Characterization, Recombinant Production, and Bioactivity of a Novel Immunomodulatory Protein from Hypsizygus marmoreus
Abstract
:1. Introduction
2. Results and Discussion
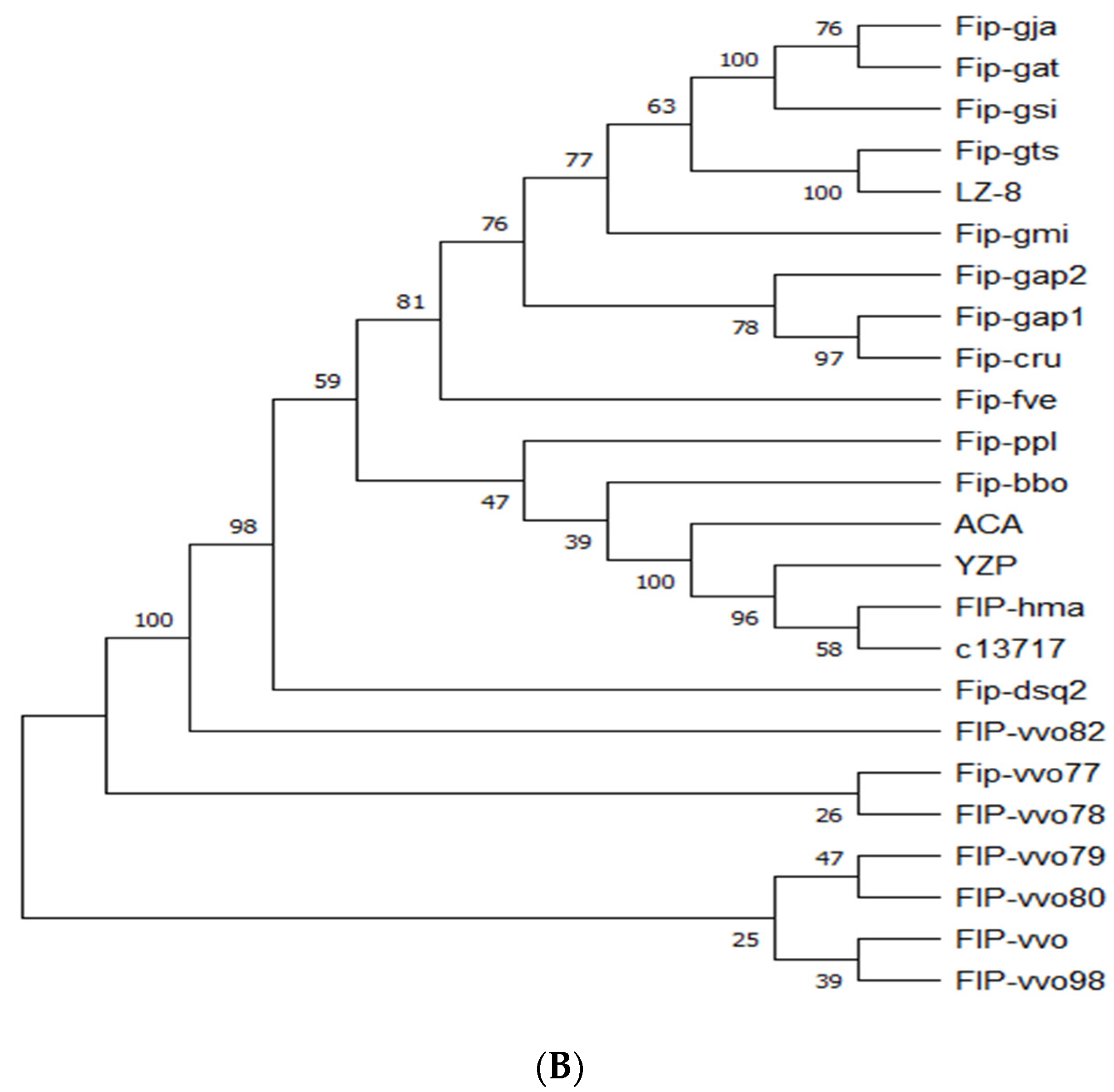
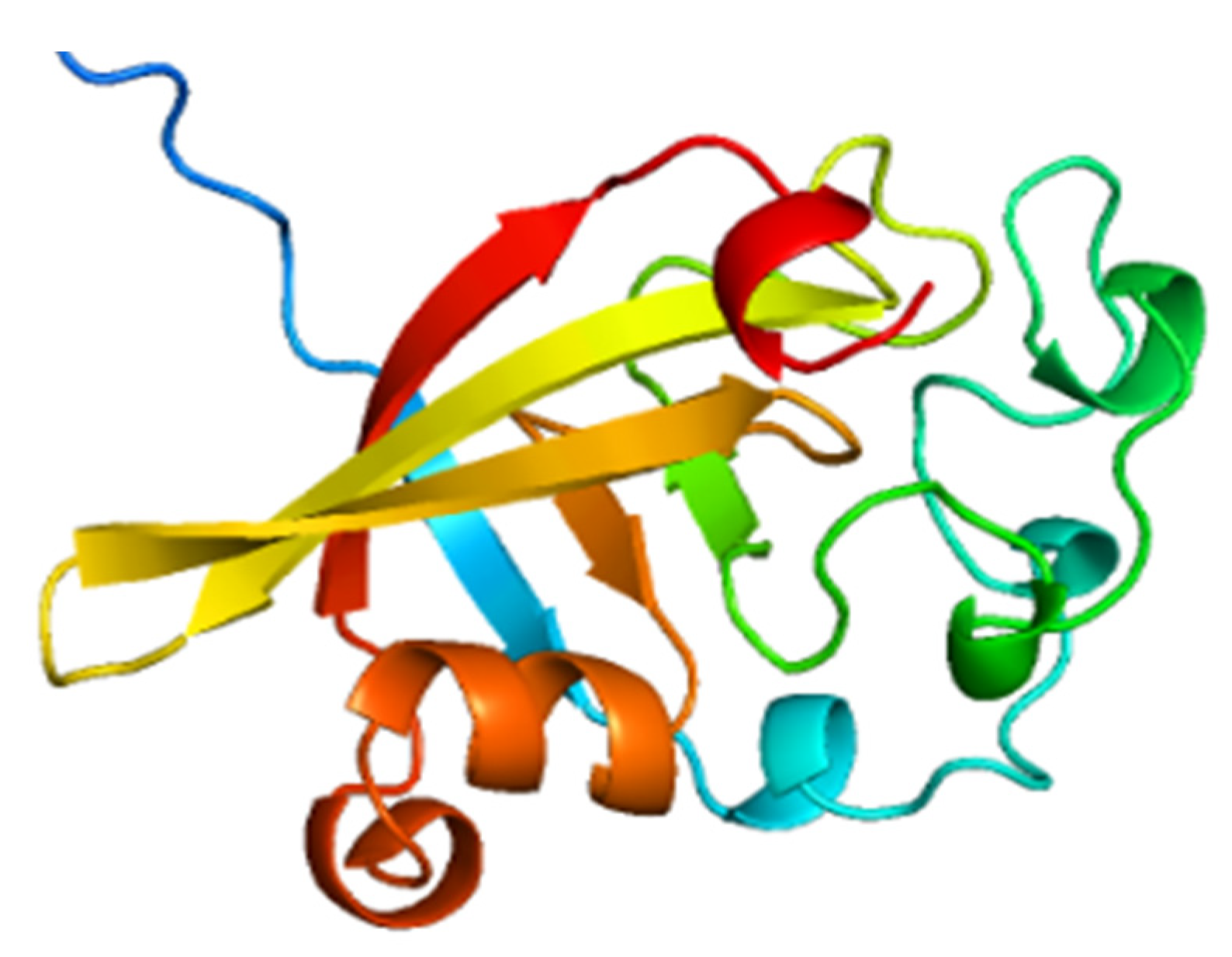
2.1. Structural and Biochemical Properties of FIP-hma
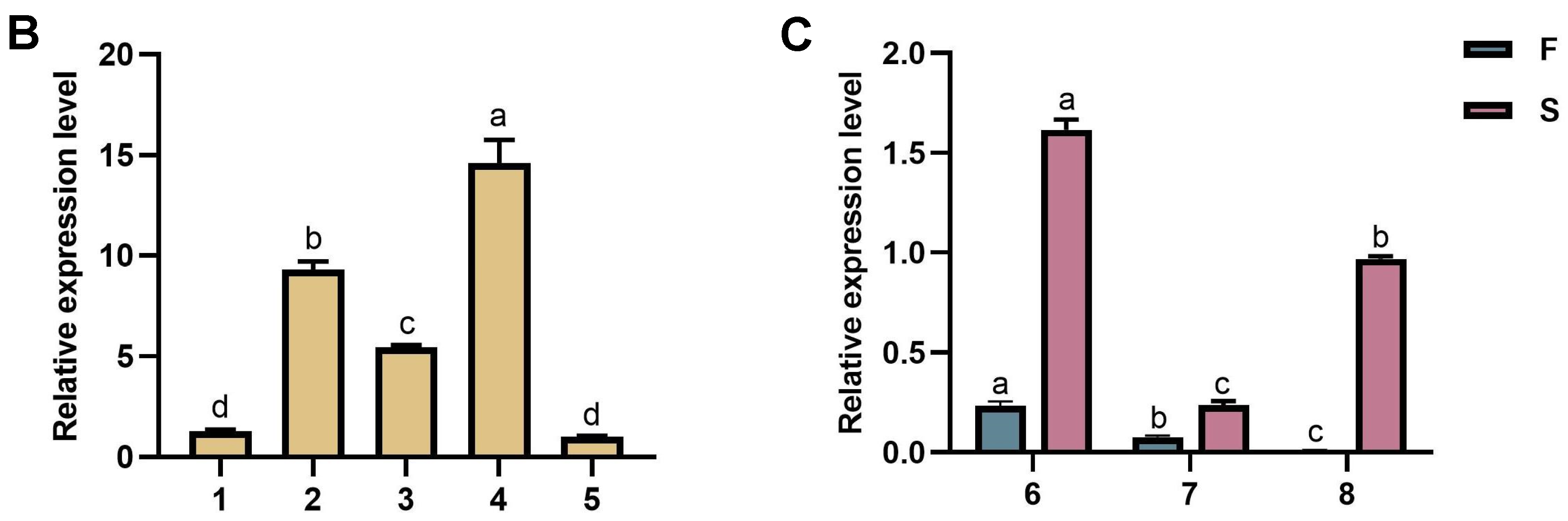
2.2. Real-Time RT-PCR Detection of FIP-hma Gene Expression Levels during the Development of H. marmoreus
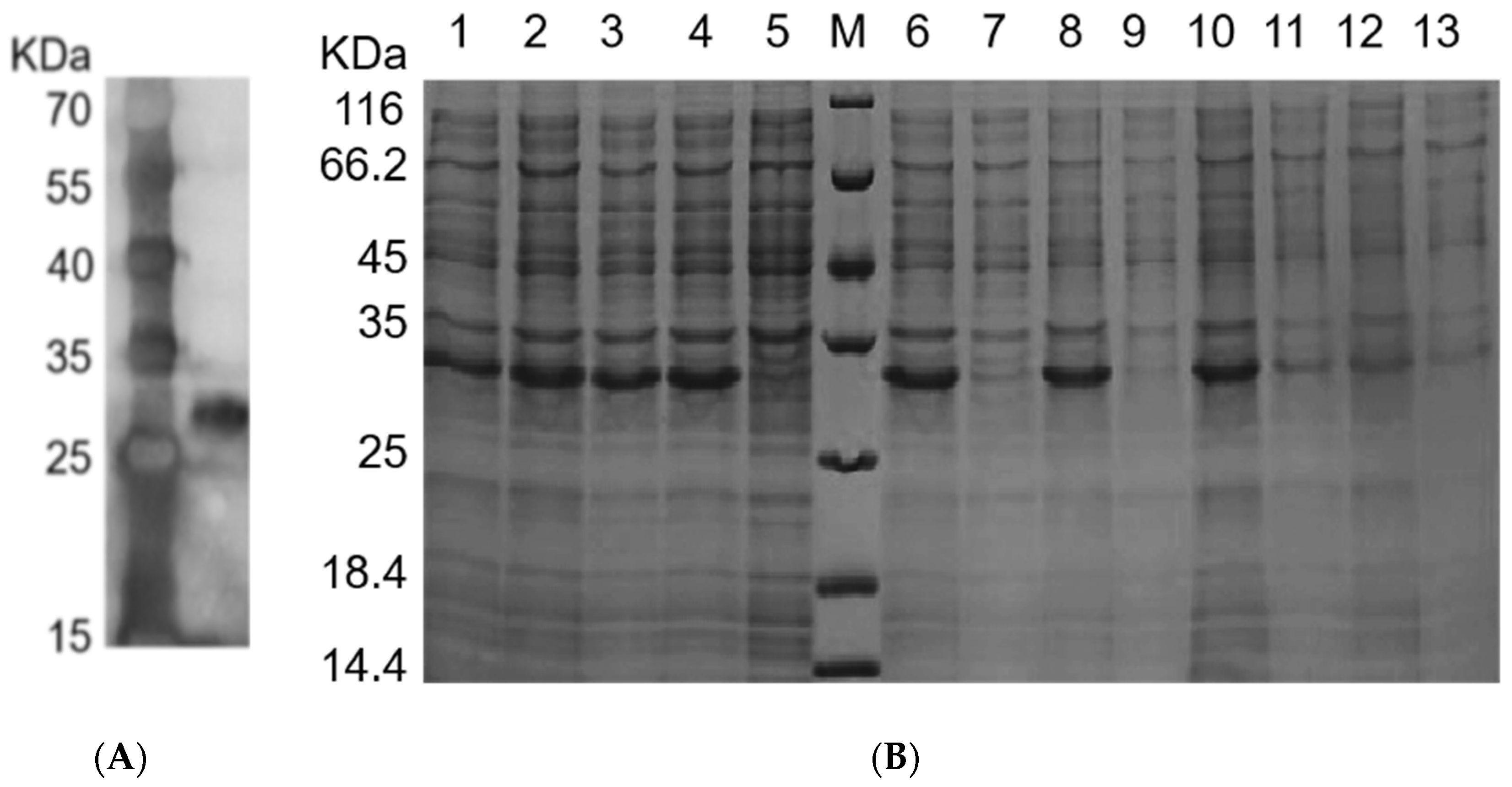
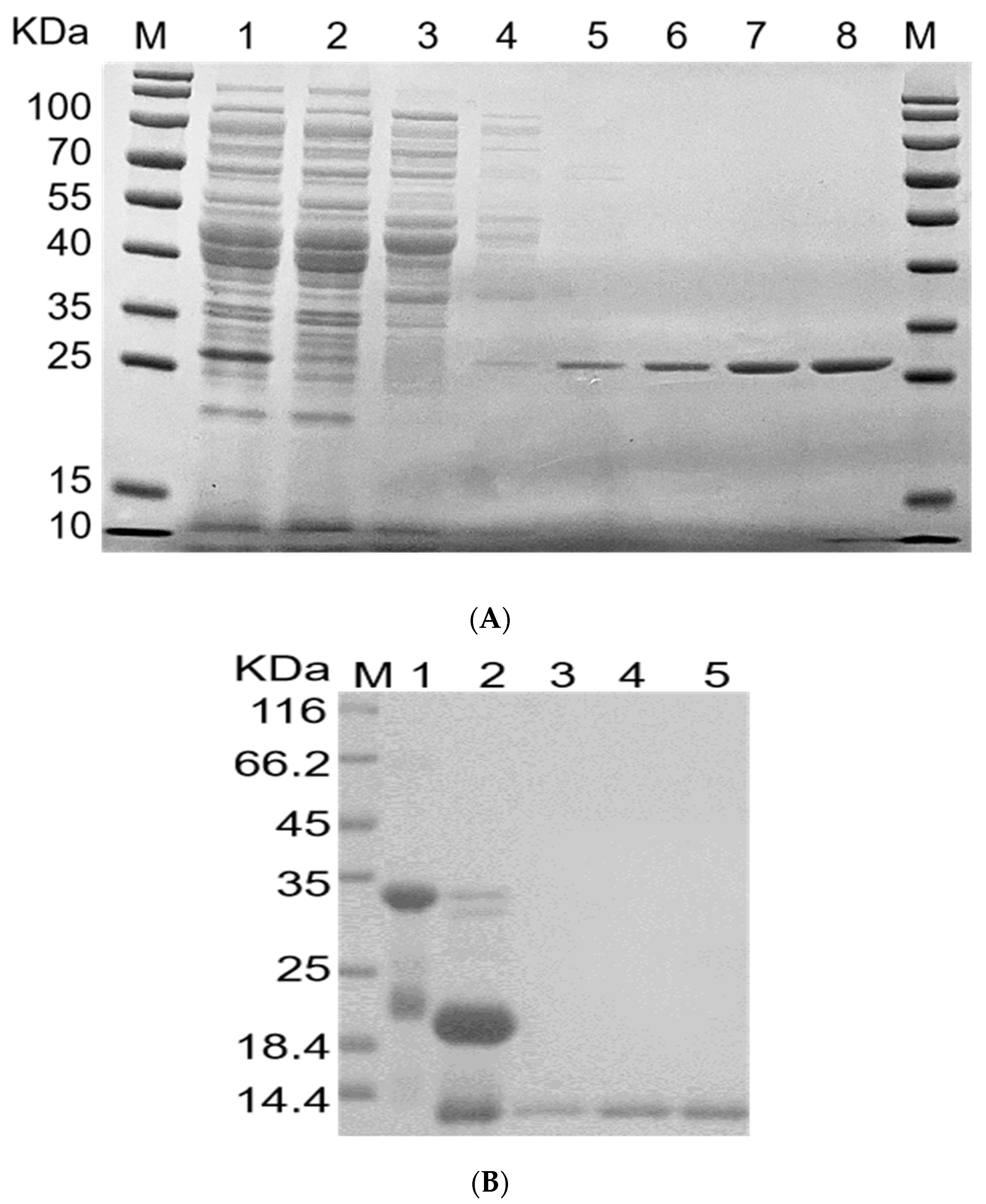
2.3. Expression of rFIP-hma in E. coli
2.4. Purification of rFIP-hma
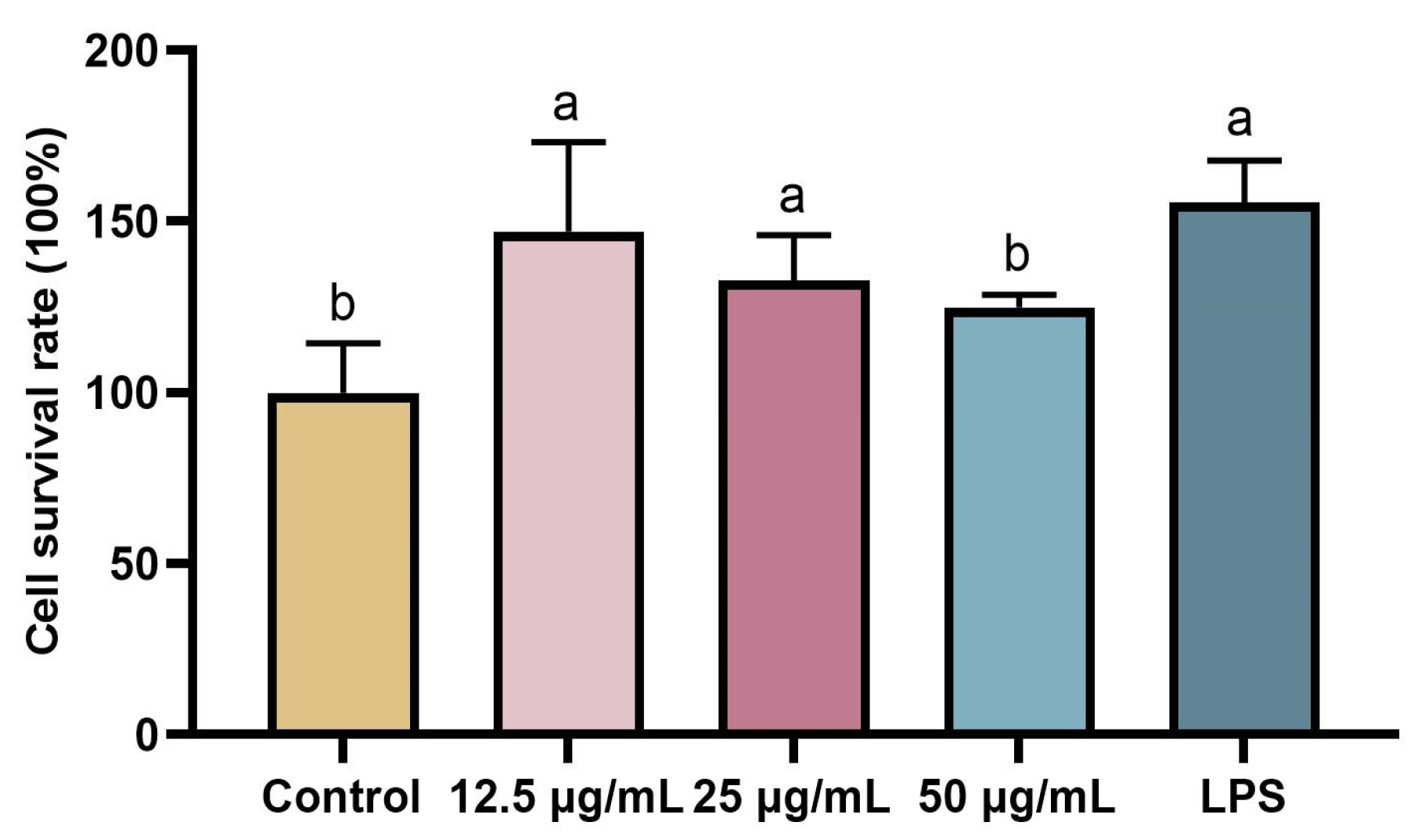
2.5. Detecting the Toxicity of rFIP-hma to RAW 264.7 by MTT
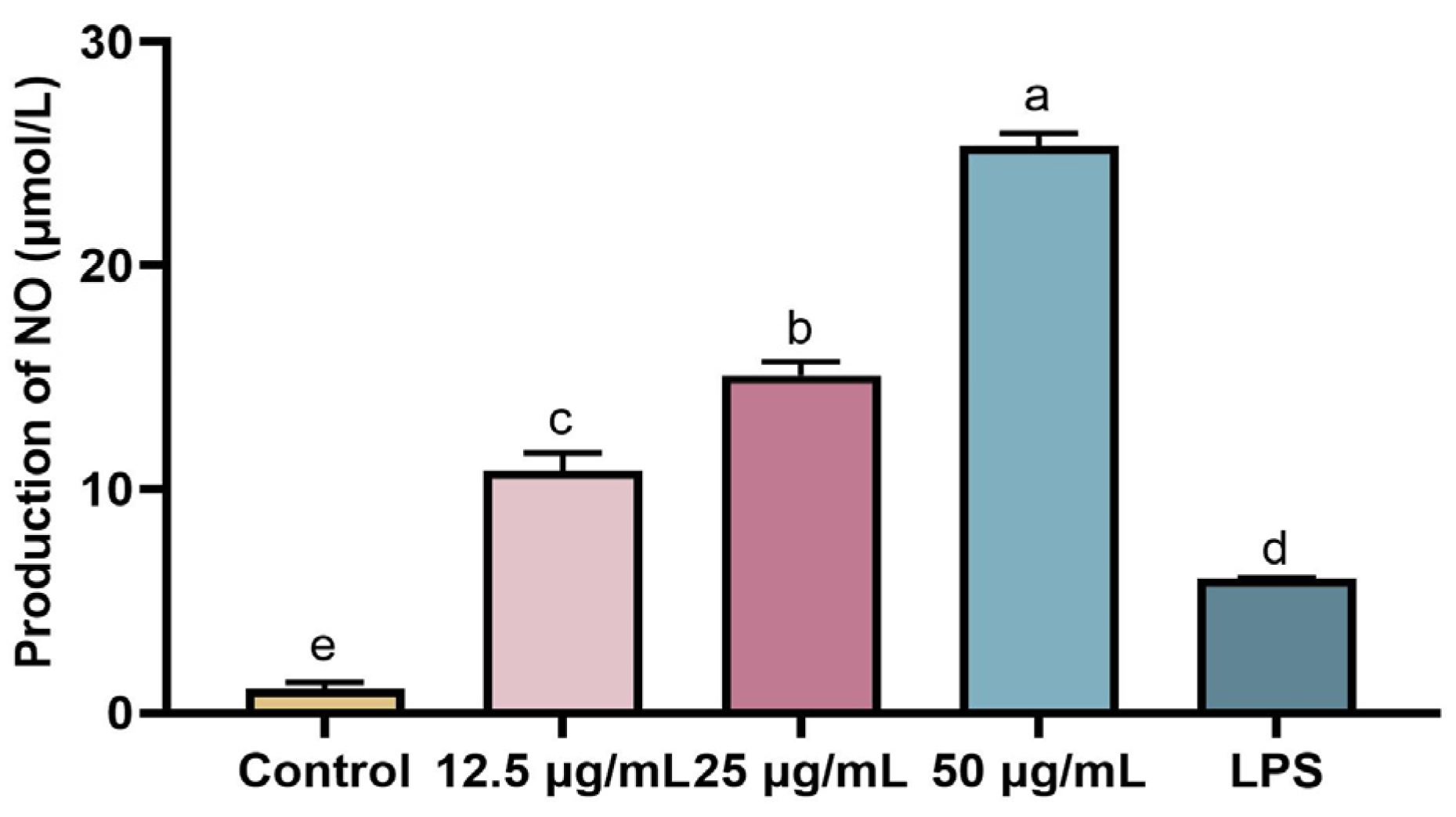
2.6. Effect of rFIP-hma on NO Production
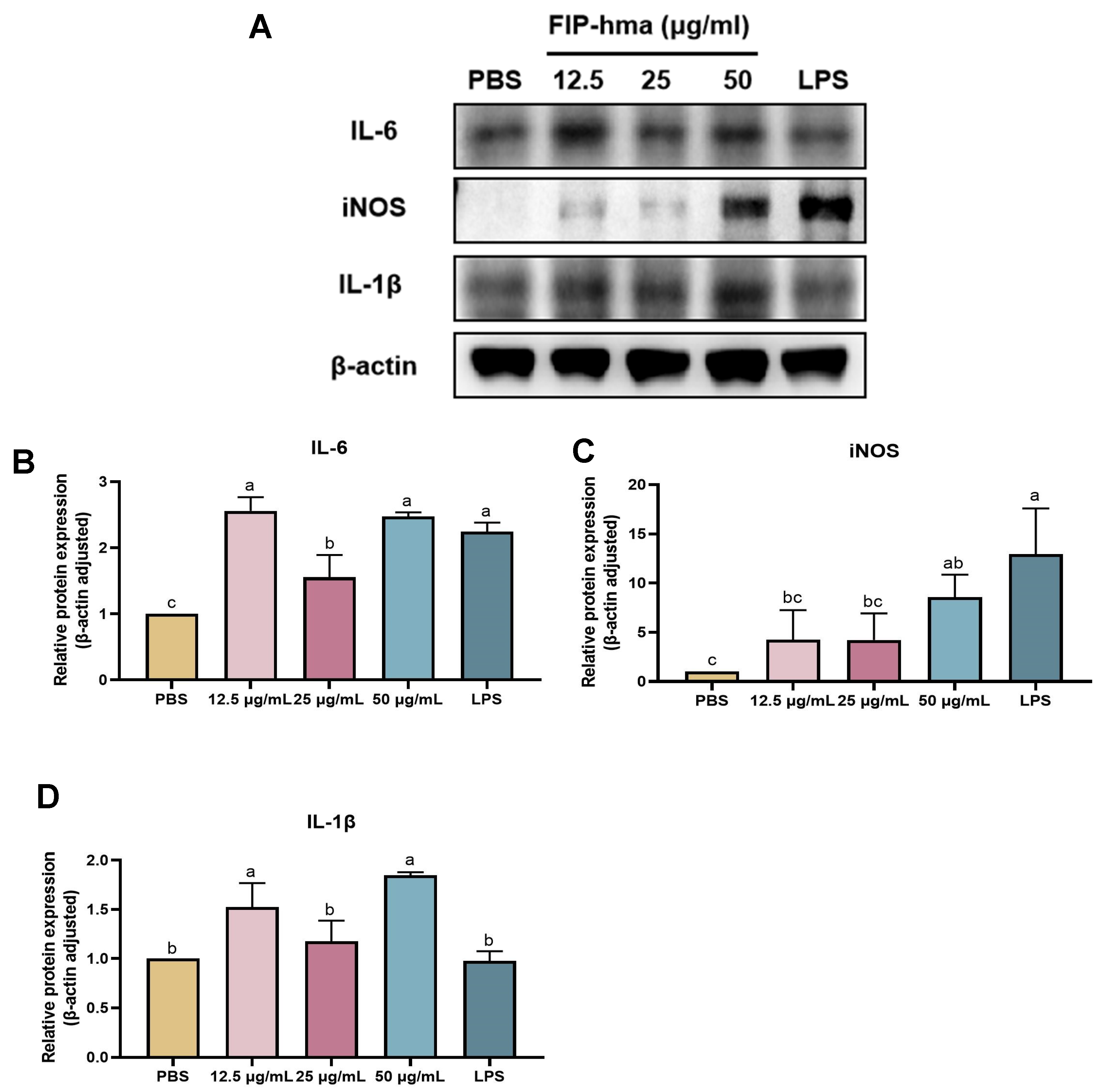
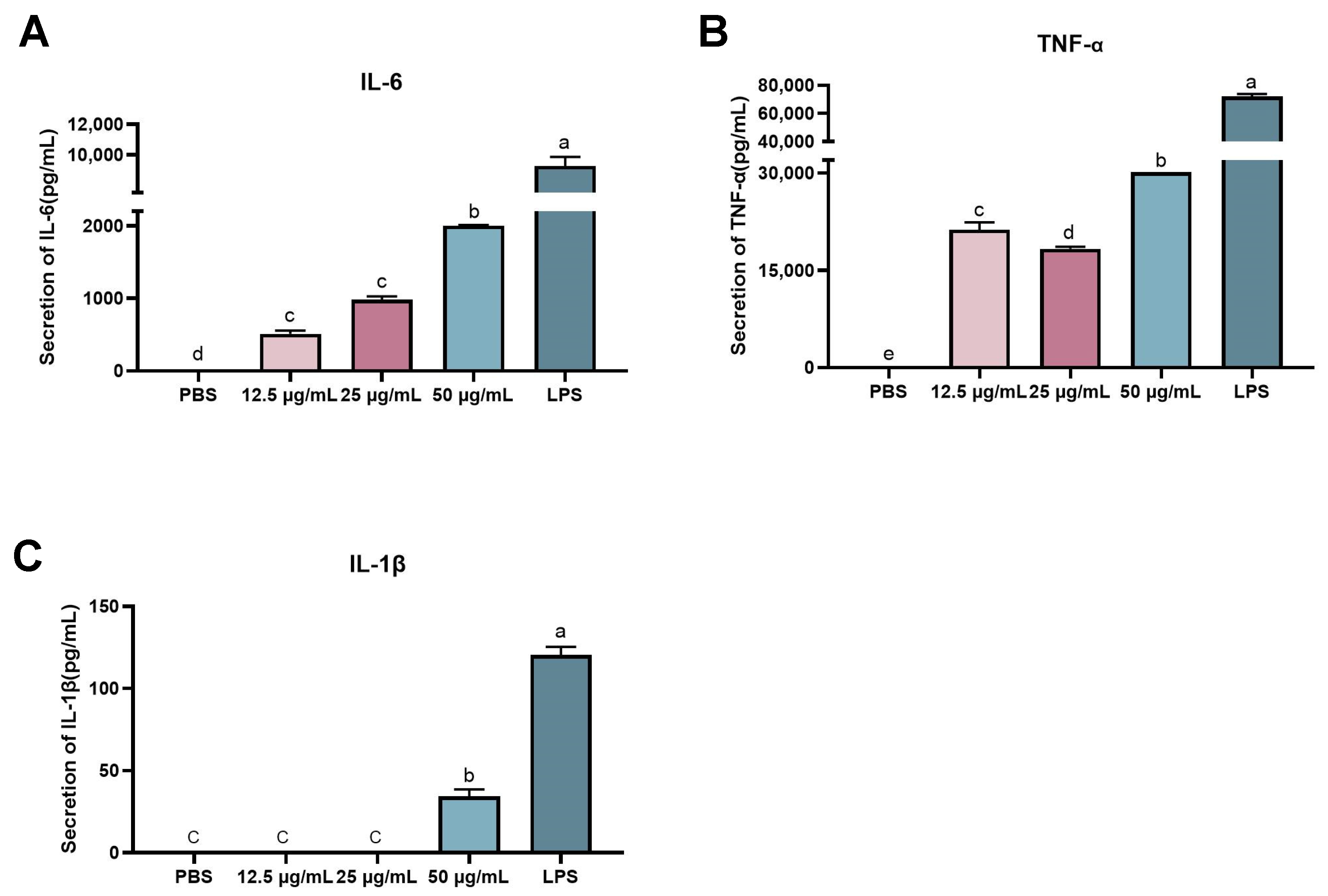
2.7. Effect of rFIP-hma on Cytokine Expression and Release
3. Materials and Methods
3.1. Materials
3.2. Bioinformatics Analysis
3.3. Real-Time PCR Assay
3.4. Expression of rFIP-hma
3.5. Purification of rFIP-hma
3.6. Cell Culture
3.7. MTT Assay
3.8. NO Determination Assay
3.9. Effect of rFIP-hma on Cytokine Expression and Release
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kala, K.; Pajak, W.; Sulkowska-Ziaja, K.; Krakowska, A.; Lazur, J.; Fidurski, M.; Marzec, K.; Zieba, P.; Fijalkowska, A.; Szewczyk, A.; et al. Hypsizygus marmoreus as a Source of Indole Compounds and Other Bioactive Substances with Health-Promoting Activities. Molecules 2022, 27, 8917. [Google Scholar] [CrossRef] [PubMed]
- El Enshasy, H.A.; Hatti-Kaul, R. Mushroom immunomodulators: Unique molecules with unlimited applications. Trends Biotechnol. 2013, 31, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Ayeka, P.A. Potential of Mushroom Compounds as Immunomodulators in Cancer Immunotherapy: A Review. Evid.-Based Complement. Altern. Med. 2018, 2018, 7271509. [Google Scholar] [CrossRef] [PubMed]
- Motta, F.; Gershwin, M.E.; Selmi, C. Mushrooms and immunity. J. Autoimmun. 2021, 117, 102576. [Google Scholar] [CrossRef]
- Xie, Y.; Li, S.; Sun, L.; Liu, S.; Wang, F.; Wen, B.; Sun, L.; Fang, X.; Chai, Y.; Cao, H.; et al. Fungal immunomodulatory protein from Nectria haematococca suppresses growth of human lung adenocarcinoma by inhibiting the PI3K/Akt Pathway. Int. J. Mol. Sci. 2018, 19, 3429. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Yao, W.J.S.; Zhu, Y.F.; Liu, H.; Zhang, J.J.; Jia, L. Characterization, antioxidant and antiinflammation of mycelia selenium polysaccharides from Hypsizygus marmoreus SK-03. Carbohydr. Polym. 2018, 201, 566–574. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Wang, X.; Zhu, Y.; Zhang, J.; Liu, H.; Jia, L. Characterization, anti-oxidation and anti-inflammation of polysaccharides by Hypsizygus marmoreus against LPS-induced toxicity on lung. Int. J. Biol. Macromol. 2018, 111, 121–128. [Google Scholar] [CrossRef]
- Pan, H.H.; Ko, J.L.; Wu, C.T.; Sun, H.L.; Quek, Y.W.; Ku, M.S.; Lue, K.H. Effect of the Fungal Immunomodulatory Protein FIP-fve in the Neutrophilic Asthma Animal Model. Int. Arch Allergy Immunol. 2021, 182, 1143–1154. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Biscaia, S.M.P.; Bellan, D.L.; Viana, S.R.F.; Di-Medeiros Leal, M.C.; Vasconcelos, A.F.D.; Liao, L.M.; Trindade, E.S.; Carbonero, E.R. Structure elucidation of a bioactive fucomannogalactan from the edible mushroom Hypsizygus marmoreus. Carbohydr. Polym. 2019, 225, 115203. [Google Scholar] [CrossRef]
- Kino, K.; Yamashita, A.; Yamaoka, K.; Watanabe, J.; Tanaka, S.; Ko, K.; Shimizu, K.; Tsunoo, H. Isolation and characterization of a new immunomodulatory protein, Ling Zhi-8 (LZ-8), from Ganoderma lucidium. J. Biol. Chem. 1989, 264, 472–478. [Google Scholar] [CrossRef]
- Ko, J.L.; Hsu, C.I.; Lin, R.H.; Kao, C.L.; Lin, J.Y. A New Fungal Immunomodulatory Protein, FIP-fve Isolated from the Edible Mushroom, Flammulina velutipes and its Complete Amino Acid Sequence. Eur. J. Biochem. 1995, 228, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.C.; Hsu, C.I.; Lin, R.H.; Kao, C.L.; Lin, J.Y. Fip-vvo, a new fungal immunomodulatory protein isolated from Volvariella volvacea. Biochem. J. 1997, 323, 557–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.Z.; Wang, X.F.; Chen, Y.Y.; Lin, J.; Zhou, X.W. Cytokines expression induced by Ganoderma sinensis fungal immunomodulatory proteins (FIP-gsi) in mouse spleen cells. Appl. Biochem. Biotechnol. 2010, 162, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bastiaan-Net, S.; Wichers, H.J. Current Understanding of the Structure and Function of Fungal Immunomodulatory Proteins. Front. Nutr. 2020, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Echeverry, P.T.; Lopez-Gartner, G.A. Fungal immunomodulatory proteins in the context of biomedicine. Front. Biosci. 2017, 9, 286–306. [Google Scholar]
- Li, Q.Z.; Zheng, Y.Z.; Zhou, X.W. Fungal immunomodulatory proteins: Characteristic, potential antitumor activities and their molecular mechanisms. Drug Discov. Today 2019, 24, 307–314. [Google Scholar] [CrossRef]
- Gordon, S.; Pluddemann, A. Tissue macrophages: Heterogeneity and functions. BMC Biol. 2017, 15, 53. [Google Scholar] [CrossRef]
- Poppell, M.; Hammel, G.; Ren, Y. Immune Regulatory Functions of Macrophages and Microglia in Central Nervous System Diseases. Int. J. Mol. Sci. 2023, 24, 5925. [Google Scholar] [CrossRef]
- Lendeckel, U.; Venz, S.; Wolke, C. Macrophages: Shapes and functions. ChemTexts 2022, 8, 12. [Google Scholar] [CrossRef]
- Lin, J.W.; Hao, L.X.; Xu, G.X.; Sun, F.; Gao, F.; Zhang, R.; Liu, L.X. Molecular cloning and recombinant expression of a gene encoding a fungal immunomodulatory protein from Ganoderma lucidum in Pichia pastoris. World J. Microbiol. Biotechnol. 2008, 25, 383–390. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, J.; Han, X.; Zhang, P.; Dai, X.; Liu, J.; Zhang, X.; Lee, I.; Liu, S. High-Yield Production in Escherichia coli of Fungal Immunomodulatory Protein Isolated from Flammulina velutipes and Its Bioactivity Assay in Vivo. Int. J. Mol. Sci. 2013, 14, 2230–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ejike, U.C.; Chan, C.J.; Lim, C.S.Y.; Lim, R.L.H. Functional evaluation of a recombinant fungal immunomodulatory protein from L. rhinocerus produced in P. pastoris and E. coli host expression systems. Appl. Microbiol. Biotechnol. 2021, 105, 2799–2813. [Google Scholar] [CrossRef]
- Lin, C.H.; Sheu, G.T.; Lin, Y.W.; Yeh, C.S.; Huang, Y.H.; Lai, Y.C.; Chang, J.G.; Ko, J.L. A new immunomodulatory protein from Ganoderma microsporum inhibits epidermal growth factor mediated migration and invasion in A549 lung cancer cells. Process Biochem. 2010, 45, 1537–1542. [Google Scholar] [CrossRef]
- Hao, M.; Yang, S.; Han, S.; Che, H.J.F.S.; Wellness, H. The amino acids differences in epitopes may promote the different allergenicity of ovomucoid derived from hen eggs and quail eggs. Food Sci. Hum. Wellness 2023, 12, 861–870. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.N.; Wang, Y.; Li, Y.; Tang, L.H.; Bai, R.; Bao, D.P. Bioinformatics analysis of a novel immunoregulatory protein from Ganoderma lucidum. Acta Edulis Fungi 2016, 23, 25–29. [Google Scholar]
- Sheu, F.; Chien, P.J.; Hsieh, K.Y.; Chin, K.L.; Huang, W.T.; Tsao, C.Y.; Chen, Y.F.; Cheng, H.C.; Chang, H.H. Purification, cloning, and functional characterization of a novel immunomodulatory protein from Antrodia camphorata (bitter mushroom) that exhibits TLR2-dependent NF-KB activation and M1 polarization within murine macrophages. J. Agric Food Chem. 2009, 57, 4130–4141. [Google Scholar] [CrossRef]
- Kuan, Y.C.; Wu, Y.J.; Hung, C.L.; Sheu, F. Trametes versicolor protein YZP activates regulatory B lymphocytes—Gene identification through de novo assembly and function analysis in a murine acute colitis model. PLoS ONE 2013, 8, e72422. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.Y.; Hsu, M.F.; Huang, C.S.; Fu, H.Y.; Wang, H.J.; Hseu, R.S.; Huang, C.T.; Yang, C.S. A 2.0 Å Structure of the Fungal Immunomodulatory Protein GMI from Ganoderma microsporum. Protein Crystallogr. 2007, 2, 132. [Google Scholar]
- Paaventhan, P.; Joseph, J.S.; Seow, S.V.; Vaday, S.; Robinson, H.; Chua, K.Y.; Kolatkar, P.R. A 1.7Å Structure of Fve, a Member of the New Fungal Immunomodulatory Protein Family. J. Mol. Biol. 2003, 332, 461–470. [Google Scholar] [CrossRef]
- Chen, S.; Ge, W.; Buswell, J.A. Biochemical and molecular characterization of a laccase from the edible straw mushroom, Volvariella volvacea. Eur. J. Biochem. 2004, 271, 318–328. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Hao, H.B.; Zhao, J.; Wang, Q.; Juan, J.X.; Chen, M.J.; Feng, Z.Y.; Zhang, J.J. Expression analysis of laccase isozyme gene during Hypsizygus marmoreus growth and development. Mycosystema 2020, 39, 1038–1048. [Google Scholar] [CrossRef]
- Li, Q.; Lei, Y.; Hu, G.; Lei, Y.; Dan, D. Effects of Tween 80 on the liquid fermentation of Lentinus edodes. Food Sci. Biotechnol. 2018, 27, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wang, Q.; Pu, H.; Gu, S.; Luo, L.; Yin, Z. Optimization of soluble human interferon-gamma production in Escherichia coli using SUMO fusion partner. World J. Microbiol. Biotechnol. 2013, 29, 319–325. [Google Scholar] [CrossRef]
- Kuo, D.; Nie, M.; Courey, A.J. SUMO as a solubility tag and in vivo cleavage of SUMO fusion proteins with Ulp1. Methods Mol. Biol. 2014, 1177, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.G.; Sun, Y.; Deng, T.S.; Song, L.L.; Li, P.; Zeng, H.J.; Tang, X.M. Identification and functional characterization of a novel immunomodulatory protein from Morchella conica SH. Front. Immunol. 2020, 11, 559770. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, Z.; Xu, W.; Xie, Y.; Zhao, L.; Tang, X.; Wang, F.; Xin, F. FIP-sch2, a new fungal immunomodulatory protein from Stachybotrys chlorohalonata, suppresses proliferation and migration in lung cancer cells. Appl. Microbiol. Biotechnol. 2017, 101, 3227–3235. [Google Scholar] [CrossRef]
- Rosano, G.L.; Morales, E.S.; Ceccarelli, E.A. New tools for recombinant protein production in Escherichia coli: A 5-year update. Protein Sci. 2019, 28, 1412–1422. [Google Scholar] [CrossRef]
- Xu, H.; Kong, Y.Y.; Chen, X.; Guo, M.Y.; Bai, X.H.; Lu, Y.J.; Li, W.; Zhou, X.W. Recombinant FIP-gat, a Fungal Immunomodulatory Protein from Ganoderma atrum, Induces Growth Inhibition and Cell Death in Breast Cancer Cells. J. Agric Food Chem. 2016, 64, 2690–2698. [Google Scholar] [CrossRef]
- Xu, X.F.; Yan, H.D.; Chen, J.; Zhang, X.W. Bioactive proteins from mushrooms. Biotechnol. Adv. 2011, 29, 667–674. [Google Scholar] [CrossRef]
- Lee, M.; Rey, K.; Besler, K.; Wang, C.; Choy, J. Immunobiology of Nitric Oxide and Regulation of Inducible Nitric Oxide Synthase. Results Probl. Cell Differ. 2017, 62, 181–207. [Google Scholar] [CrossRef]
- Zhou, S.; Guan, S.; Duan, Z.; Han, X.; Zhang, X.; Fan, W.; Li, H.; Chen, L.; Ma, H.; Liu, H.; et al. Molecular cloning, codon-optimized gene expression, and bioactivity assessment of two novel fungal immunomodulatory proteins from Ganoderma applanatum in Pichia. Appl. Microbiol. Biotechnol. 2018, 102, 5483–5494. [Google Scholar] [CrossRef]
- Ejike, U.C.; Chan, C.J.; Okechukwu, P.N.; Lim, R.L.H. New advances and potentials of fungal immunomodulatory proteins for therapeutic purposes. Crit. Rev. Biotechnol. 2020, 40, 1172–1190. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Shang, J.J.; Li, Y.; Zhou, C.L.; Hou, D.; Li, J.L.; Tan, Q.; Bao, D.P.; Yang, R.H. The complete mitochondrial genome of the Basidiomycete edible fungus Hypsizygus marmoreus. Mitochondrial DNA Part B Resour. 2018, 3, 1241–1243. [Google Scholar] [CrossRef] [Green Version]
- González Muñoz, A.; Botero Orozco, K.J.; López Gartner, G.A. Finding of a novel fungal immunomodulatory protein coding sequence in Ganoderma australe. Rev. Colomb. Biotecnol. 2014, 16, 90–95. [Google Scholar] [CrossRef]
- Kyte, J.; Doolittle, R. A Simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Qian, J.; Gao, Y.N.; Wang, Y.; Wu, Y.Y.; Wang, Y.; Zhao, Y.C.; Chen, H.Y.; Bao, D.P.; Xu, J.Y.; Bian, X.H. Selection and Evaluation of Appropriate Reference Genes for RT-qPCR Normalization of Volvariella volvacea Gene Expression under Different Conditions. BioMed Res. Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ren, D.; Lin, D.; Alim, A.; Zheng, Q.; Yang, X. Chemical characterization of a novel polysaccharide ASKP-1 from Artemisia sphaerocephala Krasch seed and its macrophage activation via MAPK, PI3k/Akt and NF-kappaB signaling pathways in RAW264.7 cells. Food Funct. 2017, 8, 1299–1312. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Gao, Y.; Li, Y.; Wan, J.N.; Yang, R.H.; Mao, W.J.; Zhou, C.L.; Tang, L.H.; Gong, M.; et al. Discovery and characterization of the highly active fungal immunomodulatory protein fip-vvo82. J. Chem. Inf. Model. 2016, 56, 2103–2114. [Google Scholar] [CrossRef]
- Lin, W.H.; Hung, C.H.; Hsu, C.I.; Lin, J.Y. Dimerization of the N-terminal amphipathic α-helix domain of the fungal immunomodulatory protein from Ganoderma tsugae (fip-gts) defined by a yeast two-hybrid system and site-directed mutagenesis. J. Biol. Chem. 1997, 272, 20044–20048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.W.; Guan, S.Y.; Duan, Z.W.; Shen, Y.H.; Fan, W.L.; Chen, L.J.; Zhang, L.; Zhang, L.; Li, T.L. Gene cloning of a novel fungal immunomodulatory protein from Chroogomphis rutilus and its expression in Pichia pastoris. J. Chem. Technol. Biotechnol. 2016, 91, 2761–2768. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.N.; Bai, R.; Chen, H.Y.; Wu, Y.Y.; Shang, J.J.; Bao, D.P. Identification of a novel anti-cancer protein, fip-bbo, from Botryobasidium botryosum and protein structure analysis using molecular dynamic simulation. Sci. Rep. 2019, 9, 5818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.Y.; Shi, L.J.; Ding, Y.; Nie, Y.; Tang, X.M. Identification and functional characterization of a novel fungal immunomodulatory protein from Postia placenta. Food Chem. Toxicol. 2015, 78, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, Z.; Sun, L.; Liu, X.; Huang, Y.; Wang, F.; Xin, F. Characterization of a new fungal immunomodulatory protein, fip-dsq2 from Dichomitus squalens. J. Biotechnol. 2017, 246, 45–51. [Google Scholar] [CrossRef]


| Number | Primer Name | Primer Sequence (5′-3′) |
|---|---|---|
| 1 | α-tubulinF | TTGTCCAACACCACCGCCATC |
| 2 | α-tubulinR | TGCCGACCTCCTCATAGTCCTT |
| 3 | FIP-hmaF | GGACATGCTGGCAGCTCACTTA |
| 4 | FIP-hmaR | TCAGAGCCCGCACACAGATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Wang, Y.; Wu, Y.; Bao, D.; Bing, W.; Li, Y.; Chen, H. Characterization, Recombinant Production, and Bioactivity of a Novel Immunomodulatory Protein from Hypsizygus marmoreus. Molecules 2023, 28, 4796. https://doi.org/10.3390/molecules28124796
Yu S, Wang Y, Wu Y, Bao D, Bing W, Li Y, Chen H. Characterization, Recombinant Production, and Bioactivity of a Novel Immunomodulatory Protein from Hypsizygus marmoreus. Molecules. 2023; 28(12):4796. https://doi.org/10.3390/molecules28124796
Chicago/Turabian StyleYu, Shuhui, Ying Wang, Yingying Wu, Dapeng Bao, Wei Bing, Yan Li, and Hongyu Chen. 2023. "Characterization, Recombinant Production, and Bioactivity of a Novel Immunomodulatory Protein from Hypsizygus marmoreus" Molecules 28, no. 12: 4796. https://doi.org/10.3390/molecules28124796






