Molecular Mechanism Study on the Effect of Microstructural Differences of Octylphenol Polyoxyethylene Ether (OPEO) Surfactants on the Wettability of Anthracite
Abstract
:1. Introduction
2. Results and Discussion
2.1. Surface Tension Analysis
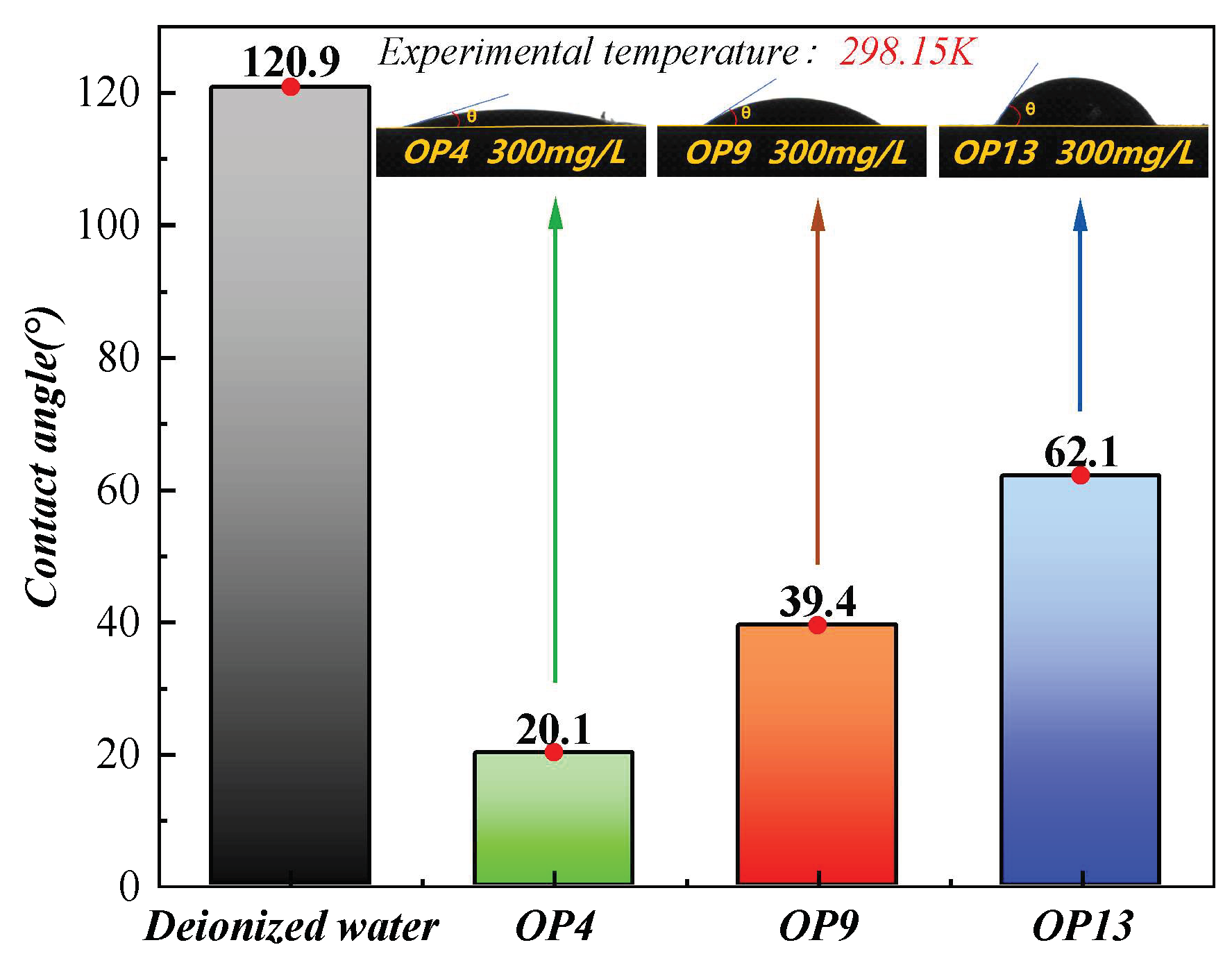
2.2. Contact Angle Analysis
2.3. FTIR Analysis
2.4. XPS Analysis
2.5. UV Spectrophotometer Measurement Analysis
2.6. N2 Adsorption Curves, Specific Surface Area and Pore Distribution Analysis
2.7. SEM Analysis
2.8. Molecular Dynamics Simulation Results
2.8.1. Contact Surface Area
2.8.2. Interaction Energy
2.8.3. Relative Concentration Distribution
2.8.4. Mean Square Displacement
3. Experiment and Simulation
3.1. Experimental Materials
3.2. Experimental Facilities and Parameters
3.2.1. Adsorption Experiment
3.2.2. Surface Tension Measurement
3.2.3. Contact Angle Measurement
3.2.4. FTIR Measurement
3.2.5. XPS Measurement
3.2.6. UV Spectrophotometer Measurement
3.2.7. Specific Surface Area and Porosity Distribution Test
3.2.8. SEM Measurement
3.3. Molecular Dynamics Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- International Energy Agency. Coal 2022; IEA: Paris, France, 2022; Available online: https://www.iea.org/reports/coal-2022 (accessed on 16 December 2022).
- Wang, X.; Yuan, S.; Jiang, B. Experimental investigation of the wetting ability of surfactants to coals dust based on physical chemistry characteristics of the different coal samples. Adv. Powder Technol. 2019, 30, 1696–1708. [Google Scholar] [CrossRef]
- Ahmed, M.; Guo, X.; Zhao, X.-M. Spectroscopic and microscopic characterization of atmospheric particulate matter. Instrum. Sci. Technol. 2017, 45, 659–682. [Google Scholar] [CrossRef]
- Fan, T.; Zhou, G.; Wang, J. Preparation and characterization of a wetting-agglomeration-based hybrid coal dust suppressant. Process Saf. Environ. Prot. 2018, 113, 282–291. [Google Scholar] [CrossRef]
- Wang, K.; Ding, C.; Jiang, S.; Zhengyan, W.; Shao, H.; Zhang, W. Application of the addition of ionic liquids using a complex wetting agent to enhance dust control efficiency during coal mining. Process Saf. Environ. Prot. 2019, 122, 13–22. [Google Scholar] [CrossRef]
- Chu, C.; Muradian, N. Safety and environmental implications of coal mining. Int. J. Environ. Pollut. 2016, 59, 250–268. [Google Scholar] [CrossRef]
- Xiu, Z.; Nie, W.; Yan, J.; Chen, D.; Cai, P.; Liu, Q.; Du, T.; Yang, B. Numerical simulation study on dust pollution characteristics and optimal dust control air flow rates during coal mine production. J. Clean. Prod. 2020, 248, 119197. [Google Scholar] [CrossRef]
- Zheng, Y.-P.; Feng, C.-G.; Jing, G.-X.; Qian, X.-M.; Li, X.-J.; Liu, Z.-Y.; Huang, P. A statistical analysis of coal mine accidents caused by coal dust explosions in China. J. Loss Prev. Process Ind. 2009, 22, 528–532. [Google Scholar] [CrossRef]
- Khan, A.M.; Ray, S.K.; Mohalik, N.K.; Mishra, D.; Mandal, S.; Pandey, J.K. Experimental and CFD Simulation Techniques for Coal Dust Explosibility: A Review. Min. Metall. Explor. 2022, 39, 1445–1463. [Google Scholar] [CrossRef]
- Wade, W.A.; Petsonk, E.L.; Young, B.; Mogri, I. Severe Occupational Pneumoconiosis among West Virginian Coal Miners: One Hundred Thirty-eight Cases of Progressive Massive Fibrosis Compensated between 2000 and 2009. Chest 2011, 139, 1458–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halldin, C.N.; Blackley, D.J.; Markle, T.; Cohen, R.A.; Laney, A.S. Patterns of progressive massive fibrosis on modern coal miner chest radiographs. Arch. Environ. Occup. Health 2020, 75, 152–158. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Division on Earth and Life Studies, Board on Health Sciences Policy; Board on Environmental Studies and Toxicology; Board on Earth Sciences and Resources; Committee on the Study of the Control of Respirable Coal Mine Dust Exposure in Underground Mines. Monitoring and Sampling Approaches to Assess Underground Coal Mine Dust Exposures; National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Derickson, A. “Nuisance Dust”: Unprotective Limits for Exposure to Coal Mine Dust in the United States, 1934–1969. Am. J. Public Health 2013, 103, 238–249. [Google Scholar] [CrossRef]
- Hu, S.; Huang, Y.; Feng, G.; Shao, H.; Liao, Q.; Gao, Y.; Hu, F. Investigation on the design of atomization device for coal dust suppression in underground roadways. Process Saf. Environ. Prot. 2019, 129, 230–237. [Google Scholar] [CrossRef]
- Xu, G.; Chen, Y.; Eksteen, J.; Xu, J. Surfactant-aided coal dust suppression: A review of evaluation methods and influencing factors. Sci. Total Environ. 2018, 639, 1060–1076. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Qiu, H.; Zhang, Q.; Xu, M.; Wang, J.; Wang, G. Experimental Investigation of Coal Dust Wettability Based on Surface Contact Angle. J. Chem. 2016, 2016, 9452303. [Google Scholar] [CrossRef] [Green Version]
- Rosen, M.J. The Relationship of Structure to Properties in Surfactants; Wiley Online Library: Hoboken, NJ, USA, 1972. [Google Scholar]
- Manglik, R.M.; Wasekar, V.M.; Zhang, J. Dynamic and equilibrium surface tension of aqueous surfactant and polymeric solutions. Exp. Therm. Fluid Sci. 2001, 25, 55–64. [Google Scholar] [CrossRef]
- Shi, G.; Qi, J.; Wang, Y.; Shen, H. Synergistic influence of noncationic surfactants on the wettability and functional groups of coal. Powder Technol. 2021, 385, 92–105. [Google Scholar] [CrossRef]
- Zhang, Q.; Xing, X.; Zhou, G.; Hu, Y.; Shang, S.; Fu, M.; Ma, H.; Li, H.; Men, Y. Preparation and micro-wetting mechanism analysis of highly permeable-moistening additive for coal seam water injection based on plant extraction technology. Fuel 2022, 322, 124125. [Google Scholar] [CrossRef]
- Sheng, R.; Quan, X.F.; Ren, Z.H.; Huang, J.; Li, D.N.; Wang, J.R.; Qian, Z.B.; Zhang, Y.X.; Cai, L.L.; Li, B.B.; et al. Molecular interaction between sodium dodecylbenzene sulfonate and octylphenol polyoxyethylene ether and effect of hydrophilic chain. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127048. [Google Scholar] [CrossRef]
- Sis, H.; Birinci, M. Effect of nonionic and ionic surfactants on zeta potential and dispersion properties of carbon black powders. Colloids Surf. A Physicochem. Eng. Asp. 2009, 341, 60–67. [Google Scholar] [CrossRef]
- Cristovici, M.A. Investigation to control mine dust using surfactants and a new approach for eliminating their negative effect on flotation. Min. Metall. Explor. 1991, 8, 38–42. [Google Scholar] [CrossRef]
- You, X.; He, M.; Zhang, W.; Wei, H.; He, Q.; Lyu, X.; Li, L. Molecular dynamics simulations and contact angle of surfactant at the coal–water interface. Mol. Simul. 2018, 44, 722–727. [Google Scholar] [CrossRef]
- Li, Z.; Ma, C.; Wang, J.; Lyu, X.; Zhang, Q.; You, X.; Li, L. Investigation of nonylphenol ethoxylate on the surface characteristics of low rank coal. Part. Sci. Technol. 2019, 38, 1012–1018. [Google Scholar] [CrossRef]
- Li, L.; He, M.; Liu, M.; Lin, M.; Hu, S.; Yu, H.; Wang, Q.; You, X. Effect of the degree of polymerization of nonylphenol polyoxyethylene ether on the dewatering of low-rank coal. Physicochem. Probl. Miner. Process. 2020, 56, 723–736. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Q.; Zhou, G.; Wang, H.; Bai, Y.; Liu, Y. Influence Mechanism of Surfactants on Wettability of Coal with Different Metamorphic Degrees Based on Infrared Spectrum Experiments. ACS Omega 2021, 6, 22248–22258. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Yan, G.; Li, J.; Bai, X. Molecular mechanism of hydrophobic tail chain saturation in nonionic surfactants changing the wettability of anthracite. J. Mol. Liq. 2022, 368, 120732. [Google Scholar] [CrossRef]
- Yan, G.; Ren, G.; Bai, L.; Feng, J.; Zhang, Z. Molecular Model Construction and Evaluation of Jincheng Anthracite. ACS Omega 2020, 5, 10663–10670. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Guo, T.; Shu, C.-M.; Li, Q.-W.; Li, D.-J.; Chen, L.-G. Effects of oxygen concentrations on the coal oxidation characteristics and functional groups. J. Therm. Anal. Calorim. 2020, 142, 899–912. [Google Scholar] [CrossRef]
- Chen, X.; Yan, G.; Xu, G.; Yang, X.; Dong, J.; Liang, Z.; Wei, S.; Ali, M.S. Computational Study on the Microscopic Adsorption Characteristics of Linear Alkylbenzene Sulfonates with Different Chain Lengths on Anthracite Surface. J. Chem. 2022, 2022, 1–17. [Google Scholar] [CrossRef]
- Chen, X.; Yan, G.; Xu, G.; Yang, X.; Li, J.; Bai, X.; Kyzas, G. Influence of the Branched Structure of Polyoxyethylene Units in Nonionic Surfactants on the Wettability of Anthracite: A Combined Modeling and Experimental Study. Adsorpt. Sci. Technol. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Chen, X.; Yan, G.; Yang, X.; Xu, G.; Wei, S. Microscopic Diffusion Characteristics of Linear Alkylbenzene Sulfonates on the Surface of Anthracite: The Influence of Different Attachment Sites of Benzene Ring in the Backbone. Minerals 2021, 11, 1045. [Google Scholar] [CrossRef]
- Chen, X.; Yan, G.; Yang, X.; Xu, G. Study on Adsorption Characteristics of Sulfonate Gemini Surfactant on Lignite Surface. Minerals 2021, 11, 1401. [Google Scholar] [CrossRef]
- Parekh, B.; Aplan, F. The critical surface tension of wetting of coal. Recent Dev. Sep. Sci. 1978, 4, 107–113. [Google Scholar]
- Sis, H.; Birinci, M. Wetting and rheological characteristics of hydrophobic organic pigments in water in the presence of non-ionic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2014, 455, 58–66. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, G.; Li, S.; Wang, C.; Liu, R.; Jiang, W. Molecular dynamics simulation and experimental characterization of anionic surfactant: Influence on wettability of low-rank coal. Fuel 2020, 279, 118323. [Google Scholar] [CrossRef]
- Otzen, D. Protein–surfactant interactions: A tale of many states. Biochim. Et Biophys. Acta BBA—Proteins Proteom. 2011, 1814, 562–591. [Google Scholar] [CrossRef]
- Huang, J.; Ren, Z.H. Synergistic Interaction between Nonionic Octylphenol Polyoxyethylene Ethers and Effect of Hydrophilic Chain. J. Surfactants Deterg. 2017, 20, 1197–1203. [Google Scholar] [CrossRef]
- Huang, J.; Ren, Z.H. Micellization and interactions for ternary mixtures of amino sulfonate surfactant and nonionic octylphenol polyoxyethylene ethers in aqueous solution: 1 Blending with nonionic surfactants with smaller numbers of hydrophilic unit. J. Mol. Liq. 2019, 278, 53–60. [Google Scholar] [CrossRef]
- Liu, A.; Fan, P.-P.; Qiao, X.-X.; Li, Z.-H.; Wang, H.-F.; Fan, M.-Q. Synergistic effect of mixed DDA/surfactants collectors on flotation of quartz. Miner. Eng. 2020, 159, 106605. [Google Scholar] [CrossRef]
- Perkins, K.M.; Gupta, C.; Charleson, E.N.; Washburn, N.R. Surfactant properties of PEGylated lignins: Anomalous interfacial activities at low grafting density. Colloids Surf. A Physicochem. Eng. Asp. 2017, 530, 200–208. [Google Scholar] [CrossRef]
- Calvo, E.; Bravo, R.; Amigo, A.; Gracia-Fadrique, J. Dynamic surface tension, critical micelle concentration, and activity coefficients of aqueous solutions of nonyl phenol ethoxylates. Fluid Phase Equilibria 2009, 282, 14–19. [Google Scholar] [CrossRef]
- Srinivasan, V.; Blankschtein, D. Effect of Counterion Binding on Micellar Solution Behavior: 1. Molecular−Thermodynamic Theory of Micellization of Ionic Surfactants. Langmuir 2003, 19, 9932–9945. [Google Scholar] [CrossRef]
- Jonstroemer, M.; Joensson, B.; Lindman, B. Self-diffusion in nonionic surfactant-water systems. J. Phys. Chem. 1991, 95, 3293–3300. [Google Scholar] [CrossRef]
- Hehe, J.; Guanhua, N.; Chuanjie, Z.; Xiangfei, Z.; Gongshuai, S.; Zhenyang, W.; Qiming, H. Molecular dynamics simulations and experimental characterization of the effect of different concentrations of [Bmim][Cl] in aqueous solutions on the wettability of anthracite. Fuel 2022, 324, 124618. [Google Scholar] [CrossRef]
- Li, Q.; Lin, B.; Zhao, S.; Dai, H. Surface physical properties and its effects on the wetting behaviors of respirable coal mine dust. Powder Technol. 2013, 233, 137–145. [Google Scholar] [CrossRef]
- Xi, X.; Jiang, S.; Zhang, W.; Wang, K.; Shao, H.; Wu, Z. An experimental study on the effect of ionic liquids on the structure and wetting characteristics of coal. Fuel 2019, 244, 176–183. [Google Scholar] [CrossRef]
- Chen, X.; Gao, J.; Deng, C.; Ge, S.; Fan, C.; Zhang, W. Experimental study on chemical structure and wetting influence of imidazole ionic liquids on coal. Fuel 2022, 330, 125545. [Google Scholar] [CrossRef]
- Zhao, B.; Li, S.; Lin, H.; Cheng, Y.; Kong, X.; Ding, Y. Experimental study on the influence of surfactants in compound solution on the wetting-agglomeration properties of bituminous coal dust. Powder Technol. 2022, 395, 766–775. [Google Scholar] [CrossRef]
- Li, J.; Yan, G.; Zhou, L.; Bai, X.; Chen, X. Molecular mechanism of the effect of benzene ring structure in nonionic surfactants on the wettability of anthracite. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130634. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, S.; Longhurst, P.; Yang, W.; Zheng, S. Molecular structure characterization of bituminous coal in Northern China via XRD, Raman and FTIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 255, 119724. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, S.; Yuan, X.; Kong, R. Effects of nonionic collectors with oxygen-containing functional groups on flotation performance of low-rank coal. Fuel 2022, 330, 125585. [Google Scholar] [CrossRef]
- Xia, W.; Niu, C.; Li, Y. Effect of heating process on the wettability of fine coals of various ranks. Can. J. Chem. Eng. 2017, 95, 475–478. [Google Scholar] [CrossRef]
- Wen, B.; Xia, W.; Niu, C. Comparison of pyrolysis and oxidation actions on chemical and physical property of anthracite coal surface. Adv. Powder Technol. 2020, 31, 2447–2455. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Fan, M.; Guo, J.; Li, B. Decrease in hydrophilicity and moisture readsorption of Manglai lignite using lauryl polyoxyethylene ether: Effects of the HLB and coverage on functional groups and pores. Fuel Process. Technol. 2018, 174, 33–40. [Google Scholar] [CrossRef]
- Bandforuzi, S.R.; Hadjmohammadi, M.R. Application of non-ionic surfactant as a developed method for the enhancement of two-phase solvent bar microextraction for the simultaneous determination of three phthalate esters from water samples. J. Chromatogr. A 2018, 1561, 39–47. [Google Scholar] [CrossRef]
- Ballesteros-Gómez, A.; Sicilia, M.D.; Rubio, S. Supramolecular solvents in the extraction of organic compounds. A review. Anal. Chim. Acta 2010, 677, 108–130. [Google Scholar] [CrossRef] [PubMed]
- Misra, P.K.; Panigrahi, S.; Dash, U.; Mandal, A.B. Organization of amphiphiles. Part XI: Physico-chemical aspects of mixed micellization involving normal conventional surfactant and a non-ionic gemini surfactant. J. Colloid Interface Sci. 2010, 345, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Levitz, P.; El Miri, A.; Keravis, D.; Van Damme, H. Adsorption of nonionic surfactants at the solid—Solution interface and micellization: A comparative fluorescence decay study. J. Colloid Interface Sci. 1984, 99, 484–492. [Google Scholar] [CrossRef]
- Mäntele, W.; Deniz, E. UV–VIS absorption spectroscopy: Lambert-Beer reloaded. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 965–968. [Google Scholar] [CrossRef]
- Aktas, Z.; Woodburn, E.T. The adsorption behaviour of nonionic reagents on two low rank British coals. Miner. Eng. 1994, 7, 1115–1126. [Google Scholar] [CrossRef]
- You, Q.; Wang, C.; Ding, Q.; Zhao, G.; Fang, J.; Liu, Y.; Zhao, M.; Dai, C.; Geng, M. Impact of surfactant in fracturing fluid on the adsorption–desorption processes of coalbed methane. J. Nat. Gas Sci. Eng. 2015, 26, 35–41. [Google Scholar] [CrossRef]
- Zhao, L.; Guanhua, N.; Lulu, S.; Qian, S.; Shang, L.; Kai, D.; Jingna, X.; Gang, W. Effect of ionic liquid treatment on pore structure and fractal characteristics of low rank coal. Fuel 2020, 262, 116513. [Google Scholar] [CrossRef]
- Mishra, D.K.; Samad, S.K.; Varma, A.K.; Mendhe, V.A. Pore geometrical complexity and fractal facets of Permian shales and coals from Auranga Basin, Jharkhand, India. J. Nat. Gas Sci. Eng. 2018, 52, 25–43. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Li, Q.; Liu, D.; Zhou, Y.; Lv, D. Insights into matrix compressibility of coals by mercury intrusion porosimetry and N2 adsorption. Int. J. Coal Geol. 2018, 200, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Liu, Z.; Lin, S.; Li, X.; Ali, M. Study on microstructure characteristics of material evidence in coal dust explosion and its significance in accident investigation. Fuel 2020, 265, 116992. [Google Scholar] [CrossRef]
- Yi, M.; Cheng, Y.; Wang, Z.; Wang, C.; Hu, B.; He, X. Effect of particle size and adsorption equilibrium time on pore structure characterization in low pressure N2 adsorption of coal: An experimental study. Adv. Powder Technol. 2020, 31, 4275–4281. [Google Scholar] [CrossRef]
- Niu, C.; Xia, W.; Peng, Y. Analysis of coal wettability by inverse gas chromatography and its guidance for coal flotation. Fuel 2018, 228, 290–296. [Google Scholar] [CrossRef]
- Chen, X.; Yan, G.; Zhou, Y.; Xu, G.; Bai, X.; Li, J. Molecular mechanism study on the effect of nonionic surfactants with different degrees of ethoxylation on the wettability of anthracite. Chemosphere 2023, 310, 136902. [Google Scholar] [CrossRef]
- Li, B.; Guo, J.; Liu, S.; Albijanic, B.; Zhang, L.; Sun, X. Molecular insight into the mechanism of benzene ring in nonionic surfactants on low-rank coal floatability. J. Mol. Liq. 2020, 302, 112563. [Google Scholar] [CrossRef]
- Anvari, M.H.; Liu, Q.; Xu, Z.; Choi, P. Molecular Dynamics Study of Hydrophilic Sphalerite (110) Surface as Modified by Normal and Branched Butylthiols. Langmuir 2018, 34, 3363–3373. [Google Scholar] [CrossRef]
- Chang, H.; Zhang, H.; Jia, Z.; Li, X.; Gao, W.; Wei, W. Wettability of coal pitch surface by aqueous solutions of cationic Gemini surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2016, 494, 59–64. [Google Scholar] [CrossRef]
- You, X.; He, M.; Zhang, W.; Wei, H.; Lyu, X.; He, Q.; Li, L. Molecular dynamics simulations of nonylphenol ethoxylate on the Hatcher model of subbituminous coal surface. Powder Technol. 2018, 332, 323–330. [Google Scholar] [CrossRef]
- Zhou, G.; Xing, M.; Wang, K.; Wang, Q.; Xu, Z.; Li, L.; Cheng, W. Study on wetting behavior between CTAC and BS-12 with gas coal based on molecular dynamics simulation. J. Mol. Liq. 2022, 357, 118996. [Google Scholar] [CrossRef]
- Xue, X. Prediction for the burnout of pulverized coal in a coal-fired power plant by combining proximate analysis, coal petrography, and pulverized-coal size distribution. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 69–74. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Yan, K. Adsorption of collectors on model surface of Wiser bituminous coal: A molecular dynamics simulation study. Miner. Eng. 2015, 79, 31–39. [Google Scholar] [CrossRef]











| Reagents | Ethylene Oxide Number | Surface Tension (mN/m) | CMC (10−2 mol/L) | HLB |
|---|---|---|---|---|
| OP4 | 4 | 27.182 | 0.00223 | 9.4 |
| OP9 | 9 | 33.391 | 0.00246 | 13.5 |
| OP13 | 13 | 35.900 | 0.00275 | 14.0 |
| Deionized water | / | 71.558 | / | / |
| Surfactant | Fitting Formula | K | R2 |
|---|---|---|---|
| OP4 | θ = 2020.05/(100.5 − 80.4 × exp(−0.25000 × K × t)) | 1.91781 ± 0.08048 | 0.94557 |
| OP9 | θ = 4223.68/(107.2 − 67.8 × exp(−0.58112 × K × t)) | 1.16375 ± 0.02970 | 0.97389 |
| OP13 | θ = 6880.68/(110.8 − 48.7 × exp(−1.27515 × K × t)) | 0.68961 ± 0.01311 | 0.98127 |
| Functional Groups | Raw Coal | OP4 | OP9 | OP13 | |
|---|---|---|---|---|---|
| C 1s | C-C/C-H (%) | 88.87 | 68.82 | 74.20 | 75.76 |
| C-O (%) | 4.11 | 23.48 | 21.11 | 16.26 | |
| C=O (%) | 7.00 | 0.29 | 2.33 | 1.21 | |
| O=C-O (%) | 0.02 | 7.41 | 2.36 | 6.77 | |
| O 1s | C-O/OH (%) | 18.60 | 86.31 | 56.10 | 50.87 |
| C=O (%) | 59.05 | 10.15 | 25.57 | 34.90 | |
| COO/COOH (%) | 22.35 | 3.54 | 18.32 | 14.23 |
| Surfactant | Absorbance | C1 (mg/L) | C0 (mg/L) | V Liquid (L) | m (g) | w (mg/g) |
|---|---|---|---|---|---|---|
| OP4 | 1.1213 | 166.55 | 300 | 0.5 | 0.5 | 133.45 |
| OP9 | 0.8870 | 227.72 | 300 | 0.5 | 0.5 | 72.28 |
| OP13 | 0.4822 | 236.32 | 300 | 0.5 | 0.5 | 63.68 |
| Model | EV/(kcal·mol−1) | EE/(kcal·mol−1) | E/(kcal·mol−1) |
|---|---|---|---|
| OP4–Anthracite | −1017.792 (95.64%) | −46.38 (4.36%) | −1064.172 |
| OP9–Anthracite | −441.722 (86.35%) | −69.797 (13.65%) | −511.519 |
| OP13–Anthracite | −436.561 (94.09%) | −27.392 (5.91%) | −463.953 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yan, G.; Kong, S.; Bai, X.; Li, G.; Zhang, J. Molecular Mechanism Study on the Effect of Microstructural Differences of Octylphenol Polyoxyethylene Ether (OPEO) Surfactants on the Wettability of Anthracite. Molecules 2023, 28, 4748. https://doi.org/10.3390/molecules28124748
Li J, Yan G, Kong S, Bai X, Li G, Zhang J. Molecular Mechanism Study on the Effect of Microstructural Differences of Octylphenol Polyoxyethylene Ether (OPEO) Surfactants on the Wettability of Anthracite. Molecules. 2023; 28(12):4748. https://doi.org/10.3390/molecules28124748
Chicago/Turabian StyleLi, Jiajun, Guochao Yan, Shaoqi Kong, Xuyang Bai, Gang Li, and Jiawei Zhang. 2023. "Molecular Mechanism Study on the Effect of Microstructural Differences of Octylphenol Polyoxyethylene Ether (OPEO) Surfactants on the Wettability of Anthracite" Molecules 28, no. 12: 4748. https://doi.org/10.3390/molecules28124748
APA StyleLi, J., Yan, G., Kong, S., Bai, X., Li, G., & Zhang, J. (2023). Molecular Mechanism Study on the Effect of Microstructural Differences of Octylphenol Polyoxyethylene Ether (OPEO) Surfactants on the Wettability of Anthracite. Molecules, 28(12), 4748. https://doi.org/10.3390/molecules28124748







