Hibiscus acetosella: An Unconventional Alternative Edible Flower Rich in Bioactive Compounds
Abstract
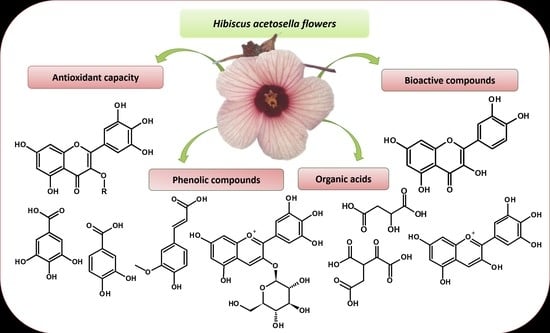
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization, Centesimal Composition, and Antioxidant Capacity
2.2. Analysis of Hibiscus acetosella Flowers Extract by 1H NMR
2.3. Determination of Bioactive Compounds by HRMS and HPLC-DAD
2.4. Cytotoxicity Evaluation
3. Materials and Methods
3.1. Collection and Processing Flower Sample
3.2. Chemical Physical Analysis and Centesimal Composition
3.3. DPPH• and ABTS•+ Radicals Scavenging Capacity Assay
3.4. Total Phenolic Composition
3.5. Bioactive Compounds Identification
3.5.1. Chemical Profile by Nuclear Magnetic Resonance
3.5.2. Identification by High Resolution Mass Spectrometry (HRMS)
3.6. Quantification of Bioactive Compounds by HPLC-DAD
Quantification by Relative Response Factor
3.7. Cytotoxicity Evaluation
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nowicka, P.; Wojdyło, A. Anti-Hyperglycemic and Anticholinergic Effects of Natural Antioxidant Contents in Edible Flowers. Antioxidants 2019, 8, 308. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, J.A.; Rezende, F.A.G.G.; Moura, M.A.F.; Dominguete, L.C.B.; Sande, D. Edible flowers: Bioactive profile and its potential to be used in food development. Food Res. Int. 2019, 129, 108868. [Google Scholar] [CrossRef]
- De Morais, J.S.; Sant’Ana, A.S.; Dantas, A.M.; Silva, B.S.; Lima, M.S.; Borges, G.C.; Magnani, M. Antioxidant activity and bioaccessibility of phenolic compounds in white, red, blue, purple, yellow and orange edible flowers through a simulated intestinal barrier. Food Res. Int. 2020, 131, 109046. [Google Scholar] [CrossRef]
- Dos Santos, I.C.; Reis, S.N. Edible flowers: Traditional and current use. Ornam. Hortic. 2021, 27, 438–445. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Purohit, S.R.; Rana, S.S.; Idrishi, R.; Sharma, V.; Ghosh, P. A review on nutritional, bioactive, toxicological properties and preservation of edible flowers. Futur. Foods 2021, 4, 100078. [Google Scholar] [CrossRef]
- Zhen, J.; Villani, T.S.; Guo, Y.; Qi, Y.; Chin, K.; Pan, M.-H.; Ho, C.-T.; Simon, J.E.; Wu, Q. Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem. 2015, 190, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Al-Yousef, H.M.; Siddiqui, N.A.; Alhowiriny, T.A.; Alqasoumi, S.I.; Amina, M.; Hassan, W.H.B.; Abdelaziz, S.; Abdalla, R.H. Anticancer activity and concurrent analysis of ursolic acid, β-sitosterol and lupeol in three different Hibiscus species (aerial parts) by validated HPTLC method. Saudi Pharm. J. 2018, 26, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Janson, B.; Prasomthong, J.; Malakul, W.; Boonsong, T.; Tunsophon, S. Hibiscus sabdariffa L. calyx extract prevents the adipogenesis of 3T3-L1 adipocytes, and obesity-related insulin resistance in high-fat diet-induced obese rats. Biomed. Pharmacother. 2021, 138, 111438. [Google Scholar] [CrossRef]
- Riaz, G.; Chopra, R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018, 102, 575–586. [Google Scholar] [CrossRef]
- Kinupp, V.F.; Lorenzi, H.H. Unconventional Food Plants (PANC) in Brazil: Identification Guide, Nutritional Aspects and Illustrated Recipes, 1st ed.; Plantarum Institute: São Paulo, Brazil, 2014; ISBN 978-85-86714-46-7. [Google Scholar]
- Ball, D.W. Concentration Scales for Sugar Solutions. J. Chem. Educ. 2006, 83, 1489. [Google Scholar] [CrossRef]
- Chensom, S.; Okumura, H.; Mishima, T. Primary Screening of Antioxidant Activity, Total Polyphenol Content, Carotenoid Content, and Nutritional Composition of 13 Edible Flowers from Japan. Prev. Nutr. Food Sci. 2019, 24, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Yang, W.-K.; Kim, H.Y.; Min, B.; Caturla, N.; Jones, J.; Park, Y.-C.; Lee, Y.-C.; Kim, S.-H. Metabolaid® Combination of Lemon Verbena and Hibiscus Flower Extract Prevents High-Fat Diet-Induced Obesity through AMP-Activated Protein Kinase Activation. Nutrients 2018, 10, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef] [Green Version]
- Sim, Y.Y.; Nyam, K.L. Hibiscus cannabinus L. (kenaf) studies: Nutritional composition, phytochemistry, pharmacology, and potential applications. Food Chem. 2020, 344, 128582. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Z.; Zhang, C.-C.; Fu, Y.-J.; Cui, Q. Comparative analysis of phytochemical profile, antioxidant and anti-inflammatory activity from Hibiscus manihot L. flower. Arab. J. Chem. 2021, 15, 103503. [Google Scholar] [CrossRef]
- Mar, J.M.; da Silva, L.S.; Moreira, W.P.; Biondo, M.M.; Pontes, F.L.D.; Campos, F.R.; Kinupp, V.F.; Campelo, P.H.; Sanches, E.A.; Bezerra, J.d.A. Edible flowers from Theobroma speciosum: Aqueous extract rich in antioxidant compounds. Food Chem. 2021, 356, 129723. [Google Scholar] [CrossRef]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef] [Green Version]
- Ormazabal, P.; Scazzocchio, B.; Varì, R.; Santangelo, C.; D’archivio, M.; Silecchia, G.; Iacovelli, A.; Giovannini, C.; Masella, R. Effect of protocatechuic acid on insulin responsiveness and inflammation in visceral adipose tissue from obese individuals: Possible role for PTP1B. Int. J. Obes. 2018, 42, 2012–2021. [Google Scholar] [CrossRef] [Green Version]
- Izcara, S.; Perestrelo, R.; Morante-Zarcero, S.; Câmara, J.S.; Sierra, I. High throughput analytical approach based on μQuEChERS combined with UHPLC-PDA for analysis of bioactive secondary metabolites in edible flowers. Food Chem. 2022, 393, 133371. [Google Scholar] [CrossRef]
- Montalvo-González, E.; Villagrán, Z.; González-Torres, S.; Iñiguez-Muñoz, L.E.; Isiordia-Espinoza, M.A.; Ruvalcaba-Gómez, J.M.; Arteaga-Garibay, R.I.; Acosta, J.L.; González-Silva, N.; Anaya-Esparza, L.M. Physiological Effects and Human Health Benefits of Hibiscus sabdariffa: A Review of Clinical Trials. Pharmaceuticals 2022, 15, 464. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Tenuta, M.C.; Menichini, F.; Xiao, J.; Tundis, R. Edible Flowers: A Rich Source of Phytochemicals with Antioxidant and Hypoglycemic Properties. J. Agric. Food Chem. 2015, 64, 2467–2474. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Salas, E.; Barouh, N.; Baréa, B.; Figueroa-Espinoza, M.C. Lipophilization and MS characterization of the main anthocyanins purified from hibiscus flowers. Food Chem. 2017, 230, 189–194. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Chong, S.; Yan, S.; Yu, R.; Chen, R.; Si, J.; Zhang, X. Identification and quantitative analysis of anthocyanins composition and their stability from different strains of Hibiscus syriacus L. flowers. Ind. Crops Prod. 2022, 177, 114457. [Google Scholar] [CrossRef]
- Mar, J.M.; da Silva, L.S.; Lira, A.C.; Kinupp, V.F.; Yoshida, M.I.; Moreira, W.P.; Bruginski, E.; Campos, F.R.; Machado, M.B.; de Souza, T.P.; et al. Bioactive compounds-rich powders: Influence of different carriers and drying techniques on the chemical stability of the Hibiscus acetosella extract. Powder Technol. 2020, 360, 383–391. [Google Scholar] [CrossRef]
- Ngan, L.; Tan, M.; Hoang, N.; Thanh, D.; Linh, N.; Hoa, T.; Nuong, N.; Hieu, T. Antibacterial activity of Hibiscus rosa-sinensis L. red flower against antibiotic-resistant strains of Helicobacter pylori and identification of the flower constituents. Braz. J. Med. Biol. Res. 2021, 54, e10889. [Google Scholar] [CrossRef]
- Rengarajan, S.; Melanathuru, V.; Govindasamy, C.; Chinnadurai, V.; Elsadek, M.F. Antioxidant activity of flavonoid compounds isolated from the petals of Hibiscus rosa sinensis. J. King Saud Univ.-Sci. 2020, 32, 2236–2242. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.R.; Hassan, H.M.M. Free radical scavenging activity of three different flowers—Hibiscus rosa-sinensis, Quisqualis indica and Senna surattensis. Asian Pac. J. Trop. Biomed. 2016, 6, 771–777. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Cai, Z.; Chen, C.; Mei, Y.; Wei, L.; Liu, S.; Zou, L.; Wu, N.; Yuan, J.; Liu, X.; et al. Comparative Study on Chemical Constituents of Medicinal and Non-Medicinal Parts of Flos Abelmoschus manihot, Based on Metabolite Profiling Coupled with Multivariate Statistical Analysis. Horticulturae 2022, 8, 317. [Google Scholar] [CrossRef]
- Kapepula, P.M.; Ngombe, N.K.; Tshibangu, P.T.; Tsumbu, C.; Franck, T.; Mouithys-Mickalad, A.; Mumba, D.; Tshala-Katumbay, D.; Serteyn, D.; Tits, M.; et al. Comparison of metabolic profiles and bioactivities of the leaves of three edible Congolese Hibiscus species. Nat. Prod. Res. 2017, 31, 2885–2892. [Google Scholar] [CrossRef]
- Lyu, J.I.; Ryu, J.; Jin, C.H.; Kim, D.-G.; Kim, J.M.; Seo, K.-S.; Kim, J.-B.; Kim, S.H.; Ahn, J.-W.; Kang, S.-Y.; et al. Phenolic Compounds in Extracts of Hibiscus acetosella (Cranberry Hibiscus) and Their Antioxidant and Antibacterial Properties. Molecules 2020, 25, 4190. [Google Scholar] [CrossRef]
- Parmenter, B.H.; Croft, K.D.; Hodgson, J.M.; Dalgaard, F.; Bondonno, C.P.; Lewis, J.R.; Cassidy, A.; Scalbert, A.; Bondonno, N.P. An overview and update on the epidemiology of flavonoid intake and cardiovascular disease risk. Food Funct. 2020, 11, 6777–6806. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Liu, Y.L.; Zheng, Y.; Ivey, K.; Willett, W.C.; Stampfer, M.J.; Rimm, E.B.; Cassidy, A. Change in habitual intakes of flavonoid-rich foods and mortality in US males and females. BMC Med. 2023, 21, 181. [Google Scholar] [CrossRef]
- Abdul-Awal, S.M.; Nazmir, S.; Nasrin, S.; Nurunnabi, T.R.; Uddin, S.J. Evaluation of pharmacological activity of Hibiscus tiliaceus. Springerplus 2016, 5, 1209. [Google Scholar] [CrossRef] [Green Version]
- Μatsumoto, T.; Imahori, D.; Achiwa, K.; Saito, Y.; Ohta, T.; Yoshida, T.; Kojima, N.; Yamashita, M.; Nakayama, Y.; Watanabe, T. Chemical structures and cytotoxic activities of the constituents isolated from Hibiscus tiliaceus. Fitoterapia 2020, 142, 104524. [Google Scholar] [CrossRef]
- Adolfo Lutz Institute. Physico-Chemical Methods for Food Analysis, 4th ed.; Zenebon, O., Pascuet, N.S., Tiglea, P., Eds.; Adolfo Lutz Institute: São Paulo, Brazil, 2008. [Google Scholar]
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicryl-Hydrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Ramos, A.S.; Mar, J.M.; da Silva, L.S.; Acho, L.D.; da Silva, B.J.P.; Lima, E.S.; Campelo, P.H.; Sanches, E.A.; Bezerra, J.A.; Chaves, F.C.M.; et al. Pedra-ume caá fruit: An Amazon cherry rich in phenolic compounds with antiglycant and antioxidant properties. Food Res. Int. 2019, 123, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.S.; Souza, R.O.; Boleti, A.P.D.A.; Bruginski, E.R.; Lima, E.S.; Campos, F.R.; Machado, M.B. Chemical characterization and antioxidant capacity of the araçá-pera (Psidium acutangulum): An exotic Amazon fruit. Food Res. Int. 2015, 75, 315–327. [Google Scholar] [CrossRef]
- Lima, L.G.B.; Montenegro, J.; de Abreu, J.P.; Santos, M.C.B.; Nascimento, T.P.D.; Santos, M.d.S.; Ferreira, A.G.; Cameron, L.C.; Ferreira, M.S.L.; Teodoro, A.J. Metabolite Profiling by UPLC-MSE, NMR, and Antioxidant Properties of Amazonian Fruits: Mamey Apple (Mammea americana), Camapu (Physalis angulata), and Uxi (Endopleura uchi). Molecules 2020, 25, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Provan, G.J.; Helliwell, K. HPLC determination of catechins in tea leaves and tea extracts using relative response factors. Food Chem. 2003, 81, 307–312. [Google Scholar] [CrossRef]
- da Silva, U.P.; Ferreira, B.W.; de Sousa, B.L.; Barreto, R.W.; Martins, F.T.; Neto, J.H.D.A.; Vaz, B.G.; da Silva, R.R.; Martins, T.V.F.; Mendes, T.A.D.O.; et al. Synthesis of bis(ylidene) cyclohexanones and their antifungal activity against selected plant pathogenic fungi. Mol. Divers. 2022, 27, 281–297. [Google Scholar] [CrossRef]
- Nascimento, F.R.; Baeta, J.V.D.P.B.; de França, A.A.P.; e Oliveira, M.A.B.R.; Pizziolo, V.R.; dos Santos, A.A.; Mendes, T.A.D.O.; Diaz-Muñoz, G.; Diaz, M.A.N. Dibenzoylmethane derivative inhibits melanoma cancer in vitro and in vivo through induction of intrinsic and extrinsic apoptotic pathways. Chem. Interact. 2021, 351, 109734. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Mean ± SD |
|---|---|
| pH | 2.8 ± 0.0 |
| Soluble solids (°Brix) | 3.4 ± 0.0 |
| Moisture (g/100 g) | 91.76 ± 0.27 |
| Ashes (g/100 g) | 0.45 ± 0.01 |
| Lipids (g/100 g) | 0.90 ± 0.17 |
| Protein (g/100 g) | ND |
| Carbohydrates (g/100 g) | 6.89 ± 0.19 |
| Calories (kcal) | 34.7 ± 0.45 |
| DPPH (μM TE) | 507.8 ± 2.7 c |
| ABTS (μM TE) | 783.9 ± 30.8 a |
| TPC (mg GAE/g) | 568.8 ± 0.8 b |
| Compound | Molecular Ion | [M − H]− m/z | [M − H]− m/z (Error in ppm) |
| Mallic acid | C4H5O5 | 133.0142 | 133.0142 (0.5) |
| Oxalosuccinic acid | C6H5O7 | 189.0041 | 189.0043 (−1.0) |
| Quercetin | C15H9O7 | 301.0354 | 301.0352 (0.7) |
| Myricetin | C15H9O8 | 317.0303 | 317.0295 (2.3) |
| Gallic acid 3-O-β-glucoside | C13H15O10 | 331.0671 | 331.0640 (9.4) |
| 5-(3-Carboxy-2,5-dihydroxyphenyl)-2,4-dihydroxy-3-methoxybenzoic acid | C15H11O9 | 335.0409 | 335.0410 (−0.3) |
| Caffeoyl-hydroxycitric acid | C15H13O11 | 369.0463 | 369.0476 (−3.4) |
| Quercetin 3-O-rhamnoside (quercitrin) | C21H19O11 | 447.0933 | 447.0930 (0.5) |
| Quercetin 3-O-glucoside | C21H19O12 | 463.0882 | 463.0863 (4.1) |
| Myricetin 3-O-glucoside | C21H19O13 | 479.0831 | 479.0833 (0.3) |
| 3,5-di-O-galloylquinic acid | C21H19O14 | 495.0780 | 495.0788 (1.6) |
| Quercetin 3-O-β-D-(1″-O-malonyl)-xylopyranoside | C23H17O14 | 517.0624 | 517.0627 (0.7) |
| Quercetin 3-O-(6′-O-malonyl)galactoside | C24H19O15 | 547.0729 | 547.0731 (0.3) |
| Delphinidin 3-O-(6″-O-malonyl)-β-glucoside | C24H21O15 | 549.0886 | 549.0896 (−1.8) |
| Kaempferol-3-O-sambubioside | C26H27O15 | 579.1355 | 579.1351 (−0.7) |
| Quercetin-3-O-sambubioside | C26H27O16 | 595.1305 | 595.1298 (1.1) |
| Cyanidin 3-O-β-D-caffeoylglucoside | C30H25O14 | 609.1250 | 609.1258 (−1.3) |
| Myricetin-3-arabinogalactoside | C26H27O17 | 611.1254 | 611.1264 (−1.7) |
| Miricetin 3-O-β-D-glucosil-(1→2)-β-D-glucoside | C27H29O18 | 641.1359 | 641.1388 (4.4) |
| Compound | Molecular Ion | [M + H]+ m/z | [M + H]+ m/z (error in ppm) |
| Delphinidin | C15H11O7 | 303.0499 | 303.0409 (−3.1) |
| Cyanidin 3-O-β-D-glucoside | C21H21O11 | 449.1078 | 449.1088 (−2.1) |
| Delphinidin 3-glucoside | C21H21O12 | 465.1028 | 465.1035 (−1.6) |
| Cyanidin 3-sambubioside | C26H29O15 | 581.1501 | 581.1495 (1.1) |
| Delphinidin 3-sambubioside | C26H29O16 | 597.1450 | 597.1453 (0.5) |
| Delphinidin 3,5-O-diglucoside | C27H31O17 | 627.1556 | 627.1558 (0.4) |
| RT (min) | Bioactive Compound | λmax (nm) | Conc. (μg/mL) | SD * | RSD% ǂ | R | R2 | Calibration Curve |
|---|---|---|---|---|---|---|---|---|
| 7.68 | Gallic acid | 271 | 322 | 0.06 | 19.91 | 0.998 | 0.996 | y = 0.75 × 106 X + 12,790.7 |
| 10.19 | Protocatechuic acid | 293 | 44 | 0.01 | 18.64 | 0.998 | 0.996 | y = 2.85 × 106 X + 50,231.1 |
| 11.62 | Cyanidin 3-O-glucoside | 279/529 | 201 | 0.09 | 42.33 | 0.968 | 0.937 | y = 0.44 × 106 X − 11,172 |
| 12.54 | Cyanidin | 519 | 10 | 0.00 | 19.85 | 0.993 | 0.987 | y = 3.88 × 106 X + 85,884 |
| 13.20 | Delphinidin 3-O-glucoside | 521 | 243 | 0.43 | 23.84 | 0.994 | 0.988 | y = 0.10 × 106 X + 3339.92 |
| 13.76 | Caffeic acid | 324 | 237 | 0.04 | 18.08 | 0.993 | 0.987 | y = 1.57 × 106 X + 26,680 |
| 17.00 | Sinapic acid | 324 | 65 | 0.01 | 17.79 | 0.998 | 0.996 | y = 2.19 × 106 X 36,087.4 |
| 20.32 | Myricetin | 256/375 | 363 | 0.15 | 25.24 | 0.996 | 0.992 | y = 0.05 × 106 X − 9668.84 |
| 21.63 | Quercitrin | 254/371 | 65 | 0.00 | 15.93 | 0.998 | 0.997 | y = 4.02 × 106 X + 54,454.1 |
| 22.12 | Luteolin | 255/349 | 13 | 0.00 | 16.36 | 0.999 | 0.999 | y = 0.83 × 106 X + 5952.23 |
| RT (min) | Bioactive Compound | λmax (nm) | * RF | ** Content | *** LOQ | **** LOD |
|---|---|---|---|---|---|---|
| Anthocyanidin standard | 1.92974 × 10−6 | −1.73 × 10−9 | −5.70 × 10−9 | |||
| 11.95 | Anthocyanin derivative | 277/529 | 9.90959 × 10−7 | 0.080 | ||
| Cinnamic standard | 837,181.4346 | 6.03 × 10 | 1.99 × 10 | |||
| 12.70 | Cinnamic acid | 326 | 72,848.10127 | 0.021 | ||
| 14.67 | Cinnamic derivative | 326 | 32,050.63291 | 0.009 | ||
| Flavonol standard | 20,175.78773 | 4.27 × 10 | 1.41 × 10 | |||
| 15.27 | Flavonol rutinoside derivative | 355 | 184,461.5385 | 0.070 | ||
| 15.48 | Flavonol rutinoside derivative | 346 | 267,748.7179 | 0.102 | ||
| 18.11 | Flavonol derivative | 369 | 117,369.146 | 0.082 | ||
| 19.27 | Flavonol derivative | 254/370 | 1.28163 × 10−7 | 5.46 | ||
| 19.70 | Flavonol derivative | 252/370 | 154,988.9807 | 0.108 | ||
| Flavone standard | 518,203.8567 | 9.28 × 10 | 3.06 × 10 | |||
| 20.81 | Flavone derivative | 369 | 3870.52091 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos Silva, L.Y.; da Silva Ramos, A.; Cavalcante, D.N.; Kinupp, V.F.; da Silva Rodrigues, J.V.; Ventura, B.M.L.; de Oliveira Mendes, T.A.; Sanches, E.A.; Campelo, P.H.; de Araújo Bezerra, J. Hibiscus acetosella: An Unconventional Alternative Edible Flower Rich in Bioactive Compounds. Molecules 2023, 28, 4819. https://doi.org/10.3390/molecules28124819
dos Santos Silva LY, da Silva Ramos A, Cavalcante DN, Kinupp VF, da Silva Rodrigues JV, Ventura BML, de Oliveira Mendes TA, Sanches EA, Campelo PH, de Araújo Bezerra J. Hibiscus acetosella: An Unconventional Alternative Edible Flower Rich in Bioactive Compounds. Molecules. 2023; 28(12):4819. https://doi.org/10.3390/molecules28124819
Chicago/Turabian Styledos Santos Silva, Laila Yasmim, Andrezza da Silva Ramos, Débora Nogueira Cavalcante, Valdely Ferreira Kinupp, João Vitor da Silva Rodrigues, Bianca Muniz Lacerda Ventura, Tiago Antônio de Oliveira Mendes, Edgar Aparecido Sanches, Pedro Henrique Campelo, and Jaqueline de Araújo Bezerra. 2023. "Hibiscus acetosella: An Unconventional Alternative Edible Flower Rich in Bioactive Compounds" Molecules 28, no. 12: 4819. https://doi.org/10.3390/molecules28124819
APA Styledos Santos Silva, L. Y., da Silva Ramos, A., Cavalcante, D. N., Kinupp, V. F., da Silva Rodrigues, J. V., Ventura, B. M. L., de Oliveira Mendes, T. A., Sanches, E. A., Campelo, P. H., & de Araújo Bezerra, J. (2023). Hibiscus acetosella: An Unconventional Alternative Edible Flower Rich in Bioactive Compounds. Molecules, 28(12), 4819. https://doi.org/10.3390/molecules28124819