Extraction and Encapsulation of Phytocompounds of Poniol Fruit via Co-Crystallization: Physicochemical Properties and Characterization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Activity, Entrapment Yield, and Loading Capacity of the Co-Crystallized (Cc-Pe) Sample
2.2. Bulk Density and Tapped Density
2.3. Flowability
2.4. Hygroscopicity
2.5. Solubilization Time
2.6. X-ray Diffraction Analysis
2.7. Microstructure of Microencapsulated PONIOL (F. Jangomas) Powder after Co-Crystallization
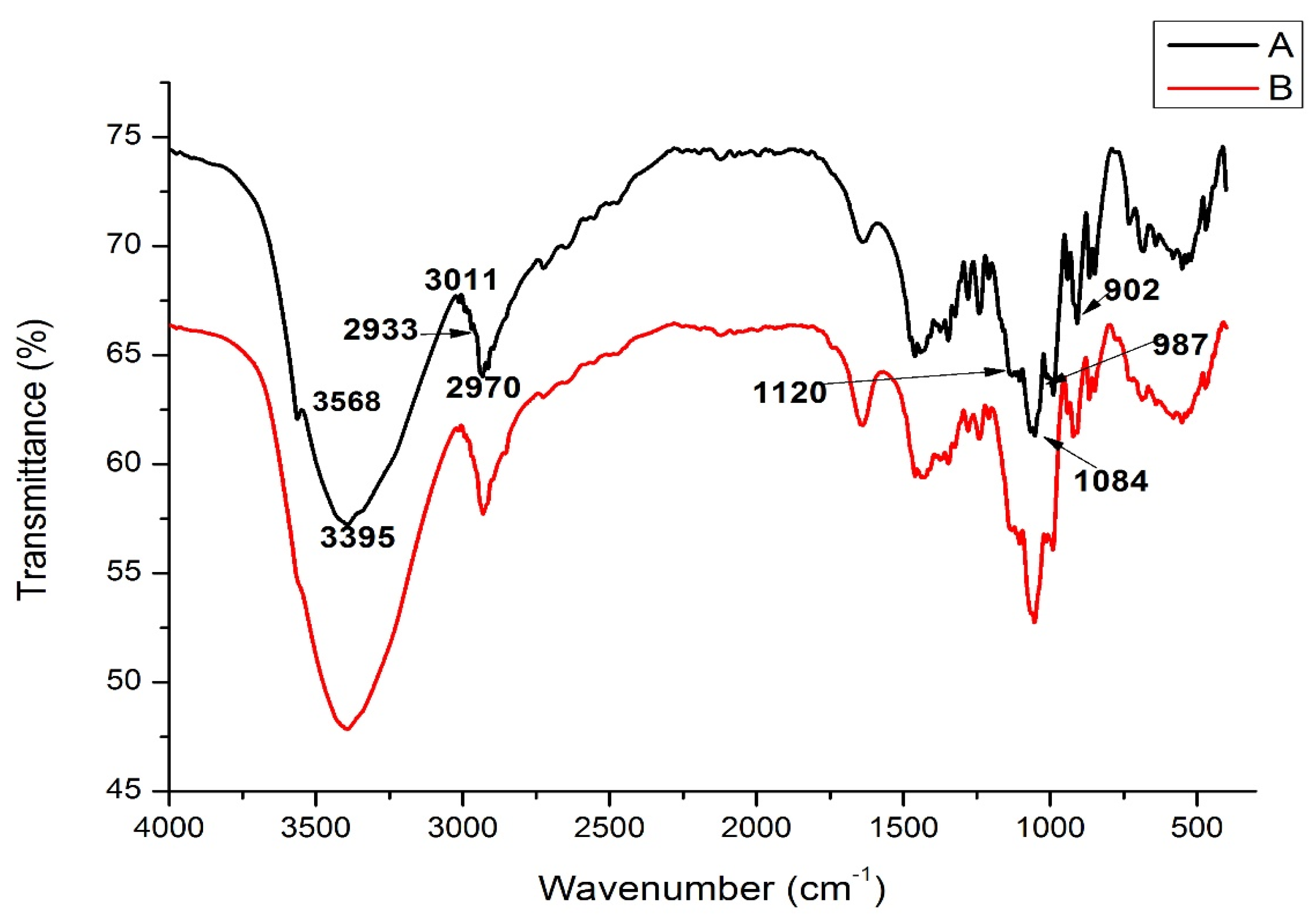
2.8. Fourier Transform Infrared (FT-IR) Spectroscopy
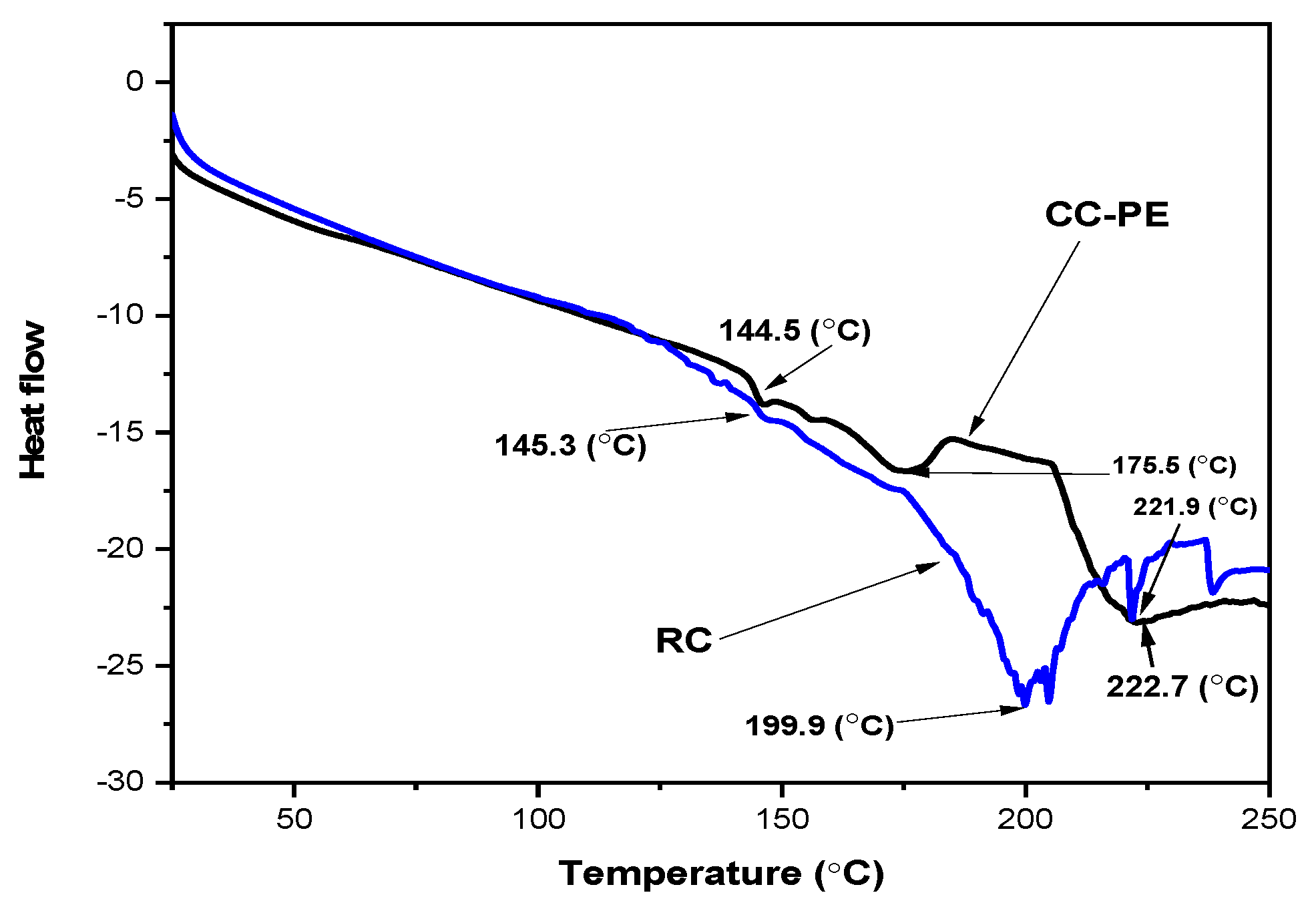
2.9. Thermal (DSC) Analysis of Co-Crystallized Poniol Extract Powder
3. Material and Methods
3.1. Raw Material and Chemicals and Reagents
3.2. Fruit Extract Preparation
3.3. Preparation of the Co-Crysta–Llization Products
3.3.1. Determination of Total Phenolic Content
3.3.2. Determination of Antioxidant Activity
3.3.3. Loading Capacity and Entrapment Yield [11]
3.3.4. Bulk Density, Tapped Density, and Flowability
3.3.5. Hygroscopicity
3.3.6. Solubilization Time
3.3.7. X-ray Diffraction (XRD)
3.3.8. Scanning Electron Microscopy (SEM)
3.3.9. FTIR (Fourier Transform Infrared Spectroscopy) Analysis
3.3.10. Thermal Analysis (Differential Scanning Calorimetry)
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sasi, S.; Anjum, N.; Tripathi, Y.C. Ethnomedicinal, Phytochemical and Pharmacological Aspects of Flacourtia Jangomas: A Review. Int. J. Pharm. Pharm. Sci. 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Ara, R.; Jahan, S.; Abdullah, A.; Fakhruddin, A.N.M.; Saha, B. Physico-chemical properties and mineral content of selected tropical fruits in Bangladesh. Bangladesh J. Sci. Ind. Res. 2015, 49, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Barua, U.; Das, R.; Gogoi, B.; Baruah, B.G.S. Underutilized Fruits of Assam for Livelihood and Nutritional Security. Agric. Rev. 2019, 40, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Jeyachandran, R.; Mahesh, A. Enumeration of Antidiabetic Herbal Flora of Tamil Nadu. Res. J. Med. Plant 2007, 1, 144–148. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.K. Flacourtia jangomas. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2013; pp. 771–775. [Google Scholar] [CrossRef]
- Karangutkar, A.V.; Ananthanarayan, L. Co-crystallization of Basella rubra extract with sucrose: Characterization of co-crystals and evaluating the storage stability of betacyanin pigments. J. Food Eng. 2020, 271, 109776. [Google Scholar] [CrossRef]
- Chezanoglou, E.; Goula, A.M. Co-crystallization in sucrose: A promising method for encapsulation of food bioactive components. Trends Food Sci. Technol. 2021, 114, 262–274. [Google Scholar] [CrossRef]
- Chikodili, I.M.; Chioma, I.I.; Ukamaka, I.A.; Nnenna, O.T.; Ogechukwu, O.D.; Mmesoma, E.E.; Chikodi, E.C.; IfedibaluChukwu, E.I. Phytocompound inhibitors of caspase 3 as beta-cell apoptosis treatment development option: An In-silico approach. Sci. Phytochem. 2023, 2, 17–37. [Google Scholar] [CrossRef]
- Chezanoglou, E.; Kenanidou, N.; Spyropoulos, C.; Xenitopoulou, D.; Zlati, E.; Goula, A.M. Encapsulation of pomegranate peel extract in sucrose matrix by co-crystallization. Sustain. Chem. Pharm. 2023, 31, 100949. [Google Scholar] [CrossRef]
- Tzatsi, P.; Goula, A.M. Encapsulation of Extract from Unused Chokeberries by Spray Drying, Co-crystallization, and Ionic Gelation. Waste Biomass-Valoriz. 2021, 12, 4567–4585. [Google Scholar] [CrossRef]
- Kaur, P.; Elsayed, A.; Subramanian, J.; Singh, A. Encapsulation of carotenoids with sucrose by co-crystallization: Physicochemical properties, characterization and thermal stability of pigments. LWT 2021, 140, 110810. [Google Scholar] [CrossRef]
- Wang, H.; Gao, S.; Zhang, D.; Wang, Y.; Zhang, Y.; Jiang, S.; Li, B.; Wu, D.; Lv, G.; Zou, X.; et al. Encapsulation of catechin or curcumin in co-crystallized sucrose: Fabrication, characterization and application in beef meatballs. LWT 2022, 168, 113911. [Google Scholar] [CrossRef]
- Queiroz, M.B.; Sousa, F.R.; da Silva, L.B.; Alves, R.M.V.; Alvim, I.D. Co-crystallized sucrose-soluble fiber matrix: Physicochemical and structural characterization. LWT 2022, 154, 112685. [Google Scholar] [CrossRef]
- Rai, K.; Chhanwal, N.; Shah, N.N.; Singhal, R.S. Encapsulation of ginger oleoresin in co-crystallized sucrose: Development, characterization and storage stability. Food Funct. 2021, 12, 7964–7974. [Google Scholar] [CrossRef]
- Behnamnik, A.; Vazifedoost, M.; Didar, Z.; Hajirostamloo, B. Evaluation of physicochemical, structural, and antioxidant properties of microencapsulated seed extract from Securigera securidaca by co-crystallization method during storage time. Biocatal. Agric. Biotechnol. 2021, 35, 102090. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Deladino, L.; Agudelo-Mesa, L.; Martino, M. Yerba mate antioxidant powders obtained by co-crystallization: Stability during storage. J. Food Eng. 2014, 124, 158–165. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Matera, S.; Deladino, L.; Hoya, A.; Navarro, A.; Martino, M. Compressed tablets based on mineral-functionalized starch and co-crystallized sucrose with natural antioxidants. J. Food Eng. 2015, 146, 234–242. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Gallo, L.; Bucalá, V.; Martino, M.; Navarro, A. Co-crystallization of zinc sulfate with sucrose: A promissory strategy to render zinc solid dosage forms more palatable. J. Food Eng. 2016, 170, 100–107. [Google Scholar] [CrossRef]
- Barua, U.; Das, R.P.; Das, P.; Das, K.; Gogoi, B. Morpho-physiological, proximate composition and antioxidant activity of Flacourtia jangomas (Lour.) Raeus. Plant Physiol. Rep. 2022, 27, 56–64. [Google Scholar] [CrossRef]
- Biswas, S.C.; Kumar, P.; Kumar, R.; Das, S.; Misra, T.K.; Dey, D. Nutritional Composition and Antioxidant Properties of the Wild Edible Fruits of Tripura, Northeast India. Sustainability 2022, 14, 12194. [Google Scholar] [CrossRef]
- Hossain, M.; Rahim, A.; Haque, R. Biochemical properties of some important underutilized minor fruits. J. Agric. Food Res. 2021, 5, 100148. [Google Scholar] [CrossRef]
- Srivastava, D.; Prabhuji, S.; Tripathi, A.; Srivastava, R.; Mishra, P. In vitroantibacterial activities of Flacourtia jungomas (Lour.) Raeus. fruit extracts. Med. Plants—Int. J. Phytomed. Relat. Ind. 2012, 4, 98–100. [Google Scholar] [CrossRef]
- Das, S.; Dewan, N.; Das, K.J.; Kalita, D. Preliminary Phytochemical, Antioxidant and Antimicrobial Studies of Flacourtia jangomas Fruits. Int. J. Curr. Pharm. Res. 2017, 9, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Saikia, S.; Nikhil Kumar Mahnot, N.K.; Charu Lata Mahanta, C.L. Phytochemical content and antioxidant activities of thirteen fruits of Assam, India. Food Biosci. 2016, 13, 15–20. [Google Scholar] [CrossRef]
- BehnamNik, A.; Vazifedoost, M.; Didar, Z.; Hajirostamloo, B. The antioxidant and physicochemical properties of microencapsulated bioactive compounds in Securigera securidaca (L.) seed extract by co-crystallization. Food Qual. Saf. 2019, 3, 243–250. [Google Scholar] [CrossRef]
- García-Pérez, P.; Gallego, P.P. Plant Phenolics as Dietary Antioxidants: Insights on Their Biosynthesis, Sources, Health-Promoting Effects, Sustainable Production, and Effects on Lipid Oxidation. In Lipid Oxidation in Food and Biological Systems; Bravo-Diaz, C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 405–426. [Google Scholar] [CrossRef]
- Jesus, F.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Health Benefits of Prunus avium Plant Parts: An Unexplored Source Rich in Phenolic Compounds. Food Rev. Int. 2022, 38, 118–146. [Google Scholar] [CrossRef]
- Rajashekar, C.B. Dual Role of Plant Phenolic Compounds as Antioxidants and Prooxidants. Am. J. Plant Sci. 2023, 14, 15–28. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Sarabandi, K.; Mahoonak, A.S.; Akbari, M. Physicochemical properties and antioxidant stability of microencapsulated marjoram extract prepared by co-crystallization method. J. Food Process. Eng. 2019, 42, e12949. [Google Scholar] [CrossRef] [Green Version]
- Deladino, L.; Anbinder, P.S.; Navarro, A.S.; Martino, M.N. Co-crystallization of yerba mate extract (Ilex paraguariensis) and mineral salts within a sucrose matrix. J. Food Eng. 2007, 80, 573–580. [Google Scholar] [CrossRef]
- Irigoiti, Y.; Yamul, D.K.; Navarro, A.S. Co-crystallized sucrose with propolis extract as a food ingredient: Powder characterization and antioxidant stability. LWT 2021, 143, 111164. [Google Scholar] [CrossRef]
- Jeong, Y.; Yoo, B. Physical, Morphological, and Rheological Properties of Agglomerated Milk Protein Isolate Powders: Effect of Binder Type and Concentration. Polymers 2023, 15, 411. [Google Scholar] [CrossRef] [PubMed]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- Bhandari, B.; Hartel, R. Co-crystallization of Sucrose at High Concentration in the Presence of Glucose and Fructose. J. Food Sci. 2002, 67, 1797–1802. [Google Scholar] [CrossRef]
- Sardar, B.R.; Singhal, R.S. Characterization of co-crystallized sucrose entrapped with cardamom oleoresin. J. Food Eng. 2013, 117, 521–529. [Google Scholar] [CrossRef]
- Bajaj, S.R.; Singhal, R.S. Enhancement of stability of vitamin B12 by co-crystallization: A convenient and palatable form of fortification. J. Food Eng. 2021, 291, 110231. [Google Scholar] [CrossRef]
- Chen, A.C.; Veiga, M.F.; Rizzuto, A.B. Co-crystallization: An encapsulation process. Food Technol. 1989, 42, 87–90. [Google Scholar]
- Limm, W.; Karunathilaka, S.R.; Mossoba, M.M. Fourier Transform Infrared Spectroscopy and Chemometrics for the Rapid Screening of Economically Motivated Adulteration of Honey Spiked With Corn or Rice Syrup. J. Food Prot. 2023, 86, 100054. [Google Scholar] [CrossRef]
- Riswahyuli, Y.; Rohman, A.; Setyabudi, F.M.C.S.; Raharjo, S. Indonesian wild honey authenticity analysis using attenuated total reflectance-fourier transform infrared (ATR-FTIR) spectroscopy combined with multivariate statistical techniques. Heliyon 2020, 6, e03662. [Google Scholar] [CrossRef]
- Salelign, K.; Duraisamy, R. Sugar and ethanol production potential of sweet potato (Ipomoea batatas) as an alternative energy feedstock: Processing and physicochemical characterizations. Heliyon 2021, 7, e08402. [Google Scholar] [CrossRef]
- Sritham, E.; Gunasekaran, S. FTIR spectroscopic evaluation of sucrose-maltodextrin-sodium citrate bioglass. Food Hydrocoll. 2017, 70, 371–382. [Google Scholar] [CrossRef]
- Teklemariam, T.A.; Moisey, J.; Gotera, J. Attenuated Total Reflectance-Fourier transform infrared spectroscopy coupled with chemometrics for the rapid detection of coconut water adulteration. Food Chem. 2021, 355, 129616. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kliks, M.M.; Jun, S.; Jackson, M.; Li, Q.X. Rapid Analysis of Glucose, Fructose, Sucrose, and Maltose in Honeys from Different Geographic Regions using Fourier Transform Infrared Spectroscopy and Multivariate Analysis. J. Food Sci. 2010, 75, C208–C214. [Google Scholar] [CrossRef] [PubMed]
- Fangio, M.F.; Orallo, D.E.; Gende, L.B.; Churio, M.S. Chemical characterization and antimicrobial activity against Paenibacillus larvae of propolis from Buenos Aires province, Argentina. J. Apic. Res. 2019, 58, 626–638. [Google Scholar] [CrossRef]
- Raemy, A.; Schweizer, T.F. Thermal behaviour of carbohydrates studied by heat flow calorimetry. J. Therm. Anal. 1983, 28, 95–108. [Google Scholar] [CrossRef]
- Cevallos, P.A.P.; Buera, M.P.; Elizalde, B.E. Encapsulation of cinnamon and thyme essential oils components (cinnamaldehyde and thymol) in β-cyclodextrin: Effect of interactions with water on complex stability. J. Food Eng. 2010, 99, 70–75. [Google Scholar] [CrossRef]
- Pralhad, T.; Rajendrakumar, K. Study of freeze-dried quercetin–cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. J. Pharm. Biomed. Anal. 2004, 34, 333–339. [Google Scholar] [CrossRef]
- Vasisht, K.; Chadha, K.; Karan, M.; Bhalla, Y.; Jena, A.K.; Chadha, R. Enhancing biopharmaceutical parameters of bioflavonoid quercetin by co-crystallization. Crystengcomm 2016, 18, 1403–1415. [Google Scholar] [CrossRef]
- Deladino, L.; Navarro, A.S.; Martino, M.N. Microstructure of minerals and yerba mate extract co-crystallized with sucrose. J. Food Eng. 2010, 96, 410–415. [Google Scholar] [CrossRef]
- Bozkir, B.; Acet, T.; Özcan, K. Investigation of the effects of different extraction methods on some biological activities of Dactylorhiza romana subsp. georgica (Klinge) Soó ex Renz & Taubenheim. S. Afr. J. Bot. 2022, 149, 347–354. [Google Scholar] [CrossRef]
- Szalata, M.; Dreger, M.; Zielińska, A.; Banach, J.; Szalata, M.; Wielgus, K. Simple Extraction of Cannabinoids from Female Inflorescences of Hemp (Cannabis sativa L.). Molecules 2022, 27, 5868. [Google Scholar] [CrossRef]
- Yang, L.; Shen, S.-Y.; Wang, Z.-N.; Yang, T.; Guo, J.-W.; Hu, R.-Y.; Li, Y.-F.; Burner, D.M.; Ying, X.-M. New Value-Added Sugar and Brown Sugar Products from Sugarcane: A Commercial Approach. Sugar Technol 2020, 22, 853–857. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; pp. 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, L.; Liu, X.; Hasan, K.F.; Li, H.; Zhou, S.; Zhang, Q.; Zhou, Y. Effect of thermosonication treatment on blueberry juice quality: Total phenolics, flavonoids, anthocyanin, and antioxidant activity. LWT 2021, 150, 112021. [Google Scholar] [CrossRef]
- Cai, Y.; Corke, H. Production and Properties of Spray-dried Amaranthus Betacyanin Pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Daza, L.D.; Fujita, A.; Fávaro-Trindade, C.S.; Rodrigues-Ract, J.N.; Granato, D.; Genovese, M.I. Effect of spray drying conditions on the physical properties of Cagaita (Eugenia dysenterica DC.) fruit extracts. Food Bioprod. Process. 2016, 97, 20–29. [Google Scholar] [CrossRef]


| Products | Loading Capacity LC. (mg GAE/100 g) | TPC (mg GAE/100 g of Dried Fruit) | Entrapment Yield (%) | Antioxidant Activity (%) |
|---|---|---|---|---|
| CC-PE | 29.25 ± 0.03 | 29.25 ± 0.03 | 76.38 ± 0.07 | 65.10 ± 0.03 |
| PE | NA | 38.17 ± 0.04 | NA | 98.42 ± 0.37 |
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.7 ± 0.03 |
| Sample | Bulk Density | Tapped Density | Flowability (HR) | Hygroscopicity (%) | Solubilization Time (sec) |
|---|---|---|---|---|---|
| RC | 0.716 ± 0.034 | 0.774 ± 0.034 | 1.034 ± 0.042 | 12.345 ± 0.036 | 64.8 ± 0.1 |
| CC-PE | 0.723 ± 0.023 | 0.748 ± 0.022 | 1.082 ± 0.003 | 11.672 ± 0.023 | 62.5 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, N.A.; Dash, K.K.; Pandey, V.K.; Tripathi, A.; Mukarram, S.A.; Harsányi, E.; Kovács, B. Extraction and Encapsulation of Phytocompounds of Poniol Fruit via Co-Crystallization: Physicochemical Properties and Characterization. Molecules 2023, 28, 4764. https://doi.org/10.3390/molecules28124764
Ali NA, Dash KK, Pandey VK, Tripathi A, Mukarram SA, Harsányi E, Kovács B. Extraction and Encapsulation of Phytocompounds of Poniol Fruit via Co-Crystallization: Physicochemical Properties and Characterization. Molecules. 2023; 28(12):4764. https://doi.org/10.3390/molecules28124764
Chicago/Turabian StyleAli, N. Afzal, Kshirod Kumar Dash, Vinay Kumar Pandey, Anjali Tripathi, Shaikh Ayaz Mukarram, Endre Harsányi, and Béla Kovács. 2023. "Extraction and Encapsulation of Phytocompounds of Poniol Fruit via Co-Crystallization: Physicochemical Properties and Characterization" Molecules 28, no. 12: 4764. https://doi.org/10.3390/molecules28124764
APA StyleAli, N. A., Dash, K. K., Pandey, V. K., Tripathi, A., Mukarram, S. A., Harsányi, E., & Kovács, B. (2023). Extraction and Encapsulation of Phytocompounds of Poniol Fruit via Co-Crystallization: Physicochemical Properties and Characterization. Molecules, 28(12), 4764. https://doi.org/10.3390/molecules28124764











