Protective Effect of Astragaloside IV against Cadmium-Induced Damage on Mouse Renal Podocytes (MPC5)
Abstract
:1. Introduction
2. Results
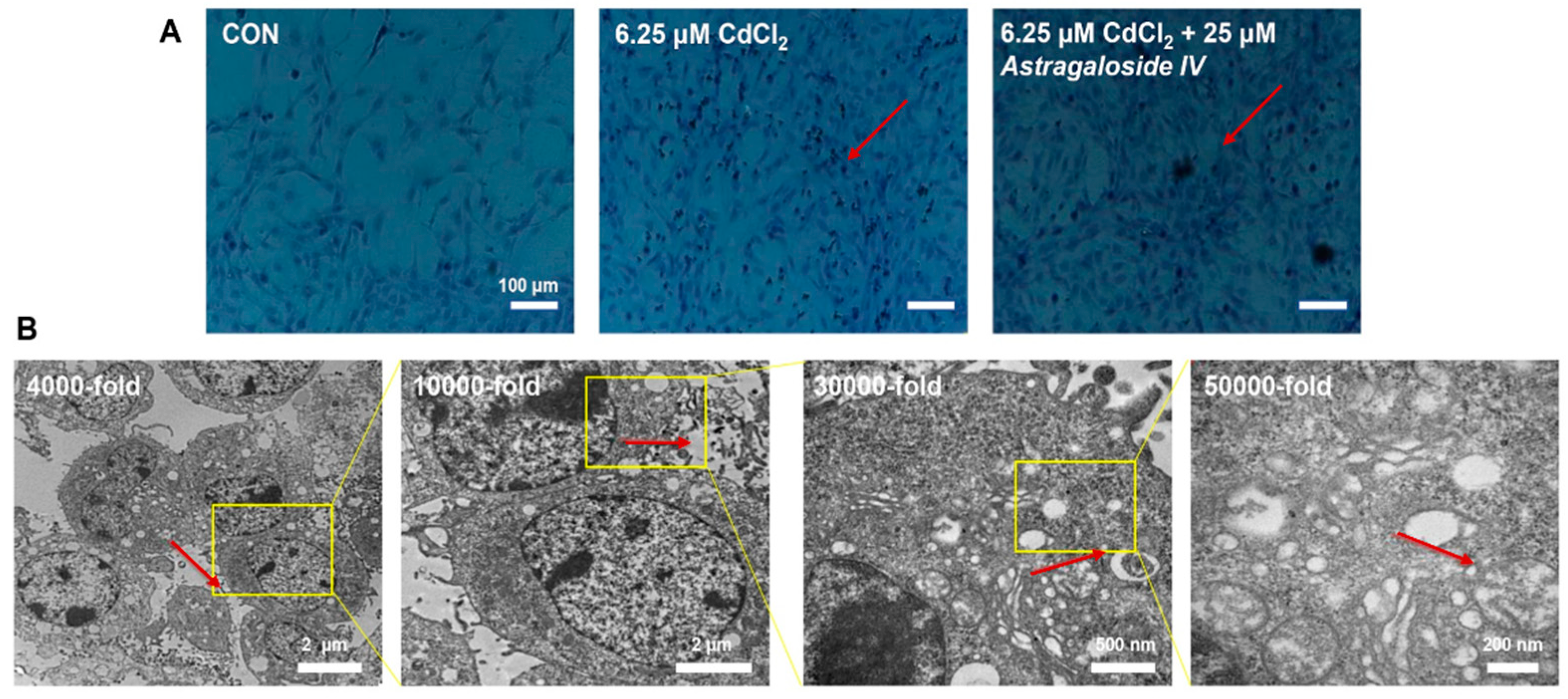
2.1. Effect of Ast Intervention on Morphological Changes in MPC5
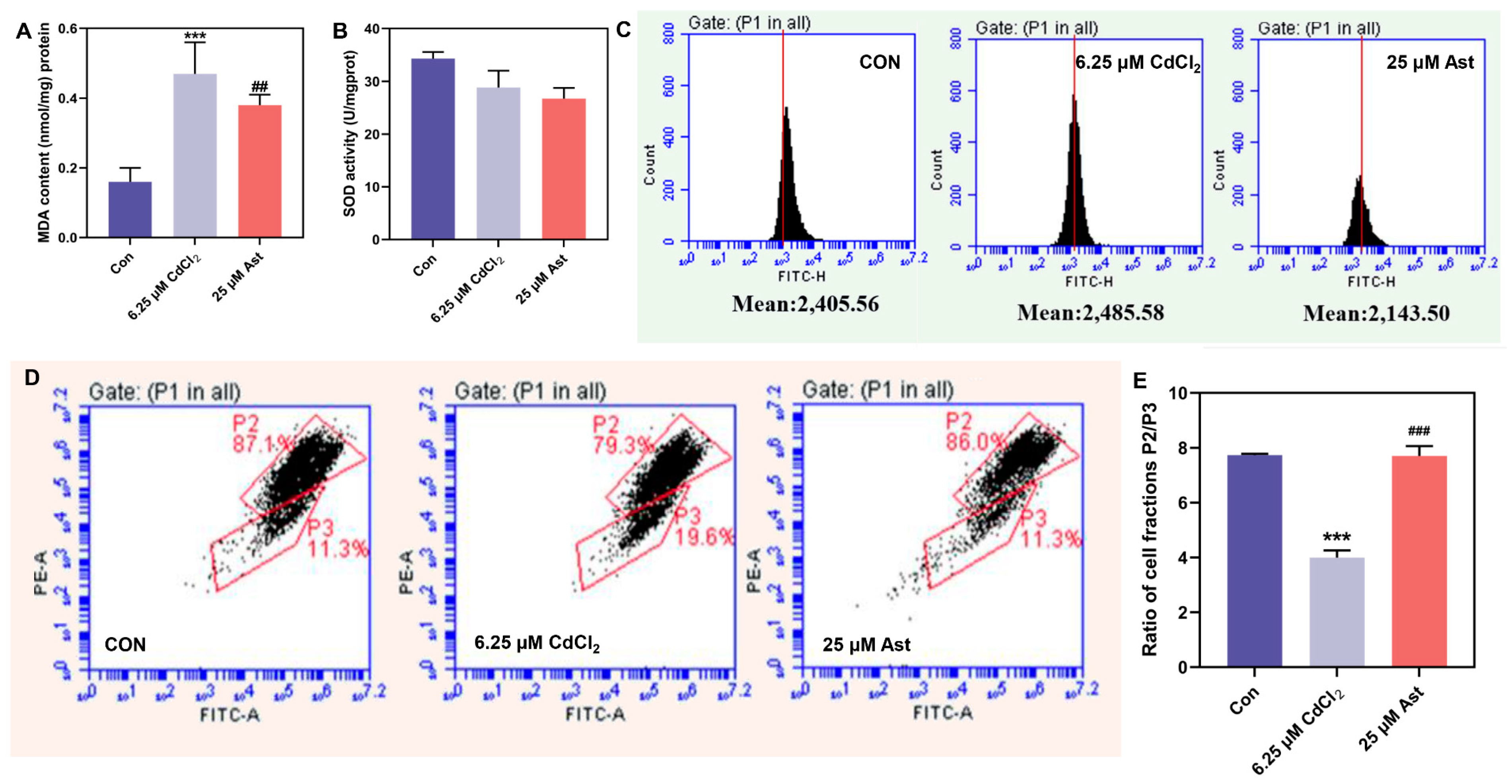
2.2. MDA, ROS Content, and SOD Activity after Ast Intervention
2.3. Effect of Ast Intervention on Mitochondrial Membrane Potential
2.4. Effect of Ast Intervention on the mRNA Expression Levels of PINK1/Parkin Pathway in Cadmium Treated MPC5 Cells at Different Times
2.5. Effect of Ast Intervention on the Protein Expression Levels of PINK1/Parkin Pathway in Cadmium-Treated MPC5 Cells at Different Times
2.6. Effect of Ast Intervention on Immunofluorescence Assay in Cadmium-Treated MPC5 Cells at Different Times
3. Discussion
4. Materials and Methods
4.1. Cell Line
4.2. Materials, Chemicals, and Antibody
4.3. Establishment of Cell Models and Cell Experiments
4.4. Influence of Oxidative Stress on MPC5 Injuries
4.4.1. Determination of Cellular Malondialdehyde (MDA) Levels and Superoxide Dismutase (SOD) Activity
4.4.2. DCFH-DA Staining for Intracellular Total ROS Levels
4.5. Characterization of Mitochondrial Membrane Potential
4.5.1. Detecting the Degree of Mitochondrial Membrane Potential Depolarization
4.5.2. Measurement of Mitochondrial Membrane Potential
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wilson, P.C.; Wu, H.J.; Kirita, Y.; Uchimura, K.; Ledru, N.; Rennke, H.G.; Welling, P.A.; Waikar, S.S.; Humphreys, B.D. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc. Natl. Acad. Sci. USA 2019, 116, 19619–19625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, G.Y.; Li, S.; Zhang, C.; Chen, H.Y.; Wang, N.; Feng, Y.B. Clinical efficacies, underlying mechanisms and molecular targets of Chinese medicines for diabetic nephropathy treatment and management. Acta Pharm. Sin. B 2021, 11, 2749–2767. [Google Scholar] [CrossRef] [PubMed]
- Sugita, E.; Hayashi, K.; Hishikawa, A.; Itoh, H. Epigenetic Alterations in Podocytes in Diabetic Nephropathy. Front. Pharmacol. 2021, 12, 759299. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, E.A.; Zhou, M.S.; Siddiqui, M.; Rezonzew, G.; Tian, R.X.; Seshan, S.V.; Muwonge, A.N.; Wong, N.J.; Azeloglu, E.U.; Fornoni, A.; et al. Nicotine, smoking, podocytes, and diabetic nephropathy. Am. J. Physiol.-Ren. Physiol. 2021, 320, F442–F453. [Google Scholar] [CrossRef]
- Sun, H.; Li, H.; Yan, J.; Wang, X.D.; Xu, M.Y.; Wang, M.X.; Fan, B.Z.; Liu, J.Y.; Lin, N.H.; Wang, X.; et al. Loss of CLDN5 in podocytes deregulates WIF1 to activate WNT signaling and contributes to kidney disease. Nat. Commun. 2022, 13, 1600. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Lima-Posada, I.; Bakris, G.L.; Jaisser, F. Mineralocorticoid receptor antagonists in diabetic kidney disease—Mechanistic and therapeutic effects. Nat. Rev. Nephrol. 2022, 18, 56–70. [Google Scholar] [CrossRef]
- Tang, C.Y.; Livingston, M.J.; Liu, Z.W.; Dong, Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 2020, 16, 489–508. [Google Scholar] [CrossRef]
- Daehn, I.S.; Duffield, J.S. The glomerular filtration barrier: A structural target for novel kidney therapies. Nat. Rev. Drug Discov. 2021, 20, 770–788. [Google Scholar] [CrossRef]
- Yang, M.; Luo, S.L.; Yang, J.F.; Chen, W.; He, L.Y.; Liu, D.; Zhao, L.; Wang, X. Lipid droplet-mitochondria coupling: A novel lipid metabolism regulatory hub in diabetic nephropathy. Front. Endocrinol. 2022, 13, 1017387. [Google Scholar] [CrossRef]
- Kim, K.; Lee, E.Y. Excessively Enlarged Mitochondria in the Kidneys of Diabetic Nephropathy. Antioxidants 2021, 10, 741. [Google Scholar] [CrossRef]
- Guo, Y.X.; Chen, X.F.; Gong, P.; Li, G.L.; Yao, W.B.; Yang, W.J. The Gut-Organ-Axis Concept: Advances the Application of Gut-on-Chip Technology. Int. J. Mol. Sci. 2023, 24, 4089. [Google Scholar] [CrossRef]
- Yang, M.; Chen, W.; He, L.Y.; Liu, D.; Zhao, L.; Wang, X. Intermittent Fasting—A Healthy Dietary Pattern for Diabetic Nephropathy. Nutrients 2022, 14, 3995. [Google Scholar] [CrossRef]
- Ni, L.H.; Yuan, C. The Mitochondrial-Associated Endoplasmic Reticulum Membrane and Its Role in Diabetic Nephropathy. Oxidative Med. Cell. Longev. 2021, 2021, 8054817. [Google Scholar] [CrossRef]
- Xu, J.W.; Liu, C.; Hsu, P.C.; Zhao, J.; Wu, T.; Tang, J.; Liu, K.; Cui, Y. Remediation of heavy metal contaminated soil by asymmetrical alternating current electrochemistry. Nat. Commun. 2019, 10, 2440. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.L.; Xu, W.X.; Xie, J.Y.; Gao, Y.W.; Wu, L.L.; Sun, L.; Feng, L.; Chen, X.; Zhang, T.; Dai, C.H.; et al. Variation of a major facilitator superfamily gene contributes to differential cadmium accumulation between rice subspecies. Nat. Commun. 2019, 10, 2562. [Google Scholar] [CrossRef] [Green Version]
- Hayes, P.; Carrijo, D.; Meints, B. Towards low cadmium accumulation in barley. Nat. Food 2020, 1, 465. [Google Scholar] [CrossRef]
- McGrath, S.P. Keeping toxic cadmium out of the food chain. Nat. Food 2022, 3, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Wang, W.G.; Yamaji, N.; Fukuoka, S.; Che, J.; Ueno, D.; Ando, T.; Deng, F.L.; Hori, K.; Yano, M.; et al. Duplication of a manganese/cadmium transporter gene reduces cadmium accumulation in rice grain. Nat. Food 2022, 3, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Glicklich, D.; Frishman, W.H. The Case For Cadmium and Lead Heavy Metal Screening. Am. J. Med. Sci. 2021, 362, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Guo, C.Z.; Kong, A.Q.; Li, K.D.; Xie, Y.M.; Shi, H.F.; Gu, J. Calcium Signaling Mediates Cell Death and Crosstalk with Autophagy in Kidney Disease. Cells 2021, 10, 3204. [Google Scholar] [CrossRef]
- Gong, P.; Wang, P.P.; Pi, S.H.; Guo, Y.X.; Pei, S.Y.; Yang, W.J.; Chang, X.N.; Wang, L.; Chen, F.X. Proanthocyanidins Protect Against Cadmium-Induced Diabetic Nephropathy Through p38 MAPK and Keap1/Nrf2 Signaling Pathways. Front. Pharmacol. 2022, 12, 801048. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.J.; Ren, W.Y.; Zhang, L.N.; Zhang, Y.M.; Liu, D.L.; Liu, Y.Q. A Review of the Pharmacological Action of Astragalus Polysaccharide. Front. Pharmacol. 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.J.; Liu, L.J.; Gao, C.F.; Chen, W.J.; Vong, C.T.; Yao, P.F.; Yang, Y.H.; Li, X.Z.; Tang, X.D.; Wang, S.P.; et al. Astragali Radix (Huangqi): A promising edible immunomodulatory herbal medicine. J. Ethnopharmacol. 2020, 258, 112895. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Chen, X.F.; Gong, P.; Wang, R.T.; Han, A.Y.; Deng, Z.F.; Qi, Z.Y.; Long, H.; Wang, J.T.; Yao, W.B.; et al. Advances in the Role and Mechanisms of Essential Oils and Plant Extracts as Natural Preservatives to Extend the Postharvest Shelf Life of Edible Mushrooms. Foods 2023, 12, 801. [Google Scholar] [CrossRef]
- Chang, X.N.; Chen, X.F.; Guo, Y.X.; Gong, P.; Pei, S.Y.; Wang, D.N.; Wang, P.P.; Wang, M.R.; Chen, F.X. Advances in Chemical Composition, Extraction Techniques, Analytical Methods, and Biological Activity of Astragali Radix. Molecules 2022, 27, 1058. [Google Scholar] [CrossRef]
- Guo, Y.X.; Chen, X.F.; Gong, P. Classification, structure and mechanism of antiviral polysaccharides derived from edible and medicinal fungus. Int. J. Biol. Macromol. 2021, 183, 1753–1773. [Google Scholar] [CrossRef]
- Guo, Y.X.; Chen, X.F.; Gong, P.; Li, Z.X.; Wu, Y.P.; Zhang, J.; Wang, J.T.; Yao, W.B.; Yang, W.J.; Chen, F.X. Advances in the mechanisms of polysaccharides in alleviating depression and its complications. Phytomedicine 2023, 109, 154566–154574. [Google Scholar] [CrossRef]
- Li, L.; Gan, H.Y.; Jin, H.Q.; Fang, Y.; Yang, Y.; Zhang, J.P.; Hu, X.W.; Chu, L.S. Astragaloside IV promotes microglia/macrophages M2 polarization and enhances neurogenesis and angiogenesis through PPAR gamma pathway after cerebral ischemia/reperfusion injury in rats. Int. Immunopharmacol. 2021, 92, 107335. [Google Scholar] [CrossRef]
- Zhou, R.X.; Guo, T.K.; Li, J.L. Research progress on the antitumor effects of astragaloside IV. Eur. J. Pharmacol. 2023, 938, 175449. [Google Scholar] [CrossRef]
- Chen, T.Q.; Yang, P.Y.; Jia, Y.J. Molecular mechanisms of astragaloside-IV in cancer therapy (Review). Int. J. Mol. Med. 2021, 47, 13. [Google Scholar] [CrossRef]
- Zang, Y.B.; Wan, J.J.; Zhang, Z.; Huang, S.; Liu, X.; Zhang, W.D. An updated role of astragaloside IV in heart failure. Biomed. Pharmacother. 2020, 126, 110012. [Google Scholar] [CrossRef]
- Gong, P.; Xiao, X.Y.; Wang, S.; Shi, F.X.; Liu, N.; Chen, X.F.; Yang, W.J.; Wang, L.; Chen, F.X. Hypoglycemic effect of astragaloside IV via modulating gut microbiota and regulating AMPK/SIRT1 and PI3K/AKT pathway. J. Ethnopharmacol. 2021, 281, 114558. [Google Scholar] [CrossRef]
- Wang, B.S.; Zhang, C.Y.; Chu, D.M.; Ma, X.F.; Yu, T.; Liu, X.Z.; Hu, C.Q. Astragaloside IV improves angiogenesis under hypoxic conditions by enhancing hypoxia-inducible factor-1 alpha SUMOylation. Mol. Med. Rep. 2021, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S.; Jeong, H.Y.; Yang, H.G.; Seo, Y.R.; Jung, E.G.; Lee, Y.S.; Nam, K.W.; Kim, W.J. Astragaloside IV Suppresses Hepatic Proliferation in Regenerating Rat Liver after 70% Partial Hepatectomy via Down-Regulation of Cell Cycle Pathway and DNA Replication. Molecules 2021, 26, 2895. [Google Scholar] [CrossRef]
- Lin, Y.Q.; Xu, Y.; Zheng, X.; Zhang, J.W.; Liu, J.F.; Wu, G.T. Astragaloside IV Ameliorates Streptozotocin Induced Pancreatic beta-Cell Apoptosis and Dysfunction through SIRT1/P53 and Akt/GSK3 beta/Nrf2 Signaling Pathways. Diabetes Metab. Syndr. Obes. 2022, 15, 131–140. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, X.Q.; Hu, X.R.; Gao, L.; Zeng, H.X.; Wang, X.; Huang, Y.B.; Zhu, W.; Wang, J.A.; Wen, J.G.; et al. METTL3-mediated m(6)A modification of TIMP2 mRNA promotes podocyte injury in diabetic nephropathy. Mol. Ther. 2022, 30, 1721–1740. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Saigusa, D.; Kanemitsu, Y.; Matsumoto, Y.; Thanai, P.; Suzuki, N.; Mise, K.; Yamaguchi, H.; Nakamura, T.; Asaji, K.; et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat. Commun. 2019, 10, 1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homan, K.A.; Gupta, N.; Kroll, K.T.; Kolesky, D.B.; Skylar-Scott, M.; Miyoshi, T.; Mau, D.; Valerius, M.T.; Ferrante, T.; Bonventre, J.V.; et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 2019, 16, 255–262. [Google Scholar] [CrossRef]
- Jourde-Chiche, N.; Fakhouri, F.; Dou, L.; Bellien, J.; Burtey, S.; Frimat, M.; Jarrot, P.A.; Kaplanski, G.; Le Quintrec, M.; Pernin, V.; et al. Endothelium structure and function in kidney health and disease. Nat. Rev. Nephrol. 2019, 15, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hou, B.L.; Wang, Z.P.; Yang, Y.L. Polystyrene microplastics induce mitochondrial damage in mouse GC-2 cells. Ecotoxicol. Environ. Saf. 2022, 237, 113520. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H. Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia-reperfusion injury. Acta Pharm. Sin. B 2020, 10, 1866–1879. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Li, Y.; Shi, L.J.; Hussain, R.; Mehmood, K.; Tang, Z.X.; Zhang, H. Heavy metals induced mitochondrial dysfunction in animals: Molecular mechanism of toxicity. Toxicology 2022, 469, 153136. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zhao, Y.; Lu, S.; Zhang, T.R.; Lindenmeyer, M.T.; Nair, V.; Gies, S.E.; Wu, G.C.; Nelson, R.G.; Czogalla, J.; et al. Single-cell transcriptomics reveals a mechanosensitive injury signaling pathway in early diabetic nephropathy. Genome Med. 2023, 15, 2. [Google Scholar] [CrossRef]
- Nunes, S.; Alves, A.; Preguica, I.; Barbosa, A.; Vieira, P.; Mendes, F.; Martins, D.; Viana, S.D.; Reis, F. Crescent-Like Lesions as an Early Signature of Nephropathy in a Rat Model of Prediabetes Induced by a Hypercaloric Diet. Nutrients 2020, 12, 881. [Google Scholar] [CrossRef] [Green Version]
- Vahsen, B.F.; Ribas, V.T.; Sundermeyer, J.; Boecker, A.; Dambeck, V.; Lenz, C.; Shomroni, O.; Gomes, L.C.; Tatenhorst, L.; Barski, E.; et al. Inhibition of the autophagic protein ULK1 attenuates axonal degeneration in vitro and in vivo, enhances translation, and modulates splicing. Cell Death Differ. 2020, 27, 2810–2827. [Google Scholar] [CrossRef]
- Case, L.B.; Zhang, X.; Ditlev, J.A.; Rosen, M.K. Stoichiometry controls activity of phase-separated clusters of actin signaling proteins. Science 2019, 363, 1093–1097. [Google Scholar] [CrossRef]
- Martin, C.E.; New, L.A.; Phippen, N.J.; Chahi, A.K.; Mitro, A.E.; Takano, T.; Pawson, T.; Blasutig, I.M.; Jones, N. Multivalent nephrin-Nck interactions define a threshold for clustering and tyrosine-dependent nephrin endocytosis. J. Cell Sci. 2020, 133, jcs236877. [Google Scholar] [CrossRef]
- Su, W.; van Wijk, S.W.; Brundel, B. Desmin variants: Trigger for cardiac arrhythmias? Front. Cell Dev. Biol. 2022, 10, 986718. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.R.; Kadioglu, H.; Patel, K.; Carrier, L.; Agnetti, G. Is Desmin Propensity to Aggregate Part of its Protective Function? Cells 2020, 9, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuscu, G.C.; Gurel, C.; Buhur, A.; Oltulu, F.; Akman, L.; Kose, T.; Yavasoglu, N.U.K.; Yavasoglu, A. The regulatory effects of clomiphene and tamoxifen on mTOR and LC3-II expressions in relation to autophagy in experimental polycystic ovary syndrome (PCOS). Mol. Biol. Rep. 2022, 49, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Chen, X.F.; Gong, P.; Guo, J.; Deng, D.; He, G.L.; Ji, C.L.; Wang, R.T.; Long, H.; Wang, J.T.; et al. Effect of shiitake mushrooms polysaccharide and chitosan coating on softening and browning of shiitake mushrooms (Lentinus edodes) during postharvest storage. Int. J. Biol. Macromol. 2022, 218, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Azimee, S.; Rahmati, M.; Fahimi, H.; Moosavi, M.A. TiO2 nanoparticles enhance the chemotherapeutic effects of 5-fluorouracil in human AGS gastric cancer cells via autophagy blockade. Life Sci. 2020, 248, 117466. [Google Scholar] [CrossRef]
- Fujikawa, I.; Ando, T.; Suzuki-Karasaki, M.; Suzuki-Karasaki, M.; Ochiai, T.; Suzuki-Karasaki, Y. Aspirin Induces Mitochondrial Ca2+ Remodeling in Tumor Cells via ROS-Depolarization-Voltage-Gated Ca2+ Entry. Int. J. Mol. Sci. 2020, 21, 4771. [Google Scholar] [CrossRef]
- Michaelis, J.B.; Brunstein, M.E.; Bozkurt, S.; Alves, L.; Wegner, M.; Kaulich, M.; Pohl, C.; Munch, C. Protein import motor complex reacts to mitochondrial misfolding by reducing protein import and activating mitophagy. Nat. Commun. 2022, 13, 5164. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lai, C.C.; Chen, F.; Ding, Y.T.; Zhou, Y.Y.; Su, S.B.; Ni, R.Q.; Tang, Z. Emodin Protects SH-SY5Y Cells Against Zinc-Induced Synaptic Impairment and Oxidative Stress Through the ERK1/2 Pathway. Front. Pharmacol. 2022, 13, 821521. [Google Scholar] [CrossRef]
- Feng, Z.; Yu, X.M.; Jiang, M.X.; Zhu, L.; Zhang, Y.; Yang, W.; Xi, W.; Li, G.H.; Qian, J. Excretable IR-820 for in vivo NIR-II fluorescence cerebrovascular imaging and photothermal therapy of subcutaneous tumor. Theranostics 2019, 9, 5706–5719. [Google Scholar] [CrossRef]
- Lin, Q.S.; Li, S.; Jiang, N.; Shao, X.H.; Zhang, M.F.; Jin, H.J.; Zhang, Z.; Shen, J.X.; Zhou, Y.J.; Zhou, W.Y.; et al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 2019, 26, 101254. [Google Scholar] [CrossRef]
- Quinn, P.M.J.; Moreira, P.I.; Ambrosio, A.F.; Alves, C.H. PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathol. Commun. 2020, 8, 189. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Ge, J.; Lv, M.W.; Talukder, M.; Guo, K.; Li, Y.H.; Li, J.L. Ameliorative effects of resveratrol against cadmium-induced nephrotoxicity via modulating nuclear xenobiotic receptor response and PINK1/Parkin-mediated Mitophagy. Food Funct. 2020, 11, 1856–1868. [Google Scholar] [CrossRef]
- Gastaldi, M.; Scaranzin, S.; Businaro, P.; Mobilia, E.; Benedetti, L.; Pesce, G.; Franciotta, D. Improving laboratory diagnostics in myasthenia gravis. Expert Rev. Mol. Diagn. 2021, 21, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, F.; Cai, K.; Xu, J.X. LncRNA HOXA-AS2 facilitates prostate cancer progression by inhibiting miR-885-5p to upregulate KDM5B. Kidney Blood Press. Res. 2023, 48, 45–55. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, P.; Yue, S.; Shi, F.; Yang, W.; Yao, W.; Chen, F.; Guo, Y. Protective Effect of Astragaloside IV against Cadmium-Induced Damage on Mouse Renal Podocytes (MPC5). Molecules 2023, 28, 4897. https://doi.org/10.3390/molecules28134897
Gong P, Yue S, Shi F, Yang W, Yao W, Chen F, Guo Y. Protective Effect of Astragaloside IV against Cadmium-Induced Damage on Mouse Renal Podocytes (MPC5). Molecules. 2023; 28(13):4897. https://doi.org/10.3390/molecules28134897
Chicago/Turabian StyleGong, Pin, Shan Yue, Fuxiong Shi, Wenjuan Yang, Wenbo Yao, Fuxin Chen, and Yuxi Guo. 2023. "Protective Effect of Astragaloside IV against Cadmium-Induced Damage on Mouse Renal Podocytes (MPC5)" Molecules 28, no. 13: 4897. https://doi.org/10.3390/molecules28134897






