile compounds from Bacillus tequilensis XK29 against Botrytis cinerea causing gray mold on cherry tomatoes. Postharvest Biol. Technol. 2023, 198, 112239. [Google Scholar] [CrossRef]
- Zhou, T.; Guo, T.; Wang, Y.; Wang, A.; Zhang, M. Carbendazim: Ecological risks, toxicities, degradation pathways and potential risks to human health. Chemosphere 2023, 314, 137723. [Google Scholar] [CrossRef]
- Gaurav, V.; Bhattacharya, S.; Sharma, N.; Datt, S.; Kumar, P.; Rai, G.; Singh, P.; Taneja, B.; Das, S. Terbinafine resistance in dermatophytes: Time to revisit alternate antifungal therapy. J. Med Mycol. 2020, 31, 101087. [Google Scholar] [CrossRef]
- Gupta, A.K.; Renaud, H.J.; Quinlan, E.M.; Shear, N.H.; Piguet, V. The Growing Problem of Antifungal Resistance in Onychomycosis and Other Superficial Mycoses. J. Am. Acad. Dermatol. 2021, 22, 149–157. [Google Scholar] [CrossRef]
- Shankarnarayan, S.A.; Shaw, D.; Sharma, A.; Chakrabarti, A.; Dogra, S.; Kumaran, M.S.; Kaur, H.; Ghosh, A.; Rudramurthy, S.M. Rapid detection of terbinafine resistance in Trichophyton species by Amplified refractory mutation system-polymerase chain reaction. Sci. Rep. 2020, 10, 1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, A.I.; Furniss, B.S. Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; The Bath Press: Beccles, UK, 1989; pp. 1198–1204. ISBN 0−582−46236−3. [Google Scholar]
- Chin, C.P.; Wu, H.S.; Wang, S.S. New Approach to Pesticide Delivery Using Nanosuspensions: Research and Applications. Ind. Eng. Chem. Res. 2011, 50, 7637–7643. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Qiao, C.; Liu, F.; Peng, Q. Amino Acid Ionic Liquids as a Potential Adjuvant for Fungicide Formulations: COSMO-RS Prediction and Dissolution Mechanism Elucidation. ACS Sustain. Chem. Eng. 2022, 10, 3295–3310. [Google Scholar] [CrossRef]
- Wang, D.; Nie, Q.; Li, Y.; Wu, T.; Bu, S. Determination of terbinafine in canine plasma using HPLC method. Chin. J. Vet. Drug 2016, 50, 34–40. [Google Scholar]
- Rekiel, E.; Zdziennicka, A.; Jańczuk, B. Adsorption properties of rhamnolipid and ethanol at water/ethanol solution-air interface. J. Mol. Liq. 2020, 308, 113080. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhang, X.; Du, S.; Han, X.; Li, J.Q.; Xiao, Y.; Xu, Z.; Wu, Q.; Xu, L.; et al. Fungicidal Action of the Triphenylphosphonium-Driven Succinate Dehydrogenase Inhibitors Is Mediated by Reactive Oxygen Species and Suggests an Effective Resistance Management Strategy. J. Agric. Food Chem. 2021, 70, 111–123. [Google Scholar] [CrossRef]
- Yang, X.; Gu, C.Y.; Sun, J.Z.; Bai, Y.; Zang, H.Y.; Chen, Y. Biological Activity of Pyraclostrobin Against Coniella granati Causing Pomegranate Crown Rot. Plant Dis. 2021, 105, 3538–3544. [Google Scholar] [CrossRef]
- Lin, X.; Li, Y.; Zhong, W.; Hong, T.; Li, L.; Song, S.; He, D. Synthesis, Bioactivity, and QSAR Study of 3,4-Dichlorophenyl Isoxazole-Substituted Stilbene Derivatives against the Phytopathogenic Fungus Botrytis cinerea. J. Agric. Food Chem. 2021, 69, 9520–9528. [Google Scholar] [CrossRef]
- Li, H.; He, Y.H.; Hu, Y.M.; Chu, Q.R.; Chen, Y.J.; Wu, Z.R.; Zhang, Z.J.; Liu, Y.Q.; Yang, C.J.; Liang, H.J.; et al. Design, Synthesis, and Structure–Activity Relationship Studies of Magnolol Derivatives as Antifungal Agents. J. Agric. Food Chem. 2021, 69, 11781–11793. [Google Scholar] [CrossRef]

| API Combination | N-CH3 (ppm) | N-CH2-Ar (ppm) | N-CH2-CH (ppm) |
|---|---|---|---|
| TIS 1 (CDCl3) | 2.51 | 4.51 | 3.66 |
| TIS 2 (DMSO) | 2.19 | 3.98 | 3.24 |
| TIS 3 (CDCl3) | 2.66 | 4.66 | 3.82 |
| TIS 4 (DMSO) | 2.16 | 3.94 | 3.18 |
| TIS 5 (CDCl3) | 2.5 | 4.49 | 3.59 |
| Terbinafine (CDCl3) | 2.21 | 3.88 | 3.12 |
| Terbinafine (DMSO) | 2.11 | 3.86 | 3.1 |
| Salt | Water | Methanol | DMSO | Acetonitrile | Acetone | Isopropanol | Ethyl acetate | Chloroform | Toluene | n-Hexane |
|---|---|---|---|---|---|---|---|---|---|---|
| 9.0 a | 6.6 | 6.5 | 6.2 | 5.1 | 4.3 | 4.3 | 4.1 | 2.3 | 0.0 | |
| T | − b | + c | + | + | + | + | + | + | + | + |
| TIS 1 | − | + | + | + | + | − | + | + | + | − |
| TIS 2 | − | − | + | − | − | − | − | − | − | − |
| TIS 3 | − | + | + | − | − | − | − | + | − | − |
| TIS 4 | − | − | + | − | − | − | − | − | − | − |
| TIS 5 | − | + | + | + | + | + | + | + | + | − |
| Drug | Inhibition Rate at 50 mg/L (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| V. m | B. c | P. o | P. c | A. s | R. s | F. g | P. a | |
| T | 100 ± 0 | 100 ± 0 | 99.64 ± 0.36 | 48.75 ± 0.45 | 100 ± 0 | 84.21 ± 0.53 | 100 ± 0 | 68.21 ± 1.87 |
| TIS 1 | 100 ± 0 | 100 ± 0 | 99.05 ± 0.21 | 46.97 ± 1.18 | 100 ± 0 | 84.79 ± 1.25 | 100 ± 0 | 74.61 ± 0.38 |
| TIS 2 | 100 ± 0 | 100 ± 0 | 96.09 ± 0.36 | 43.85 ± 1.61 | 98.8 ± 0 | 80.75 ± 0.72 | 100 ± 0 | 69.65 ± 1.7 |
| TIS 3 | 100 ± 0 | 100 ± 0 | 99.29 ± 0 | 33.16 ± 2.52 | 100 ± 0 | 83.4 ± 0.35 | 100 ± 0 | 69.54 ± 0 |
| TIS 4 | 100 ± 0 | 100 ± 0 | 98.7 ± 0.54 | 47.64 ± 1.58 | 100 ± 0 | 85.82 ± 1.51 | 100 ± 0 | 65.23 ± 0.47 |
| TIS 5 | 100 ± 0 | 100 ± 0 | 96.68 ± 0.42 | 55.88 ± 1.89 | 98.67 ± 0.61 | 82.02 ± 0.91 | 100 ± 0 | 71.96 ± 2.68 |
| Fungi | Drugs | Regression Equation | r2 | EC50 | 95% Confidence Intervals (mg/L) | |
|---|---|---|---|---|---|---|
| (mg/L) | (μmol/L) | |||||
| V. m | Terbinafine | y = 2.62x − 0.22 | 0.969 | 1.25 | 4.28 | 0.98–1.57 |
| TIS 1 | y = 2.12x − 0.77 | 0.985 | 2.24 | 5.21 | 1.93–2.57 | |
| TIS 2 | y = 2.87x − 1.87 | 0.955 | 4.28 | 6.29 | 3.16–6.00 | |
| TIS 3 | y = 2.15x − 0.66 | 0.986 | 2.03 | 4.98 | 1.77–2.34 | |
| TIS 4 | y = 2.17x − 0.59 | 0.997 | 1.88 | 4.60 | 1.64–2.14 | |
| TIS 5 | y = 2.28x − 0.39 | 0.986 | 1.49 | 3.69 | 1.30–1.73 | |
| Pyraclostrobin | y = 0.59x + 0.06 | 0.995 | 0.79 | 2.02 | 0.03–1.71 | |
| B. c | Terbinafine | y = 3.18x + 3.16 | 0.976 | 0.10 | 0.34 | 0.09–0.11 |
| TIS 1 | y = 3.03x + 2.6 | 0.959 | 0.14 | 0.32 | 0.11–0.17 | |
| TIS 2 | y = 3.53x + 2.12 | 0.990 | 0.25 | 0.37 | 0.23–0.28 | |
| TIS 3 | y = 3.53x + 3.09 | 0.942 | 0.13 | 0.33 | 0.10–0.18 | |
| TIS 4 | y = 3.7x + 3.07 | 0.974 | 0.15 | 0.36 | 0.12–0.18 | |
| TIS 5 | y = 4.27x + 3.77 | 0.966 | 0.13 | 0.33 | 0.11–0.15 | |
| Pyraclostrobin | y = 1.88x + 1.74 | 0.993 | 0.12 | 0.31 | 0.01–0.15 | |
| P. o | Terbinafine | y = 0.98x + 0.55 | 0.998 | 0.28 | 0.95 | 0.13–0.43 |
| TIS 1 | y = 1.02x + 0.33 | 0.991 | 0.47 | 1.10 | 0.28–0.66 | |
| TIS 2 | y = 1.15x + 0.07 | 0.991 | 0.88 | 1.29 | 0.65–1.11 | |
| TIS 3 | y = 1.17x + 0.28 | 0.969 | 0.58 | 1.41 | 0.39–0.76 | |
| TIS 4 | y = 1.32x + 0.17 | 0.993 | 0.75 | 1.84 | 0.57–0.94 | |
| TIS 5 | y = 1.1x + 0.34 | 0.997 | 0.49 | 1.22 | 0.31–0.68 | |
| Pyraclostrobin | y = 0.56x + 0.13 | 0.991 | 0.60 | 1.54 | 0.25–0.99 | |
| A. s | Terbinafine | y = 1.25x + 1.43 | 0.998 | 0.07 | 0.25 | 0.05–0.09 |
| TIS 1 | y = 1.42x + 1.25 | 0.936 | 0.13 | 0.30 | 0.07–0.19 | |
| TIS 2 | y = 0.92x + 0.68 | 0.987 | 0.18 | 0.27 | 0.13–0.24 | |
| TIS 3 | y = 0.99x + 0.83 | 0.965 | 0.15 | 0.36 | 0.11–0.19 | |
| TIS 4 | y = 1.23x + 1.16 | 0.986 | 0.11 | 0.28 | 0.09–0.14 | |
| TIS 5 | y = 1.1x + 1.19 | 0.996 | 0.08 | 0.20 | 0.06–0.11 | |
| Pyraclostrobin | y = 0.3x + 0.2 | 0.981 | 0.23 | 0.59 | 0.07–0.56 | |
| R. s | Terbinafine | y = 1.29x − 0.33 | 0.961 | 1.73 | 5.94 | 1.41–2.10 |
| TIS 1 | y = 1.8x − 0.78 | 0.931 | 2.49 | 5.81 | 1.69–3.72 | |
| TIS 2 | y = 1.48x − 0.78 | 0.975 | 3.32 | 4.88 | 2.78–4.03 | |
| TIS 3 | y = 1.44x − 0.39 | 0.984 | 1.84 | 4.51 | 1.53–2.19 | |
| TIS 4 | y = 1.49x − 0.56 | 0.972 | 2.30 | 5.65 | 1.93–2.75 | |
| TIS 5 | y = 1.49x − 0.59 | 0.980 | 2.43 | 6.02 | 2.04–2.90 | |
| Pyraclostrobin | y = 0.55x + 0.62 | 0.999 | 0.07 | 0.19 | 0.01–0.21 | |
| F. g | Terbinafine | y = 1.4x + 1.66 | 0.943 | 0.07 | 0.25 | 0.06–0.09 |
| TIS 1 | y = 1.25x + 1.27 | 0.988 | 0.10 | 0.23 | 0.07–0.12 | |
| TIS 2 | y = 1.37x + 1.08 | 0.995 | 0.16 | 0.24 | 0.12–0.21 | |
| TIS 3 | y = 1.32x + 1.35 | 0.984 | 0.10 | 0.24 | 0.08–0.12 | |
| TIS 4 | y = 1.3x + 1.38 | 0.995 | 0.09 | 0.21 | 0.06–0.11 | |
| TIS 5 | y = 1.33x + 1.29 | 0.983 | 0.11 | 0.27 | 0.08–0.13 | |
| Pyraclostrobin | y = 0.74x − 0.12 | 0.993 | 1.43 | 3.68 | 0.91–3.27 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Wang, Q.; Zhou, Y.; Shi, Y.; Gao, H. The Effect of Terbinafine and Its Ionic Salts on Certain Fungal Plant Pathogens. Molecules 2023, 28, 4722. https://doi.org/10.3390/molecules28124722
Wang T, Wang Q, Zhou Y, Shi Y, Gao H. The Effect of Terbinafine and Its Ionic Salts on Certain Fungal Plant Pathogens. Molecules. 2023; 28(12):4722. https://doi.org/10.3390/molecules28124722
Chicago/Turabian StyleWang, Tao, Qiuxiao Wang, Yifei Zhou, Yaolin Shi, and Haixiang Gao. 2023. "The Effect of Terbinafine and Its Ionic Salts on Certain Fungal Plant Pathogens" Molecules 28, no. 12: 4722. https://doi.org/10.3390/molecules28124722
APA StyleWang, T., Wang, Q., Zhou, Y., Shi, Y., & Gao, H. (2023). The Effect of Terbinafine and Its Ionic Salts on Certain Fungal Plant Pathogens. Molecules, 28(12), 4722. https://doi.org/10.3390/molecules28124722






