Not So Similar: Different Ways of Nb(V) and Ta(V) Catecholate Complexation
Abstract
:1. Introduction
2. Results and Discussion
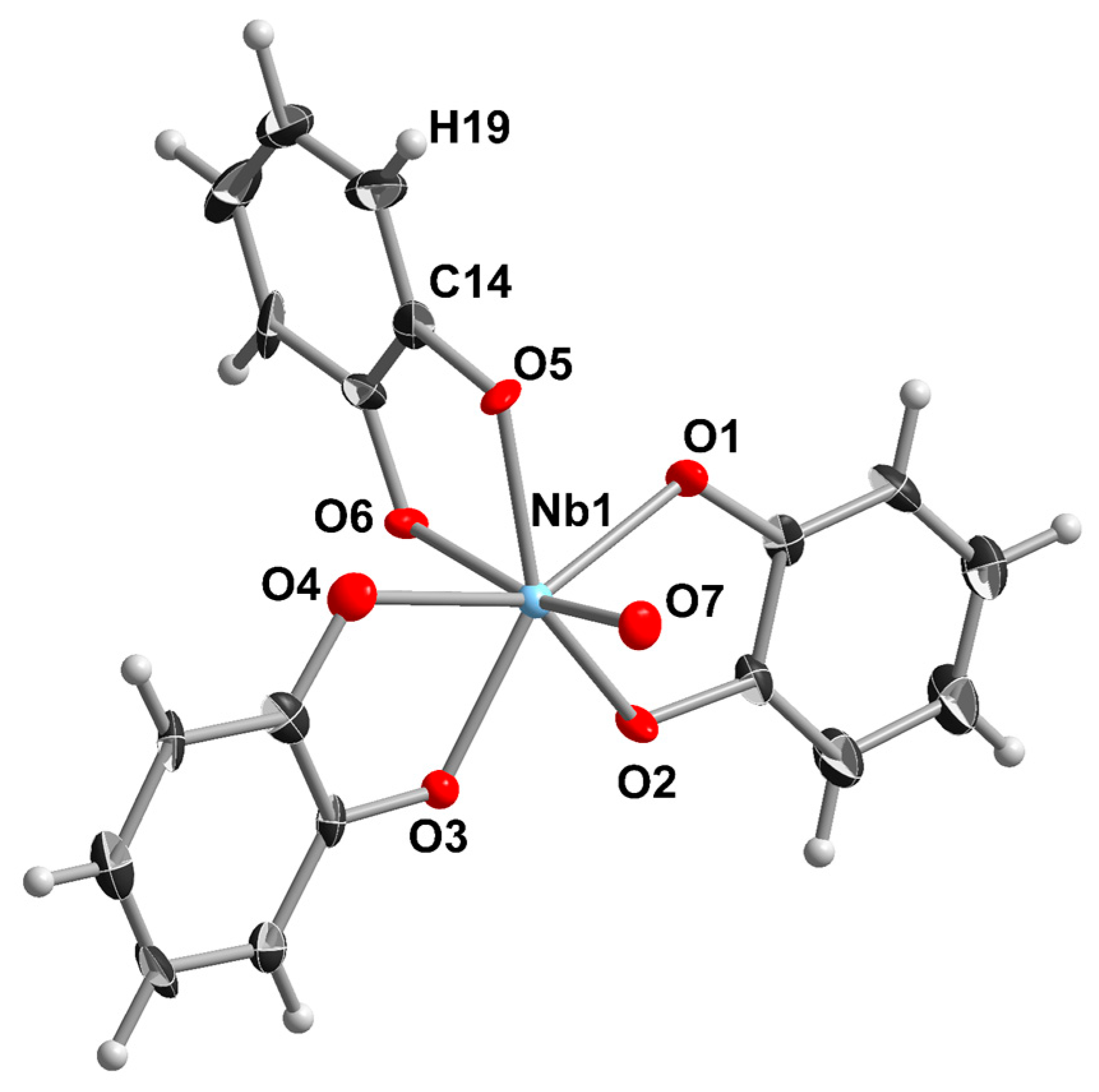
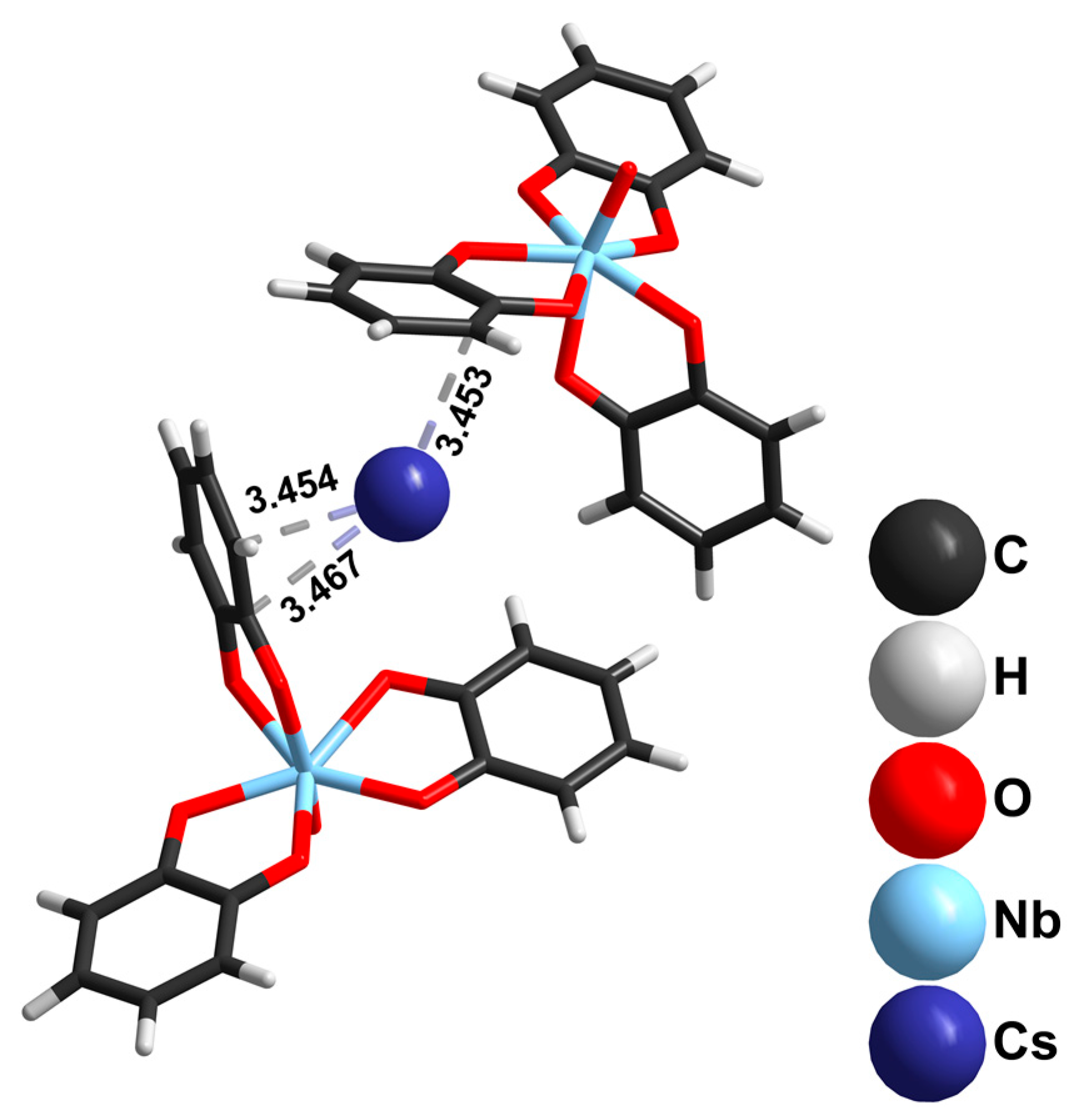
2.1. Niobium Complexes
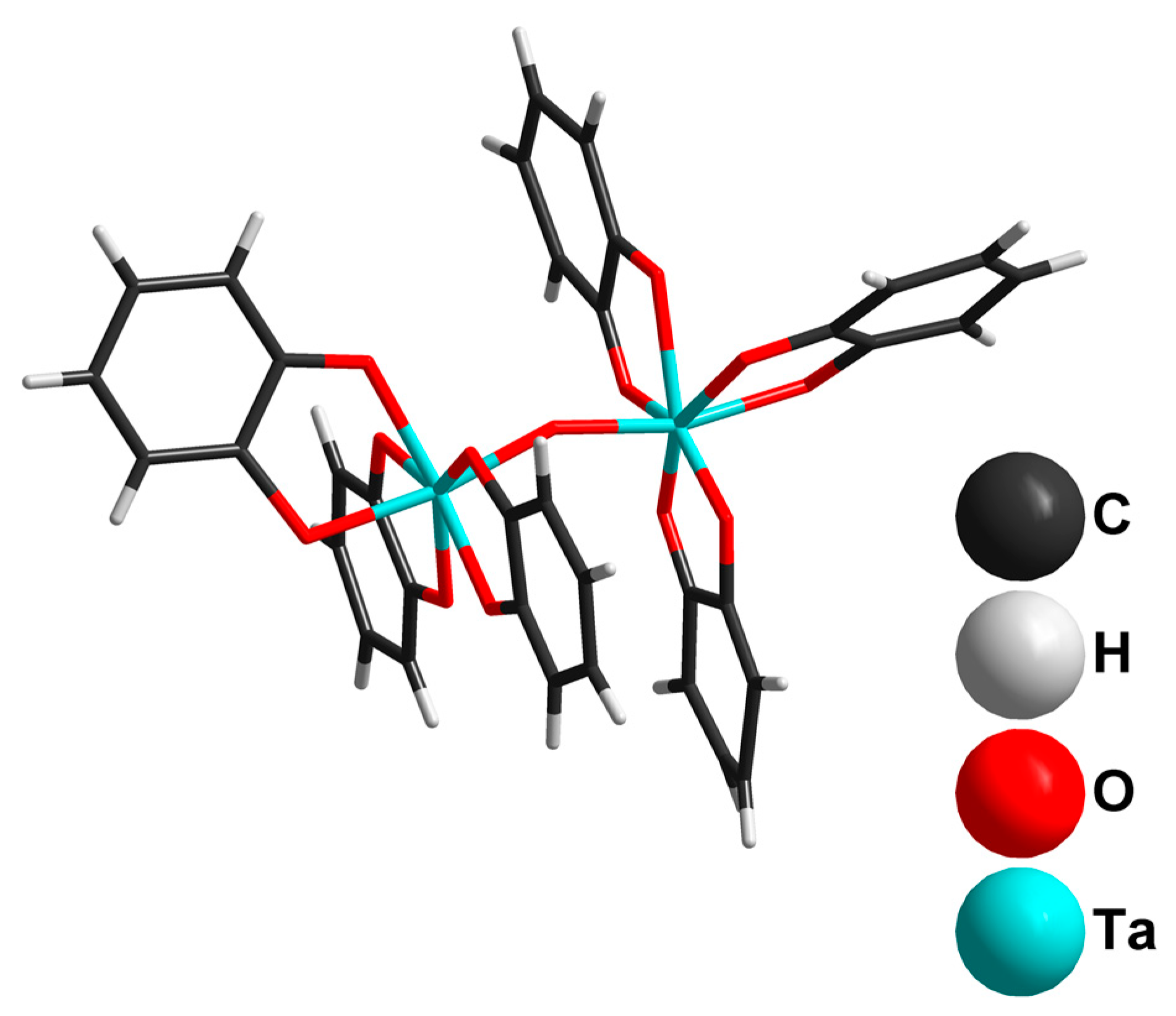
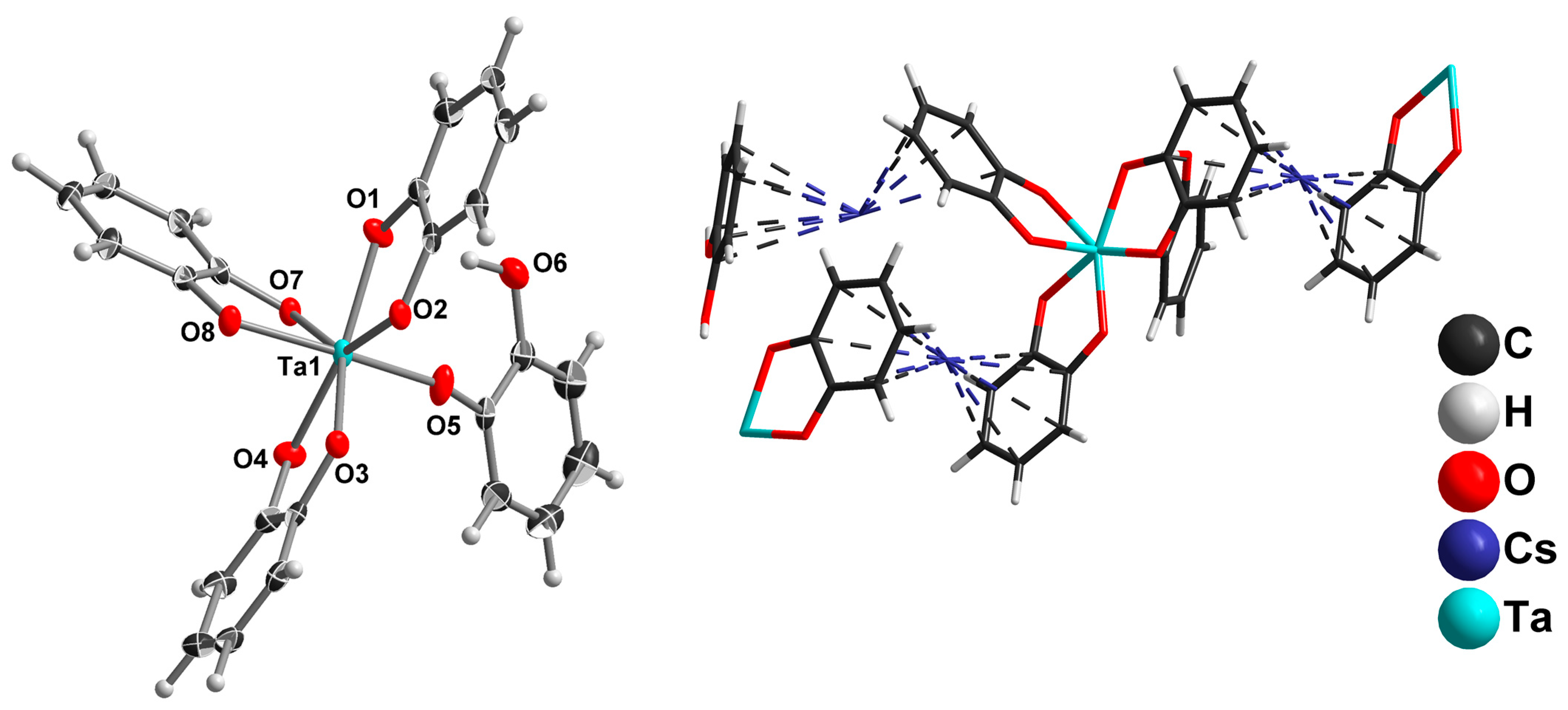
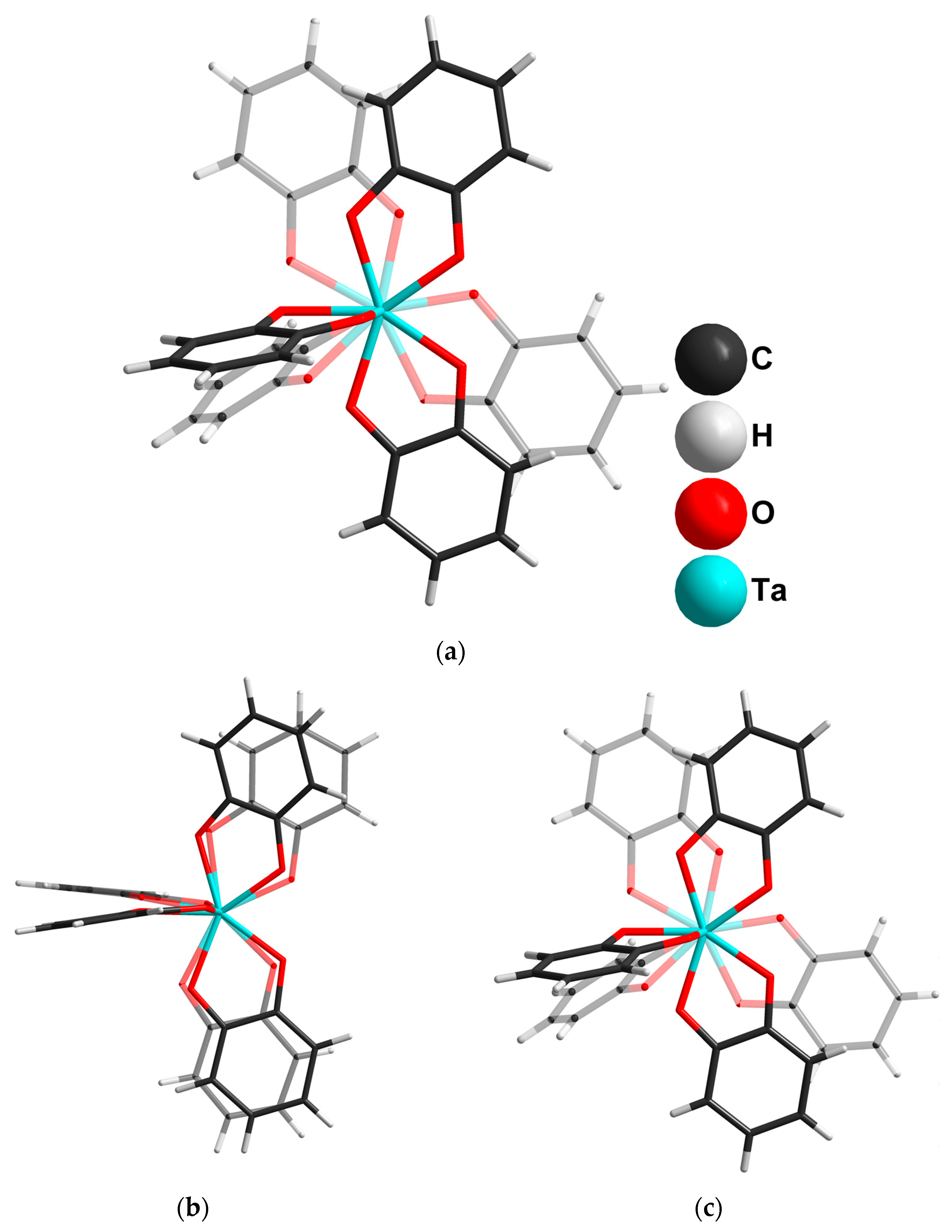
2.2. Tantalum Complexes
2.3. Comments on IR-Spectra and Stability
3. Materials and Methods
3.1. General Information
3.2. Synthesis
- Synthesis of (NH4)3[NbO(cat)3]∙4H2O (1):
- A total of 2 g of catechol (18.6 mmol) was added to a suspension of 1 g Nb2O5·xH2O (3.1 mmol) in 20 mL of H2O. After the addition of 4 mL of NH3·H2O, the resulting reaction mixture was refluxed for 1.5 h until the clear-red solution formed. Small yellowish crystals formed after the solution was kept at 5 °C for 3 days. The crude product was collected by filtration on a glass filter, washed with diethyl ether, and dried in vacuo. The typical yield was 0.740 g (23% based on Nb2O5). Anal. Calc. for C18H26N3NbO9 C, H, N (%): 41.5, 5.0, 8.1. Found C, H, N (%): 41.2, 5.2, 8.0.
- IR (ATR, ν, cm−1): 1570(w), 1481(m), 1444(w), 1391(w), 1328(w), 1251(s), 1097(m), 1020(m), 899(w), 866(w), 830(w), 806(s), 734(s), 598(vs).
- TGA: weight loss 14%—4.4H2O.
- Synthesis of K2[Nb(cat)3(Hcat)]·2H2cat·H2O(2):
- A total of 2 g of catechol (18.6 mmol) was added to a suspension of 1 g Nb2O5·xH2O (3.1 mmol) in 20 mL of H2O. After adding 4 mL of 1M KOH, the resulting mixture was refluxed for 1.5 h until a clear-brown solution formed. Small yellowish crystals formed after the solution was cooled to 5 °C and kept in such conditions for 7 days. The crude product was collected by filtration on a glass filter, washed with diethyl ether, and dried in vacuo. Yield—several crystals (manual isolation). The XRPD from the bulk sample was hard to assign.
- IR (ATR, ν, cm−1): 3522(w), 1601(w), 1512(w), 1479(s), 1452(w), 1384(w), 1356(w), 1331(w), 1280(w), 1253(s), 1208(w), 1180(w), 1165(w), 1096(m), 1034(w), 1025(w), 1017(w), 896(w), 868(w), 851(w), 805(m), 737(vs), 628(w), 615(w).
- TGA: weight loss 2.5%—1H2O.
- Synthesis of Cs3[NbO(cat)3]·H2O (3):
- A total of 2.13 g of catechol (19.3 mmol) was added to a clear solution of 2.34 g Cs8[Nb6O19]·14H2O (1.1 mmol) in 20 mL of H2O. After adding 5 mL of 2M CsOH, the resulting mixture was refluxed for 2 h until a clear-brownish solution formed. Red crystals formed after the solution was kept at 5 °C for 7 days. The crude product was collected by filtration on a glass filter, washed with diethyl ether, and dried in vacuo. Yield—several crystals (manual isolation). The XRPD from the bulk sample was hard to assign.
- IR (ATR, ν, cm−1): 3522(w), 1601(w), 1512(w), 1479(s), 1453(w), 1384(w), 1356(w), 1331(w), 1280(w), 1253(s), 1208(w), 1180(w), 1165(w), 1096(m), 1034(w), 1017(w), 908(w), 896(w), 868(w), 851(w), 805(m), 769(w), 737(vs), 628(w), 615(w), 582(w), 564(w).
- TGA: weight loss 2.5%—1H2O.
- Synthesis of (NH4)4[Ta2O(cat)6]·3H2O (4):
- A total of 1 g of catechol (9.1 mmol) was added to a solution of 1 g K8[Ta6O19]·16H2O (0.5 mmol) in 20 mL of H2O. After adding 5 mL of NH3·H2O, the resulting mixture was refluxed for 2 h. After that, the hot yellow solution was filtered from the white precipitate, and 50 mL of EtOH was added to the solution. Crystals formed after the solution was kept below 5 °C for one week. The crude product was collected by filtration on a glass filter, washed with diethyl ether, and dried in vacuo. The typical yield was 0.485 g (26% based on hexatantalate). Anal. Calc. for C36H46N4O16Ta2 C, H, N (%): 37.5, 4.0, 4.9. Found C, H, N (%): 37.5, 4.2, 4.7.
- IR (ATR, ν, cm−1): 1581(w), 1482(s), 1449(w), 1423(w), 1413(w), 1343(w), 1335(w), 1249(vs), 1150(w), 1099(w), 1044(w), 1019(w), 909(w), 882(w), 869(w), 841(w), 808(m), 769(w), 739(m), 653(w), 610(vs).
- TGA: weight loss 4%—2.6H2O.
- Synthesis of Cs2[Ta(cat)3(Hcat)]·H2cat (5):
- A total of 1.46 g of catechol (13.3 mmol) was added to a solution of 1 g Cs8[Ta6O19]·14H2O (0.37 mmol) in 20 mL of H2O. After adding 5 mL of NH3·H2O, the resulting mixture was refluxed for 2 h. After that, the hot yellow solution was filtered from the white precipitate. Crystals formed after the solution was cooled down to room temperature. The crude product was collected by filtration on a glass filter, washed with diethyl ether, and dried in vacuo. The typical yield was 0.643 g (26% based on hexatantalate). Anal. Calc. for C30H23Cs2O10Ta C, H (%): 36.4, 2.3. Found C, H (%): 36.6, 2.6.
- IR (ATR, ν, cm−1): 1478(vs), 1448(w), 1257(w), 1246(w), 1223(w), 1207(w), 1186(w), 1151(w), 1100(m), 1039(w), 1024(w), 953(w), 903(m), 879(w), 871(w), 858(w), 840(w), 821(w), 804(w), 795(w), 758(w), 739(s), 610(m), 582(w), 567(w).
- Synthesis of Cs4[Ta2O(C6H4O2)6]·7H2O (6):
- A total of 0.73 g of catechol (6.63 mmol) was added to a solution of 1.0 g Cs8[Ta6O19]·14H2O (0.37 mmol) in 20 mL of H2O. After adding 5 mL of NH3·H2O, the resulting mixture was refluxed for 2 h. After that, the hot yellow solution was filtered from the white precipitate, and 50 mL of EtOH was added to the solution. Crystals formed after the solution was kept below 5 °C for one week. The crude product was collected by filtration on a glass filter, washed with diethyl ether, and dried in vacuo. The typical yield was 0.485 g (26% based on hexatantalate). Anal. Calc. for C36H38Cs4O20Ta2 C, H (%): 25.7, 2.3. Found C, H(%): 25.3, 2.2.
- IR (ATR, ν, cm−1): 1581(w), 1549(w), 1530(w), 1513(w), 1479(s), 1450(w), 1431(w), 1413(w), 1344(w), 1334(w), 1274(w), 1248(m), 1221(w), 1153(w), 904(w), 868(w), 840(w), 806(m), 767(w), 732(m).
- TGA: weight loss 7%—6.5H2O.
3.3. X-ray Powder Diffraction
3.4. X-ray Diffraction on Single Crystals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fast, D.; Clark, M.; Fullmer, L.; Grove, K.; Nyman, M.; Gibbons, B.; Dolgos, M. Using simple aqueous precursors for a green synthetic pathway to potassium sodium niobate thin films. Thin Solid Film. 2020, 710, 138270. [Google Scholar] [CrossRef]
- Misra, A.; Kozma, K.; Streb, C.; Nyman, M. Beyond Charge Balance: Counter-Cations in Polyoxometalate Chemistry. Angew. Chemie Int. Ed. 2020, 59, 596–612. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.P.; Petrus, E.; Segado, M.; Arteaga, A.; Zakharov, L.N.; Bo, C.; Nyman, M. Strategic Capture of the {Nb 7 } Polyoxometalate. Chem. A Eur. J. 2019, 25, 10580–10584. [Google Scholar] [CrossRef] [PubMed]
- Shmakova, A.A.; Volchek, V.V.; Yanshole, V.; Kompankov, N.B.; Martin, N.P.; Nyman, M.; Abramov, P.A.; Sokolov, M.N. Niobium uptake by a [P 8 W 48 O 184 ] 40− macrocyclic polyanion. New J. Chem. 2019, 43, 9943–9952. [Google Scholar] [CrossRef]
- Zhang, D.; Li, H.; Li, C.; Wang, Z.; Li, T.; Li, N.; Cheng, M.; Wang, J.; Niu, J.; Liu, T. A large molecular cluster with high proton release capacity. Chem. Commun. 2020, 56, 12849–12852. [Google Scholar] [CrossRef]
- Liang, Z.; He, Y.; Qiao, Y.; Ma, P.; Niu, J.; Wang, J. Sandwich-Type Heteropolyniobate Templated by Mixed Heteroanions. Inorg. Chem. 2020, 59, 7895–7899. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, J.; Ma, Y.; Zhang, T.; Li, N.; Li, C.; Ma, P.; Li, T.; Wang, G.; Liu, T.; et al. Unraveling the Effects of Cobalt on Crystal Growth and Solution Behavior of Nb 6 P 2 W 12 -based Dimeric Clusters. Inorg. Chem. 2020, 59, 6747–6754. [Google Scholar] [CrossRef]
- Yang, Z.; Shang, J.; He, Y.; Qiao, Y.; Ma, P.; Niu, J.; Wang, J. A 1D Helical Chain Heterpolyniobate Templated by AsO33−. Inorg. Chem. 2020, 59, 1967–1972. [Google Scholar] [CrossRef]
- Shmakova, A.A.; Romanova, T.E.; Kompankov, N.B.; Abramov, P.A.; Sokolov, M.N. Trapping of NbV by {XW9O33}9− (X = As, Sb): Formation of New Sandwich-Type POM Complexes and Their Solution Behavior. Eur. J. Inorg. Chem. 2019, 2019, 2543–2548. [Google Scholar] [CrossRef]
- Shmakova, A.A.; Sukhikh, T.S.; Volchek, V.V.; Yanshole, V.; Stass, D.V.; Filatov, E.Y.; Glebov, E.M.; Abramov, P.A.; Sokolov, M.N. Niobium uptake by {P2W12} polyoxoanion with [NbO(C2O4)2(H2O)2]− as Nb source. Inorg. Chim. Acta 2020, 502, 119319. [Google Scholar] [CrossRef]
- Abramov, P.A.; Sokolov, M.N.; Virovets, A.V.; Floquet, S.; Haouas, M.; Taulelle, F.; Cadot, E.; Vicent, C.; Fedin, V.P. Grafting {Cp*Rh}2+ on the surface of Nb and Ta Lindqvist-type POM. Dalton Trans. 2015, 44, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Abramov, P.A.; Zemerova, T.P.; Moroz, N.K.; Kompankov, N.B.; Zhdanov, A.A.; Tsygankova, A.R.; Vicent, C.; Sokolov, M.N. Synthesis and Characterization of [(OH)TeNb5O18]6− in Water Solution, Comparison with [Nb6O19]8−. Inorg. Chem. 2016, 55, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Abramov, P.A.; Davletgildeeva, A.T.; Moroz, N.K.; Kompankov, N.B.; Santiago-Schübel, B.; Sokolov, M.N. Cation-Dependent Self-assembly of Vanadium Polyoxoniobates. Inorg. Chem. 2016, 55, 12807–12814. [Google Scholar] [CrossRef]
- Bayot, D.; Tinant, B.; Devillers, M. Water-soluble niobium peroxo complexes as precursors for the preparation of Nb-based oxide catalysts. Catal. Today 2003, 78, 439–447. [Google Scholar] [CrossRef]
- Rakhmanov, E.V.; Sinyan, Z.; Tarakanova, A.V.; Anisimov, A.V.; Akopyan, A.V.; Baleeva, N.S. Synthesis and catalytic properties of niobium indenyl peroxo complexes. Russ. J. Gen. Chem. 2012, 82, 1118–1121. [Google Scholar] [CrossRef]
- Bayot, D.; Devillers, M. Peroxo complexes of niobium(V) and tantalum(V). Coord. Chem. Rev. 2006, 250, 2610–2626. [Google Scholar] [CrossRef]
- Carniato, F.; Bisio, C.; Psaro, R.; Marchese, L.; Guidotti, M. Niobium(V) Saponite Clay for the Catalytic Oxidative Abatement of Chemical Warfare Agents. Angew. Chemie 2014, 126, 10259–10262. [Google Scholar] [CrossRef]
- Lima, A.L.D.; Fajardo, H.V.; Nogueira, A.E.; Pereira, M.C.; Oliveira, L.C.A.; de Mesquita, J.P.; Silva, A.C. Selective oxidation of aniline into azoxybenzene catalyzed by Nb-peroxo@iron oxides at room temperature. New J. Chem. 2020, 44, 8710–8717. [Google Scholar] [CrossRef]
- Androš, L.; Jurić, M.; Popović, J.; Šantić, A.; Lazić, P.; Benčina, M.; Valant, M.; Brničević, N.; Planinić, P. Ba4Ta2O9 Oxide Prepared from an Oxalate-Based Molecular Precursor—Characterization and Properties. Inorg. Chem. 2013, 52, 14299–14308. [Google Scholar] [CrossRef]
- Jurić, M.; Perić, B.; Brničević, N.; Planinić, P.; Pajić, D.; Zadro, K.; Giester, G.; Kaitner, B. Supramolecular motifs and solvatomorphism within the compounds [M(bpy)3]2[NbO(C2O4)3]Cl·nH2O (M = Fe2+, Co2+, Ni2+, Cu2+ and Zn2+; n = 11, 12). Syntheses, structures and magnetic properties. Dalton Trans. 2008, 6, 742–754. [Google Scholar] [CrossRef]
- Dubraja, L.A.; Matković-Čalogović, D.; Planinić, P. Crystal disassembly and reassembly of heterometallic NiII–TaV oxalate compounds. CrystEngComm 2015, 17, 2021–2029. [Google Scholar] [CrossRef]
- Androš Dubraja, L.; Jurić, M.; Lafargue-Dit-Hauret, W.; Pajić, D.; Zorko, A.; Ozarowski, A.; Rocquefelte, X. First crystal structures of oxo-bridged [CrIIITaV] dinuclear complexes: Spectroscopic, magnetic and theoretical investigations of the Cr–O–Ta core. New J. Chem. 2018, 42, 10912–10921. [Google Scholar] [CrossRef]
- Shmakova, A.A.; Glebov, E.M.; Korolev, V.V.; Stass, D.V.; Benassi, E.; Abramov, P.A.; Sokolov, M.N. Photochromism in oxalatoniobates. Dalton Trans. 2018, 47, 2247–2255. [Google Scholar] [CrossRef] [PubMed]
- Shmakova, A.A.; Abramov, P.A.; Sokolov, M.N. Synthesis, Structure, and Spectral Studies of the (Et4N)4[Nb2O2(C2O4)4(µ-C2O4)] Complex. J. Struct. Chem. 2019, 60, 1080–1085. [Google Scholar] [CrossRef]
- Andriotou, D.; Duval, S.; Volkringer, C.; Trivelli, X.; Shepard, W.E.; Loiseau, T. Structural Variety of Niobium(V) Polyoxo Clusters Obtained from the Reaction with Aromatic Monocarboxylic Acids: Isolation of {Nb2O}, {Nb4O4} and {Nb8O12} Cores. Chem. A Eur. J. 2022, 28, e202201464. [Google Scholar] [CrossRef] [PubMed]
- Andriotou, D.; Duval, S.; Trivelli, X.; Volkringer, C.; Loiseau, T. Molecular niobium(v) complexes with mononuclear {Nb1} and dinuclear oxo species {Nb2O} connected through aryl di- or tetra-carboxylate linker. CrystEngComm 2022, 24, 5938–5948. [Google Scholar] [CrossRef]
- Drew, M.G.B.; Rice, D.A.; Williams, D.M. Synthesis of Nb(O2H5C7)3Y (Y = O, S and Se): Crystal structure of oxo-tris-(tropolonato)niobium(V) monohydrate: A seven coordinate monomer containing a terminal Nb O bond. Inorg. Chim. Acta 1986, 118, 165–168. [Google Scholar] [CrossRef]
- Muetterties, E.L.; Wright, C.M. Chelate Chemistry. III. Chelates of High Coordination Number. J. Am. Chem. Soc. 1965, 87, 4706–4717. [Google Scholar] [CrossRef]
- Camporese, D.; Riondato, M.; Zampieri, A.; Marchiò, L.; Tapparo, A.; Mazzi, U. Tris(1,2-dimethyl-3-hydroxy-4(1H)-pyridone)oxotantalum(v): A new water-soluble tantalum complex containing the [Ta=O]3+ core. Dalton Trans. 2006, 4343–4347. [Google Scholar] [CrossRef]
- Broere, D.L.J.; Plessius, R.; van der Vlugt, J.I. New avenues for ligand-mediated processes—Expanding metal reactivity by the use of redox-active catechol, o-aminophenol and o-phenylenediamine ligands. Chem. Soc. Rev. 2015, 44, 6886–6915. [Google Scholar] [CrossRef]
- Ershova, I.V.; Piskunov, A.V.; Cherkasov, V.K. Complexes of diamagnetic cations with radical anion ligands. Russ. Chem. Rev. 2020, 89, 1157–1183. [Google Scholar] [CrossRef]
- Luca, O.R.; Crabtree, R.H. Redox-active ligands in catalysis. Chem. Soc. Rev. 2013, 42, 1440–1459. [Google Scholar] [CrossRef]
- Fairbrother, F. The Chemistry of Niobium and Tantalum; Elsevier: Amsterdam, The Netherlands, 1967; ISBN 9780444402059. [Google Scholar]
- Brown, S.A.; Land, J.E. A Spectrophotometric Study of the Niobium Catecholato Complexes 1. J. Am. Chem. Soc. 1959, 81, 3185–3187. [Google Scholar] [CrossRef]
- Brown, S.A.; Land, J.E. A spectrophotometric investigation of the niobium protocatechuic acid complex. J. Less Common Met. 1959, 1, 237–243. [Google Scholar] [CrossRef]
- Elinson, S. V Applications of Heteroligand Complexes in the Analytical Chemistry of Niobium and Tantalum. Russ. Chem. Rev. 1975, 44, 707–717. [Google Scholar] [CrossRef]
- Fairbrother, F.; Ahmed, N.; Edgar, K.; Thompson, A. Complexes of niobium and tantalum hydroxides with di-ols. J. Less Common Met. 1962, 4, 466–475. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Tan, C.-H.; Ye, X. Applications of Vanadium, Niobium, and Tantalum Complexes in Organic and Inorganic Synthesis. ACS Org. Inorg. Au 2023, 3, 74–91. [Google Scholar] [CrossRef]
- Munhá, R.F.; Zarkesh, R.A.; Heyduk, A.F. Group transfer reactions of d0 transition metal complexes: Redox-active ligands provide a mechanism for expanded reactivity. Dalton Trans. 2013, 42, 3751. [Google Scholar] [CrossRef] [PubMed]
- Zarkesh, R.A.; Ziller, J.W.; Heyduk, A.F. Four-Electron Oxidative Formation of Aryl Diazenes Using a Tantalum Redox-Active Ligand Complex. Angew. Chemie Int. Ed. 2008, 47, 4715–4718. [Google Scholar] [CrossRef]
- Šestan, M.; Perić, B.; Giester, G.; Planinić, P.; Brničević, N. Another Structure Type of Oxotris(oxalato)niobate(V): Molecular and Crystal Structure of Rb3[NbO(C2O4)3]·2H2O. Struct. Chem. 2005, 16, 409–414. [Google Scholar] [CrossRef]
- Mathern, G.; Weiss, R. Structure cristalline de l’oxotrioxalatoniobate d’ammonium à une molécule d’eau, (NH4)3NbO(C2O4)3.H2O. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1971, 27, 1610–1618. [Google Scholar] [CrossRef]
- Galešić, N.; Brničević, N.; Matković, B.; Herceg, M.; Zelenko, B.; Šljukić, M.; Prelesnik, B.; Herak, R. The crystal structure of ammonium oxobisoxalato-bisaquoniobate(V) trihydrate NH4[NbO(C2O4)2(H2O)2]·3H2O by neutron diffraction. J. Less Common Met. 1977, 51, 259–270. [Google Scholar] [CrossRef]
- Jurić, M.; Planinić, P.; Brničević, N.; Matković-Čalogović, D. Synthesis, properties and crystal structure of a Zn,Nb compound with homo- and heterodinuclear oxalate-bridged units-[{Zn(bpy)2}2(μ-C2O4)][Zn(bpy)2(μ-C2O4)NbO(C2O4)2]2·0.5bpy·7H2O: The impact of weak interactions on the crystal packing. J. Mol. Struct. 2008, 888, 266–276. [Google Scholar] [CrossRef]
- Tsunashima, R.; Long, D.; Miras, H.N.; Gabb, D.; Pradeep, C.P.; Cronin, L. The Construction of High-Nuclearity Isopolyoxoniobates with Pentagonal Building Blocks: [HNb27O76]16− and [H10Nb31O93(CO3)]23−. Angew. Chem. Int. Ed. 2010, 49, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Cotton, F.A.; Diebold, M.P.; Roth, W.J. Variable stereochemistry of the eight-coordinate tetrakis(oxalato)niobate(IV), Nb(C2O4)44−. Inorg. Chem. 1987, 26, 2889–2893. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Palkina, K.K.; Kochetov, A.N. Crystal structure of [Cs2Na(H2O)2(C23H16O3)(C23H15O3)3]. Russ. J. Inorg. Chem. 2010, 55, 1037–1041. [Google Scholar] [CrossRef]
- Mendoza-Espinosa, D.; Martinez-Ortega, B.A.; Quiroz-Guzman, M.; Golen, J.A.; Rheingold, A.L.; Hanna, T.A. Synthesis, structures and full characterization of p-tert-butylcalix[5]arene mono-, di-, tri- and pentaanionic ligand precursors. J. Organomet. Chem. 2009, 694, 1509–1523. [Google Scholar] [CrossRef]
- Yu, L.; Ren, Z.-G.; Qian, L.-W.; Zhu, Q.-Y.; Bian, G.-Q.; Dai, J. Two-step Hydrothermal Syntheses and Structures of Three Tantalum Oxyfluoride Compounds with [M(phen)3]2+ (M = Ru, Fe) Counter Ions. Z. Anorg. Allg. Chem. 2015, 641, 704–709. [Google Scholar] [CrossRef]
- Dewan, J.C.; Edwards, A.J.; Calves, J.Y.; Guerchais, J.E. Fluoride crystal structures. Part 28. Bis(tetraethylammonium)µ-oxo-bis[pentafluorotantalate(V)]. J. Chem. Soc. Dalt. Trans. 1977, 10, 978–980. [Google Scholar] [CrossRef]
- Noll, A.; Müller, U. Die Oxochlorotantalate (PPh4)2[Ta2OCl9]2·2CH2Cl2, (PPh4)2[Ta2OCl10]·2CH3CN und (K-18-Krone-6)4[Ta4O6Cl12]·12CH2Cl2. Z. Anorg. Allg. Chem. 1999, 625, 1721–1725. [Google Scholar] [CrossRef]
- Petrykin, V.; Kakihana, M.; Yoshioka, K.; Sasaki, S.; Ueda, Y.; Tomita, K.; Nakamura, Y.; Shiro, M.; Kudo, A. Synthesis and Structure of New Water-Soluble and Stable Tantalum Compound: Ammonium Tetralactatodiperoxo-μ-oxo-ditantalate(V). Inorg. Chem. 2006, 45, 9251–9256. [Google Scholar] [CrossRef] [PubMed]
- Boyle, T.J.; Tribby, L.J.; Alam, T.M.; Bunge, S.D.; Holland, G.P. Catechol derivatives of Group 4 and 5 compounds. Polyhedron 2005, 24, 1143–1152. [Google Scholar] [CrossRef]
- Pershina, V.G. Electronic Structure and Properties of the Transactinides and Their Compounds. Chem. Rev. 1996, 96, 1977–2010. [Google Scholar] [CrossRef]
- Vlček, A. Metal and Ligand Oxidation States in Dioxolene Complexes: Meaning, Assignment and Control. Comments Inorg. Chem. 1994, 16, 207–228. [Google Scholar] [CrossRef]
- Abramov, P.A.; Abramova, A.M.; Peresypkina, E.V.; Gushchin, A.L.; Adonin, S.A.; Sokolov, M.N. New polyoxotantalate salt Na8[Ta6O19]·24.5H2O and its properties. J. Struct. Chem. 2011, 52, 1012–1017. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramov, P.A.; Sokolov, M.N. Not So Similar: Different Ways of Nb(V) and Ta(V) Catecholate Complexation. Molecules 2023, 28, 4912. https://doi.org/10.3390/molecules28134912
Abramov PA, Sokolov MN. Not So Similar: Different Ways of Nb(V) and Ta(V) Catecholate Complexation. Molecules. 2023; 28(13):4912. https://doi.org/10.3390/molecules28134912
Chicago/Turabian StyleAbramov, Pavel A., and Maxim N. Sokolov. 2023. "Not So Similar: Different Ways of Nb(V) and Ta(V) Catecholate Complexation" Molecules 28, no. 13: 4912. https://doi.org/10.3390/molecules28134912





