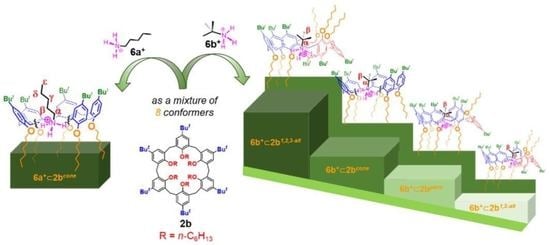
Hexahexyloxycalix[6]arene, a Conformationally Adaptive Host for the Complexation of Linear and Branched Alkylammonium Guests
Abstract
:1. Introduction
2. Results and Discussion
2.1. Binding Ability of 2b toward n-Pentylammonium Guest 6a+[B(ArF)4]–
2.2. Binding Ability of 2b toward Tert-Butylammonium Guest 6b+[B(ArF)4]–
2.3. Binding Ability of 2b toward Isopropylammonium Guest 6c+[B(ArF)4]–
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atwood, J.L.; Davies, J.E.D.; Macnicol, D.D.; Vogtle, F. Comprehensive Supramolecular Chemistry; Pergamon Press: New York, NY, USA, 1996. [Google Scholar]
- Hammes, G.G.; Chang, Y.-C.; Oas, T.G. Conformational selection or induced fit: A flux description of reaction mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 13737–13741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monod, J.; Wyman, J.; Changeux, J.-P. On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 1965, 12, 88–188. [Google Scholar] [CrossRef] [PubMed]
- Paul, F.; Weikl, T.R. How to Distinguish Conformational Selection and Induced Fit Based on Chemical Relaxation Rates. PLoS Comput. Biol. 2016, 12, e1005067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koshland, D.E. Application of a Theory of Enzyme Specificity to Protein Synthesis. Proc. Natl. Acad. Sci. USA 1958, 44, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, D.T.; Gadelle, D.; Agama, K.; Kiselev, E.; Zhang, H.; Yab, E.; Petrella, S.; Forterre, P.; Pommier, Y.; Mayer, C. Topois merase I (TOP1) Dynamics: Conformational Transition from Open to Closed States. Nat. Commun. 2022, 13, 59. [Google Scholar] [CrossRef]
- Miles, T.F.; Bower, K.S.; Lester, H.A.; Dougherty, D.A. A Coupled Array of Noncovalent Interactions Impacts the Function of the 5-HT 3 A Serotonin Receptor in an Agonist-Specific Way. ACS Chem. Neurosci. 2012, 3, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Duffy, N.H.; Lester, H.A.; Dougherty, D.A. Ondansetron and Granisetron Binding Orientation in the 5-HT3 Receptor Determined by Unnatural Amino Acid Mutagenesis. ACS Chem. Biol. 2012, 7, 1738–1745. [Google Scholar] [CrossRef] [Green Version]
- Gaeta, C.; Talotta, C.; Farina, F.; Campi, G.; Camalli, M.; Neri, P. Conformational Features and Recognition Properties of a Confo mationally Blocked Calix[7]Arene Derivative. Chem. Eur. J. 2012, 18, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, Y.; Cao, D.; Yang, Y.-W.; Jia, X.; Li, C. Host–Guest Properties of Pillar[7]Arene towards Substituted Adamantane Ammonium Cations. RSC Adv. 2014, 4, 4330–4333. [Google Scholar] [CrossRef]
- Li, C.; Shu, X.; Li, J.; Fan, J.; Chen, Z.; Weng, L.; Jia, X. Selective and Effective Binding of Pillar[5,6]Arenes toward Secondary Ammonium Salts with a Weakly Coordinating Counteranion. Org. Lett. 2012, 14, 4126–4129. [Google Scholar] [CrossRef]
- Della Sala, P.; Del Regno, R.; Talotta, C.; Capobianco, A.; Hickey, N.; Geremia, S.; De Rosa, M.; Spinella, A.; Soriente, A.; Neri, P.; et al. Prismarenes: A New Class of Macrocyclic Hosts Obtained by Templation in a Thermodynamically Controlled Synthesis. J. Am. Chem. Soc. 2020, 142, 1752–1756. [Google Scholar] [CrossRef]
- Della Sala, P.; Del Regno, R.; Di Marino, L.; Calabrese, C.; Palo, C.; Talotta, C.; Geremia, S.; Hickey, N.; Capobianco, A.; Neri, P.; et al. An Intramolecularly Self-Templated Synthesis of Macrocycles: Self-Filling Effects on the Formation of Prismarenes. Chem. Sci. 2021, 12, 9952–9961. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, P.; Del Regno, R.; Iuliano, V.; Capobianco, A.; Talotta, C.; Geremia, S.; Hickey, N.; Neri, P.; Gaeta, C. Confused Prism[5]arene: A Conformationally Adaptive Host by Stereoselective Opening of the 1,4-Bridged Naphthalene Flap. Chem. Eur. J. 2023, 29, e202203030. [Google Scholar] [CrossRef]
- Mock, W.L.; Shih, N.Y. Structure and Selectivity in Host-Guest Complexes of Cucurbituril. J. Org. Chem. 1986, 51, 4440–4446. [Google Scholar] [CrossRef]
- Yang, L.-P.; Wang, X.; Yao, H.; Jiang, W. Naphthotubes: Macrocyclic Hosts with a Biomimetic Cavity Feature. Acc. Chem. Res. 2020, 53, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; He, Z.; Yang, L.-P.; Pan, Z.-S.; Yi, M.; Jiang, R.-W.; Jiang, W. Oxatub[4]Arene: A Smart Macrocyclic Receptor with Multiple Interconvertible Cavities. Chem. Sci. 2015, 6, 6731–6738. [Google Scholar] [CrossRef] [Green Version]
- Della Sala, P.; Talotta, C.; Caruso, T.; De Rosa, M.; Soriente, A.; Neri, P.; Gaeta, C. Tuning Cycloparaphenylene Host Properties by Chemical Modification. J. Org. Chem. 2017, 82, 9885–9889. [Google Scholar] [CrossRef]
- Li, J.; Zhou, H.; Han, Y.; Chen, C. Saucer[n]arenes: Synthesis, Structure, Complexation, and Guest-Induced Circularly Polarized Luminescence Property. Angew. Chem. Int. Ed. 2021, 60, 21927–21933. [Google Scholar] [CrossRef]
- Jia, F.; Li, D.; He, S.; Yang, L.; Jiang, W. Conformational Effects on the Threading Kinetics of Dumbbell-Shaped Guests into the Cavity of Oxatub[4]Arene. Angew. Chem. Int. Ed. 2022, 61, e2022123. [Google Scholar] [CrossRef]
- Wang, X.; Jia, F.; Yang, L.-P.; Zhou, H.; Jiang, W. Conformationally Adaptive Macrocycles with Flipping Aromatic Sidewalls. Chem. Soc. Rev. 2020, 49, 4176–4188. [Google Scholar] [CrossRef]
- Del Regno, R.; Della Sala, P.; Spinella, A.; Talotta, C.; Iannone, D.; Geremia, S.; Hickey, N.; Neri, P.; Gaeta, C. Calix[2]Naphth[2]Arene: A Class of Naphthalene–Phenol Hybrid Macrocyclic Hosts. Org. Lett. 2020, 22, 6166–6170. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Talotta, C.; Gaeta, C.; Soriente, A.; Neri, P.; Pappalardo, S.; Gattuso, G.; Notti, A.; Parisi, M.F.; Pisagatti, I. Calix[5]arene through-the-Annulus Threading of Dialkylammonium Guests Weakly Paired to the TFPB Anion. J. Org. Chem. 2017, 82, 5162–5168. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, C.; Troisi, F.; Neri, P. Endo-Cavity Complexation and Through-the-Annulus Threading of Large Calixarenes Induced by Very Loose Alkylammonium Ion Pairs. Org. Lett. 2010, 12, 2092–2095. [Google Scholar] [CrossRef]
- Gaeta, C.; Talotta, C.; Farina, F.; Teixeira, F.A.; Marcos, P.M.; Ascenso, J.R.; Neri, P. Alkylammonium Cation Complexation into the Narrow Cavity of Dihomooxacalix[4]Arene Macrocycle. J. Org. Chem. 2012, 77, 10285–10293. [Google Scholar] [CrossRef]
- Talotta, C.; Gaeta, C.; De Rosa, M.; Ascenso, J.R.; Marcos, P.M.; Neri, P. Alkylammonium Guest Induced-Fit Recognition by a Flexible Dihomo oxacalix[4]Arene Derivative: Alkylammonium Guest Induced-Fit Recognition. Eur. J. Org. Chem. 2016, 2016, 158–167. [Google Scholar] [CrossRef]
- Talotta, C.; Gaeta, C.; Neri, P. Endo-Complexation of Alkylammonium Ions by Calix[4]Arene Cavity: Facilitating Cation−π Inte actions through the Weakly Coordinating Anion Approach. J. Org. Chem. 2014, 79, 9842–9846. [Google Scholar] [CrossRef]
- Kanamathareddy, S.; Gutsche, C.D. Calixarenes. 29. Aroylation and Arylmethylation of Calix[6]Arenes. J. Org. Chem. 1992, 57, 3160–3166. [Google Scholar] [CrossRef]
- Jaime, C.; De Mendoza, J.; Prados, P.; Nieto, P.M.; Sanchez, C. Carbon-13 NMR Chemical Shifts. A Single Rule to Determine the Conformation of Calix[4]Arenes. J. Org. Chem. 1991, 56, 3372–3376. [Google Scholar] [CrossRef]
- Gaeta, C.; Talotta, C.; Neri, P. Calix[6]Arene-Based Atropoisomeric Pseudo[2]Rotaxanes. Beilstein J. Org. Chem. 2018, 14, 2112–2124. [Google Scholar] [CrossRef] [Green Version]
- Hirose, K. Analytical Methods in Supramolecular Chemistry; Schalley, C.A., Ed.; Wiley-VCH: Weinheim, Germany, 2007; Chapter 2; pp. 17–54. [Google Scholar]
- Weinhold, F.; Landis, C.R. Valency and Bonding: A Natural Bond Orbital Donor-Acceptor Perspective, 1st ed.; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- Talotta, C.; Concilio, G.; de Rosa, M.; Soriente, A.; Gaeta, C.; Rescifina, A.; Ballester, P.; Neri, P. Expanding coefficient: A parameter to assess the stability of induced-fit complexes. Org. Lett. 2021, 23, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Jurcik, A.; Bednar, D.; Byska, J.; Marques, S.M.; Furmanova, K.; Daniel, L.; Kokkonen, P.; Brezovsky, J.; Strnad, O.; Stourac, J.; et al. CAVER analyst 2.0: Analysis and visualization of channels and tunnels in protein structures and molecular dynamics trajectories. Bioinformatics 2018, 34, 3586–3588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Krieger, E.; Vriend, G. YASARA View-molecular graphics for all devices-from smartphones to workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580. [Google Scholar] [CrossRef]









| Complex | Kass | +N–H···O H-Bonding Percentage of Total Binding Energy (%) |
|---|---|---|
| 6b+⊂2b1,2,3-alt | 2.6 ± 0.2 × 103 M–1 | 80 |
| 6b+⊂2bcone | 1.8 ± 0.2 × 103 M–1 | 61 |
| 6b+⊂2bpaco | 8.1 ± 0.2 × 102 M–1 | 53 |
| 6b+⊂2b1,2-alt | 4.3 ± 0.2 × 102 M–1 | 36 |
| Complex | Kass | +N–H···O H-Bonding Percentage of Total Binding Energy (%) |
|---|---|---|
| 6c+⊂2b1,2,3-alt | 3.1 ± 0.2 × 104 M–1 | 87 |
| 6c+⊂2bcone | 9.1 ± 0.2 × 103 M–1 | 77 |
| 6c+⊂2bpaco | 6.1 ± 0.2 × 103 M–1 | 66 |
| 6c+⊂2b1,2-alt | 1.2 ± 0.2 × 103 M–1 | 52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iuliano, V.; Talotta, C.; Della Sala, P.; De Rosa, M.; Soriente, A.; Neri, P.; Gaeta, C. Hexahexyloxycalix[6]arene, a Conformationally Adaptive Host for the Complexation of Linear and Branched Alkylammonium Guests. Molecules 2023, 28, 4749. https://doi.org/10.3390/molecules28124749
Iuliano V, Talotta C, Della Sala P, De Rosa M, Soriente A, Neri P, Gaeta C. Hexahexyloxycalix[6]arene, a Conformationally Adaptive Host for the Complexation of Linear and Branched Alkylammonium Guests. Molecules. 2023; 28(12):4749. https://doi.org/10.3390/molecules28124749
Chicago/Turabian StyleIuliano, Veronica, Carmen Talotta, Paolo Della Sala, Margherita De Rosa, Annunziata Soriente, Placido Neri, and Carmine Gaeta. 2023. "Hexahexyloxycalix[6]arene, a Conformationally Adaptive Host for the Complexation of Linear and Branched Alkylammonium Guests" Molecules 28, no. 12: 4749. https://doi.org/10.3390/molecules28124749