Role of Melatonin in Bovine Reproductive Biotechnology
Abstract
:1. Introduction
2. Application of Melatonin in Bovine Granulosa Cells
3. Application of Melatonin in Bovine Oocyte Cells
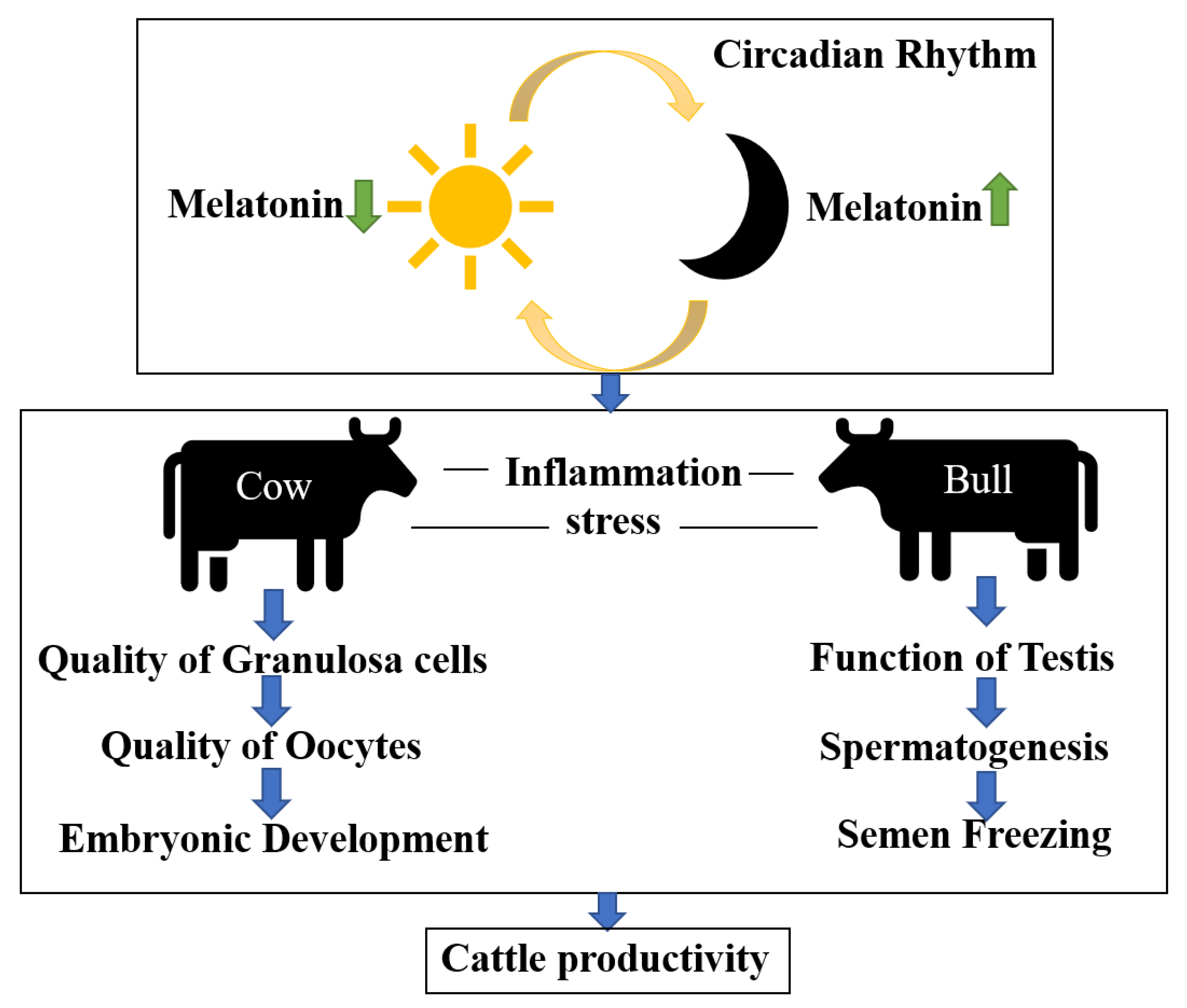
4. Melatonin Regulates Circadian Rhythms in Cattle
5. Effects of Melatonin on Inflammation in Cattle
6. Effects of Melatonin on Testicular Function, Spermatogenesis, and Semen Cryopreservation in Bulls
7. Positive Effects of Melatonin in Livestock Cells
| Species | Positive Effects of Melatonin | Concentration | References |
|---|---|---|---|
| Cattle | Melatonin promoted diameter of bovine follicles and growth of secondary oocytes | 10−7 M | [127] |
| Melatonin in cattle feed changed the β diversity of vaginal microorganisms | 20 mg | [128] | |
| Melatonin promoted proliferation of bovine theca cells and inhibited steroid production | 1 μM | [129] | |
| Melatonin promoted bovine oocyte development and maturation | 10−7 M | [12] | |
| Melatonin inhibited oxidative stress and apoptosis of bovine granulosa cells | 100 μM | [33] | |
| Melatonin increased conception rates in cattle | 0.24 mg/kg | [50] | |
| Melatonin decreased ROS production in bovine sperm and increased sperm viability, plasma membrane integrity, mitochondrial integrity, and acrosome integrity | 10−3 M | [92] | |
| Melatonin promoted development and function of bovine Sertoli cells | 320 pg/mL | [104] | |
| Pig | Melatonin regulated lipid metabolism in porcine oocytes | 10−9 M | [130] |
| Melatonin reduced ROS production in porcine oocytes and promoted mitochondrial function and embryonic development | 500 nmol/L | [131] | |
| Melatonin improved the quality of porcine embryos | 1 nM | [132] | |
| Melatonin improved semen viability and acrosome integrity in pigs | 1 μM | [133] | |
| Melatonin regulated ATP metabolism and antioxidant enzyme activity of boar sperm | 1 μM | [134] | |
| Sheep | Melatonin inhibited LPS-induced inflammation of sheep epididymal epithelial cells | 10−7 M | [135] |
| Melatonin was involved in activation of primordial follicles in sheep ovaries | 100 pg/mL | [136] | |
| Melatonin promoted development of transgenic sheep embryos and improved transgenic efficiency | 10−7 M | [137] | |
| Melatonin reduced ROS accumulation in sheep testicular interstitial cells and promoted testosterone synthesis | 10 ng/mL | [138] | |
| Dietary supplementation of melatonin increased activities of glucose amylase, isomaltase, and maltase in small intestine of sheep | 5 mg/d | [139] | |
| Melatonin reduced ROS and improved sperm quality | 10−7 M | [140] | |
| Melatonin enhanced DNA integrity and fertilization ability of sheep sperm | 1 mM | [141] |
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yasmin, F.; Sutradhar, S.; Das, P.; Mukherjee, S. Gut melatonin: A potent candidate in the diversified journey of melatonin research. Gen. Comp. Endocrinol. 2021, 303, 113693. [Google Scholar] [CrossRef]
- Herxheimer, A.; Petrie, K.J. Melatonin for preventing and treating jet lag. Cochrane Database Syst. Rev. 2002, 2002, CD001520. [Google Scholar]
- Olcese, J.M. Melatonin and Female Reproduction: An Expanding Universe. Front. Endocrinol. 2020, 11, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auld, F.; Maschauer, E.L.; Morrison, I.; Skene, D.J.; Riha, R.L. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med. Rev. 2017, 34, 10–22. [Google Scholar] [CrossRef]
- Paterson, A.M.; Foldes, A. Melatonin and farm animals: Endogenous rhythms and exogenous applications. J. Pineal Res. 1994, 16, 167–177. [Google Scholar] [CrossRef]
- Tosini, G.; Owino, S.; Guillaume, J.-L.; Jockers, R. Understanding melatonin receptor pharmacology: Latest insights from mouse models, and their relevance to human disease. Bioessays 2014, 36, 778–787. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhao, J.; Liu, H.; Wang, J.; Lu, W. Melatonin inhibits apoptosis in mouse Leydig cells via the retinoic acid-related orphan nuclear receptor α/p53 pathway. Life Sci. 2020, 246, 117431. [Google Scholar] [CrossRef] [PubMed]
- Frungieri, M.B.; Mayerhofer, A.; Zitta, K.; Pignataro, O.P.; Calandra, R.S.; Gonzalez-Calvar, S.I. Direct effect of melatonin on Syrian hamster testes: Melatonin subtype 1a receptors, inhibition of androgen production, and interaction with the local corticotropin-releasing hormone system. Endocrinology 2005, 146, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, N.R.; Marcus Leo, M.D.; Subramani, J.; Anish, D.; Sudhagar, M.; Ahmed, K.A.; Saxena, M.; Tyagi, J.S.; Sastry, K.V.H.; Saxena, V.K. Expression analysis of melatonin receptor subtypes in the ovary of domestic chicken. Vet. Res. Commun. 2009, 33, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Rosales-Corral, S.A. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update 2014, 20, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Cao, Y.; Jiang, Y.; Wei, Y.; Liu, H. Melatonin protects mouse granulosa cells against oxidative damage by inhibiting FOXO1-mediated autophagy: Implication of an antioxidation-independent mechanism. Redox Biol. 2018, 18, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, F.; He, C.; Zhang, L.; Tan, D.; Reiter, R.J.; Xu, J.; Ji, P.; Liu, G. Beneficial effects of melatonin on bovine oocytes maturation: A mechanistic approach. J. Pineal Res. 2014, 57, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Ghosal, I.; Das, D.; Chakraborty, S.B. Melatonin ameliorates HO-induced oxidative stress through modulation of Erk/Akt/NFkB pathway. Biol. Res. 2018, 51, 17. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Cui, M.; Lin, H.; Zhao, L.; Wang, J.; Chen, S.; Shao, Z. Melatonin resists oxidative stress-induced apoptosis in nucleus pulposus cells. Life Sci. 2018, 199, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, H.; Zhang, K.; Zhao, J.; Liu, H.; Ma, X.; Guo, J.; Wang, J.; Lu, W. Melatonin inhibits autophagy in TM3 cells via AKT/FOXO1 pathway. Mol. Biol. Rep. 2022, 49, 2925–2932. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y.; et al. The role of melatonin as an antioxidant in the follicle. J. Ovarian Res. 2012, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Paredes, S.D.; Manchester, L.C.; Tan, D.-X. Reducing oxidative/nitrosative stress: A newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 175–200. [Google Scholar] [CrossRef]
- Fernández, A.; Ordóñez, R.; Reiter, R.J.; González-Gallego, J.; Mauriz, J.L. Melatonin and endoplasmic reticulum stress: Relation to autophagy and apoptosis. J. Pineal Res. 2015, 59, 292–307. [Google Scholar] [CrossRef]
- Zhai, M.; Li, B.; Duan, W.; Jing, L.; Zhang, B.; Zhang, M.; Yu, L.; Liu, Z.; Yu, B.; Ren, K.; et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J. Pineal Res. 2017, 63, e12419. [Google Scholar] [CrossRef]
- Zhi, S.M.; Fang, G.X.; Xie, X.M.; Liu, L.H.; Yan, J.; Liu, D.B.; Yu, H.Y. Melatonin reduces OGD/R-induced neuron injury by regulating redox/inflammation/apoptosis signaling. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1524–1536. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, F.; Dou, Y.; Tian, X.; Liu, C.; Li, H.; Shen, H.; Chen, G. Melatonin Alleviates Intracerebral Hemorrhage-Induced Secondary Brain Injury in Rats via Suppressing Apoptosis, Inflammation, Oxidative Stress, DNA Damage, and Mitochondria Injury. Transl. Stroke Res. 2018, 9, 74–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; He, F.; Chen, Z.; Su, Q.; Yan, M.; Zhang, Q.; Tan, J.; Qian, L.; Han, Y. Melatonin modulates IL-1β-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation. Aging 2019, 11, 10499–10512. [Google Scholar] [CrossRef]
- Tamura, H.; Kawamoto, M.; Sato, S.; Tamura, I.; Maekawa, R.; Taketani, T.; Aasada, H.; Takaki, E.; Nakai, A.; Reiter, R.J.; et al. Long-term melatonin treatment delays ovarian aging. J. Pineal Res. 2017, 62, e12381. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, Y.M.; Song, K.-H.; Noh, H.; Lee, S.H. Melatonin suppresses senescence-derived mitochondrial dysfunction in mesenchymal stem cells via the HSPA1L-mitophagy pathway. Aging Cell 2020, 19, e13111. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef] [Green Version]
- D’Occhio, M.J.; Baruselli, P.S.; Campanile, G. Influence of nutrition, body condition, and metabolic status on reproduction in female beef cattle: A review. Theriogenology 2019, 125, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, F.; Sordillo, L.M.; Gandy, J.C.; Caroprese, M.; Sevi, A.; Albenzio, M.; Santillo, A. Evaluation of natural plant extracts as antioxidants in a bovine in vitro model of oxidative stress. J. Dairy Sci. 2020, 103, 8938–8947. [Google Scholar] [CrossRef]
- Dickson, M.J.; Piersanti, R.L.; Ramirez-Hernandez, R.; de Oliveira, E.B.; Bishop, J.V.; Hansen, T.R.; Ma, Z.; Jeong, K.C.C.; Santos, J.E.P.; Sheldon, M.I.; et al. Experimentally Induced Endometritis Impairs the Developmental Capacity of Bovine Oocytes. Biol. Reprod. 2020, 103, 508–520. [Google Scholar] [CrossRef]
- Fonseca Balvís, N.; Garcia-Martinez, S.; Pérez-Cerezales, S.; Ivanova, E.; Gomez-Redondo, I.; Hamdi, M.; Rizos, D.; Coy, P.; Kelsey, G.; Gutierrez-Adan, A. Cultured bovine embryo biopsy conserves methylation marks from original embryo. Biol. Reprod. 2017, 97, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Xu, F.; Bleyer, M.; Becker, S.; Melbaum, T.; Wemheuer, W.; Hirschfeld, M.; Wacker, C.; Zhao, S.; Schütz, E.; et al. Association of α/β-Hydrolase D16B with Bovine Conception Rate and Sperm Plasma Membrane Lipid Composition. Int. J. Mol. Sci. 2020, 21, 627. [Google Scholar] [CrossRef]
- Moore, S.G.; Hasler, J.F. A 100-Year Review: Reproductive technologies in dairy science. J. Dairy Sci. 2017, 100, 10314–10331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Liu, W.; Wen, A.; Yang, B.; Pang, X. Luzindole and 4P-PDOT block the effect of melatonin on bovine granulosa cell apoptosis and cell cycle depending on its concentration. PeerJ 2021, 9, e10627. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, L.; Chen, K.; Li, C.; Wang, Y.; Wang, G. Melatonin alleviates β-zearalenol and HT-2 toxin-induced apoptosis and oxidative stress in bovine ovarian granulosa cells. Environ. Toxicol. Pharmacol. 2019, 68, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zheng, Y.; Tang, X.; Gao, H.; Liu, N.; Gao, Y.; Hao, L.; Liu, S.; Jiang, Z. miR-21-3p inhibits autophagy of bovine granulosa cells by targeting VEGFA via PI3K/AKT signaling. Reproduction 2019, 158, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, W.; Pang, X.; Dai, S.; Liu, G. The Mechanism of Melatonin and Its Receptor MT2 Involved in the Development of Bovine Granulosa Cells. Int. J. Mol. Sci. 2018, 19, 2028. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, M.; Manabe, N.; Nishihara, S.; Miyamoto, H. Species-specific Differences in Apoptotic Cell Localization in Granulosa and Theca Interna Cells during Follicular Atresia in Porcine and Bovine Ovaries. J. Reprod. Dev. 2000, 46, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.-H.; Yang, C.-R.; Wang, X.-N.; Zhang, L.-L.; Gao, X.-R.; Shi, Z.-Y. Progesterone maintains the status of granulosa cells and slows follicle development partly through PGRMC1. J. Cell. Physiol. 2018, 234, 709–720. [Google Scholar] [CrossRef] [Green Version]
- Tamura, H.; Jozaki, M.; Tanabe, M.; Shirafuta, Y.; Mihara, Y.; Shinagawa, M.; Tamura, I.; Maekawa, R.; Sato, S.; Taketani, T.; et al. Importance of Melatonin in Assisted Reproductive Technology and Ovarian Aging. Int. J. Mol. Sci. 2020, 21, 1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeebaree, B.K.; Kwong, W.Y.; Mann, G.E.; Gutierrez, C.G.; Sinclair, K.D. Physiological responses of cultured bovine granulosa cells to elevated temperatures under low and high oxygen in the presence of different concentrations of melatonin. Theriogenology 2018, 105, 107–114. [Google Scholar] [CrossRef]
- Insogna, I.G.; Lanes, A.; Lee, M.S.; Ginsburg, E.S.; Fox, J.H. Association of Fresh Embryo Transfers Compared With Cryopreserved-Thawed Embryo Transfers With Live Birth Rate Among Women Undergoing Assisted Reproduction Using Freshly Retrieved Donor Oocytes. JAMA 2021, 325, 156–163. [Google Scholar] [CrossRef]
- Chen, S.U.; Lien, Y.R.; Chao, K.H.; Ho, H.N.; Yang, Y.S.; Lee, T.Y. Effects of cryopreservation on meiotic spindles of oocytes and its dynamics after thawing: Clinical implications in oocyte freezing--a review article. Mol. Cell. Endocrinol. 2003, 202, 101–107. [Google Scholar] [CrossRef]
- Sananmuang, T.; Puthier, D.; Nguyen, C.; Chokeshaiusaha, K. Novel classifier orthologs of bovine and human oocytes matured in different melatonin environments. Theriogenology 2020, 156, 82–89. [Google Scholar] [CrossRef]
- Gutiérrez-Añez, J.C.; Lucas-Hahn, A.; Hadeler, K.-G.; Aldag, P.; Niemann, H. Melatonin enhances in vitro developmental competence of cumulus-oocyte complexes collected by ovum pick-up in prepubertal and adult dairy cattle. Theriogenology 2021, 161, 285–293. [Google Scholar] [CrossRef]
- Yaacobi-Artzi, S.; Shimoni, C.; Kalo, D.; Hansen, P.J.; Roth, Z. Melatonin slightly alleviates the effect of heat shock on bovine oocytes and resulting blastocysts. Theriogenology 2020, 158, 477–489. [Google Scholar] [CrossRef]
- Pang, Y.-W.; Jiang, X.-L.; Wang, Y.-C.; Wang, Y.-Y.; Hao, H.-S.; Zhao, S.-J.; Du, W.-H.; Zhao, X.-M.; Wang, L.; Zhu, H.-B. Melatonin protects against paraquat-induced damage during in vitro maturation of bovine oocytes. J. Pineal Res. 2019, 66, e12532. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Han, J.; Pan, M.-H.; Wan, X.; Pan, Z.-N.; Sun, S.-C. Melatonin protects against defects induced by deoxynivalenol during mouse oocyte maturation. J. Pineal Res. 2018, 65, e12477. [Google Scholar] [CrossRef] [PubMed]
- El-Raey, M.; Geshi, M.; Somfai, T.; Kaneda, M.; Hirako, M.; Abdel-Ghaffar, A.E.; Sosa, G.A.; El-Roos, M.E.A.A.; Nagai, T. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol. Reprod. Dev. 2011, 78, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Brockus, K.E.; Hart, C.G.; Gilfeather, C.L.; Fleming, B.O.; Lemley, C.O. Dietary melatonin alters uterine artery hemodynamics in pregnant Holstein heifers. Domest. Anim. Endocrinol. 2016, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lemley, C.O.; Vonnahme, K.A. Physiology and endocrinology symposium: Alterations in uteroplacental hemodynamics during melatonin supplementation in sheep and cattle. J. Anim. Sci. 2017, 95, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, M.; Gao, X.; Yang, Y.; Zhao, J.; Wang, J.; Lu, W. Two melatonin treatments improve the conception rate after fixed-time artificial insemination in beef heifers following synchronisation of oestrous cycles using the CoSynch-56 protocol. Aust. Vet. J. 2021, 99, 449–455. [Google Scholar] [CrossRef]
- Zhang, C.; Clough, S.J.; Adamah-Biassi, E.B.; Sveinsson, M.H.; Hutchinson, A.J.; Miura, I.; Furuse, T.; Wakana, S.; Matsumoto, Y.K.; Okanoya, K.; et al. Impact of endogenous melatonin on rhythmic behaviors, reproduction, and survival revealed in melatonin-proficient C57BL/6J congenic mice. J. Pineal Res. 2021, 71, e12748. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Hirai, Y.; Murayama, C.; Miyamoto, A.; Miyazaki, H.; Miyazaki, K. Circadian Clock genes Per2 and clock regulate steroid production, cell proliferation, and luteinizing hormone receptor transcription in ovarian granulosa cells. Biochem. Biophys. Res. Commun. 2011, 412, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Ginther, O.J.; Pinaffi, F.L.V.; Khan, F.A.; Duarte, L.F.; Beg, M.A. Circadian influence on the preovulatory LH surge, ovulation, and prolactin concentrations in heifers. Theriogenology 2013, 79, 528–533. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Peng, J.; Yang, S.; Tong, D. Effects of melatonin on the production of GnRH and LH in luteal cells of pregnant sows. J. Mol. Endocrinol. 2022, 68, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.A.; Herlihy, M.M.; Nolan, M.B.; O’Brien, C.; Furlong, J.G.; Butler, S.T. Identification of the blue light intensity administered to one eye required to suppress bovine plasma melatonin and investigation into effects on milk production in grazing dairy cows. J. Dairy Sci. 2021, 104, 12127–12138. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.R.; Chapin, L.T.; Leining, K.B.; Tucker, H.A. Supplemental lighting stimulates growth and lactation in cattle. Science 1978, 199, 911–912. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.R.; Chapin, L.T.; Emery, R.S.; Tucker, H.A. Milk yield, feed intake, prolactin, growth hormone, and glucocorticoid response of cows to supplemented light. J. Dairy Sci. 1981, 64, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Barcelo, E.J.; Mediavilla, M.D.; Zinn, S.A.; Buchanan, B.A.; Chapin, L.T.; Tucker, H.A. Melatonin suppression of mammary growth in heifers. Biol. Reprod. 1991, 44, 875–879. [Google Scholar] [CrossRef]
- Tucker, H.A.; Ringer, R.K. Controlled photoperiodic environments for food animals. Science 1982, 216, 1381–1386. [Google Scholar] [CrossRef]
- Wyse, C.A.; Zhang, X.; McLaughlin, M.; Biello, S.M.; Hough, D.; Bellingham, M.; Curtis, A.M.; Robinson, J.E.; Evans, N.P. Circadian rhythms of melatonin and behaviour in juvenile sheep in field conditions: Effects of photoperiod, environment and weaning. Physiol. Behav. 2018, 194, 362–370. [Google Scholar] [CrossRef]
- O’Brien, C.; Darcy-Dunne, M.R.; Murphy, B.A. The effects of extended photoperiod and warmth on hair growth in ponies and horses at different times of year. PLoS ONE 2020, 15, e0227115. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.; Valekunja, U.K.; Stangherlin, A.; Howell, S.A.; Snijders, A.P.; Damodaran, G.; Reddy, A.B. Circadian rhythms in the absence of the clock gene. Science 2020, 367, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, M.; Xu, J.; Liu, X.; Duan, Y.; Zhou, C.; Xu, Y. Core clock gene Bmal1 deprivation impairs steroidogenesis in mice luteinized follicle cells. Reproduction 2020, 160, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lara, D.L.; González-Enríquez, G.V.; Torres-Mendoza, B.M.; González-Usigli, H.; Cárdenas-Bedoya, J.; Macías-Islas, M.A.; de la Rosa, A.C.; Jiménez-Delgado, A.; Pacheco-Moisés, F.; Cruz-Serrano, J.A.; et al. Effect of melatonin administration on the PER1 and BMAL1 clock genes in patients with Parkinson’s disease. Biomed. Pharmacother. 2020, 129, 110485. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Q.; Gao, W.; Li, B.-Y.; Zeng, C.; Xia, Z.; Zhao, B. Melatonin ameliorates cerebral ischemia-reperfusion injury in diabetic mice by enhancing autophagy via the SIRT1-BMAL1 pathway. FASEB J. 2021, 35, e22040. [Google Scholar] [CrossRef]
- Arellanes-Licea, E.D.C.; Pérez-Mendoza, M.; Carmona-Castro, A.; Díaz-Muñoz, M.; Miranda-Anaya, M. Obese mice exhibit sexual dimorphism in the daily profile of circulating melatonin and clock proteins PER1 and BMAL1 in the hypothalamus and peripheral oscillators. Chronobiol. Int. 2021, 38, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. BMAL1 but not CLOCK is associated with monochromatic green light-induced circadian rhythm of melatonin in chick pinealocytes. Endocr. Connect. 2019, 8, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Bian, J.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Role of BMAL1 and CLOCK in regulating the secretion of melatonin in chick retina under monochromatic green light. Chronobiol. Int. 2020, 37, 1677–1692. [Google Scholar] [CrossRef]
- Ding, H.; Zhao, J.; Liu, H.; Wang, J.; Lu, W. BMAL1 knockdown promoted apoptosis and reduced testosterone secretion in TM3 Leydig cell line. Gene 2020, 747, 144672. [Google Scholar] [CrossRef]
- Boden, M.J.; Kennaway, D.J. 297. Reproduction in the arrhythmic Bmal1 knockout mouse. Reprod. Fertil. Dev. 2005, 17, 126. [Google Scholar] [CrossRef]
- Isayama, K.; Chen, H.; Yamauchi, N.; Hattori, M.-A. REV-ERBα inhibits the PTGS2 expression in bovine uterus endometrium stromal and epithelial cells exposed to ovarian steroids. J. Reprod. Dev. 2014, 60, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Amano, T.; Tokunaga, K.; Kakegawa, R.; Yanagisawa, A.; Takemoto, A.; Tatemizo, A.; Watanabe, T.; Hatanaka, Y.; Matsushita, A.; Kishi, M.; et al. Expression analysis of circadian genes in oocytes and preimplantation embryos of cattle and rabbits. Anim. Reprod. Sci. 2010, 121, 225–235. [Google Scholar] [CrossRef]
- Nebzydoski, S.J.; Pozzo, S.; Nemec, L.; Rankin, M.K.; Gressley, T.F. The effect of dexamethasone on clock gene mRNA levels in bovine neutrophils and lymphocytes. Vet. Immunol. Immunopathol. 2010, 138, 183–192. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and inflammation-Story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Lin, J.; Tian, N.; Wu, Y.; Zhou, Y.; Wang, C.; Wang, Q.; Jin, H.; Chen, T.; Nisar, M.; et al. Melatonin protects vertebral endplate chondrocytes against apoptosis and calcification via the Sirt1-autophagy pathway. J. Cell. Mol. Med. 2019, 23, 177–193. [Google Scholar] [CrossRef]
- Zhang, W.-X.; He, B.-M.; Wu, Y.; Qiao, J.-F.; Peng, Z.-Y. Melatonin protects against sepsis-induced cardiac dysfunction by regulating apoptosis and autophagy via activation of SIRT1 in mice. Life Sci. 2019, 21, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin, noncoding RNAs, messenger RNA stability and epigenetics--evidence, hints, gaps and perspectives. Int. J. Mol. Sci. 2014, 15, 18221–18252. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J. Pineal Res. 2017, 62, e12377. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Zhang, T.; Ren, Q.; Sun, C. Melatonin alleviates adipose inflammation through elevating α-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J. Pineal Res. 2018, 64, e12455. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Qin, X.; Feng, R.; Li, Q.; Huang, F.; Li, Y.; Zhao, Q.; Huang, H. Alleviative effect of melatonin on the decrease of uterine receptivity caused by blood ammonia through ROS/NF-κB pathway in dairy cow. Ecotoxicol. Environ. Saf. 2022, 231, 113166. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Wu, H.; Ma, H.; Fu, Y.; Wei, W.; Wang, T.; Guan, S.; Yang, H.; Li, X.; Guo, J.; et al. Effects of rumen bypass melatonin feeding (RBMF) on milk quality and mastitis of Holstein cows. PeerJ 2020, 8, e9147. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, K.; Guo, J.; Zhou, J.; Loor, J.J.; Yang, Z.; Yang, Y. Melatonin Maintains Homeostasis and Potentiates the Anti-inflammatory Response in Staphylococcus aureus-Induced Mastitis through microRNA-16b/YAP1. J. Agric. Food Chem. 2022, 70, 15255–15270. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Yang, G.-H.; Zhang, L.-L.; Wang, J.; Wang, J.-F. Melatonin as Immune Potentiator for Enhancing Subunit Vaccine Efficacy against Bovine Viral Diarrhea Virus. Vaccines 2021, 9, 1039. [Google Scholar] [CrossRef]
- Regodón, S.; Ramos, A.; Morgado, S.; Tarazona, R.; Martín-Palomino, P.; Rosado, J.A.; Míguez, M.D.P. Melatonin enhances the immune response to vaccination against A1 and C strains of Dichelobacter nodosus. Vaccine 2009, 27, 1566–1570. [Google Scholar] [CrossRef]
- Ofosu, J.; Qazi, I.H.; Fang, Y.; Zhou, G. Use of melatonin in sperm cryopreservation of farm animals: A brief review. Anim. Reprod. Sci. 2021, 233, 106850. [Google Scholar] [CrossRef]
- Medrano, A.; Contreras, C.; Herrera, F.; Alcantar-Rodriguez, A. Melatonin as an antioxidant preserving sperm from domestic animals. Asian Pac. J. Reprod. 2017, 6, 241–246. [Google Scholar] [CrossRef]
- Appiah, M.O.; He, B.; Lu, W.; Wang, J. Antioxidative effect of melatonin on cryopreserved chicken semen. Cryobiology 2019, 89, 90–95. [Google Scholar] [CrossRef]
- Shahat, A.M.; Thundathil, J.C.; Kastelic, J.P. Melatonin or L-arginine in semen extender mitigate reductions in quality of frozen-thawed sperm from heat-stressed rams. Anim. Reprod. Sci. 2022, 238, 106934. [Google Scholar] [CrossRef] [PubMed]
- Inyawilert, W.; Rungruangsak, J.; Liao, Y.-J.; Tang, P.-C.; Paungsukpaibool, V. Melatonin supplementation improved cryopreserved Thai swamp buffalo semen. Reprod. Domest. Anim. 2021, 56, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Shahat, A.M.; Thundathil, J.C.; Kastelic, J.P. Melatonin improves post-thaw sperm quality after mild testicular heat stress in rams. Reprod. Domest. Anim. 2023, 58, 423–430. [Google Scholar] [CrossRef]
- Ramadan, T.A.; Kumar, D.; Ghuman, S.S.; Singh, I. Melatonin-improved buffalo semen quality during nonbreeding season under tropical condition. Domest. Anim. Endocrinol. 2019, 68, 119–125. [Google Scholar] [CrossRef]
- Su, G.; Wu, S.; Wu, M.; Wang, L.; Yang, L.; Du, M.; Zhao, X.; Su, X.; Liu, X.; Bai, C.; et al. Melatonin improves the quality of frozen bull semen and influences gene expression related to embryo genome activation. Theriogenology 2021, 176, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Frungieri, M.B.; Calandra, R.S.; Rossi, S.P. Local Actions of Melatonin in Somatic Cells of the Testis. Int. J. Mol. Sci. 2017, 18, 1170. [Google Scholar] [CrossRef] [Green Version]
- Shahat, A.M.; Thundathil, J.C.; Kastelic, J.P. Melatonin improves testicular hemodynamics and sperm quality in rams subjected to mild testicular heat stress. Theriogenology 2022, 188, 163–169. [Google Scholar] [CrossRef]
- Batmanabane, M.; Ramesh, K.G. Effect of exogenous melatonin on the onset of puberty in female albino rats. Anat. Rec. 1996, 245, 519–524. [Google Scholar] [CrossRef]
- Li, C.; Zhou, X. Melatonin and male reproduction. Clin. Chim. Acta 2015, 446, 175–180. [Google Scholar] [CrossRef]
- Vanecek, J. Melatonin inhibits release of luteinizing hormone (LH) via decrease of [Ca2+]i and cyclic AMP. Physiol. Res. 1998, 47, 329–335. [Google Scholar] [PubMed]
- Wu, C.S.; Leu, S.F.; Yang, H.Y.; Huang, B.M. Melatonin inhibits the expression of steroidogenic acute regulatory protein and steroidogenesis in MA-10 cells. J. Androl. 2001, 22, 245–254. [Google Scholar] [PubMed]
- Xu, K.; Wang, J.; Liu, H.; Zhao, J.; Lu, W. Melatonin Promotes the Proliferation of Chicken Sertoli Cells by Activating the ERK/Inhibin Alpha Subunit Signaling Pathway. Molecules 2020, 25, 1230. [Google Scholar] [CrossRef] [Green Version]
- El-Shalofy, A.; Hedia, M.; Kastelic, J. Melatonin improves testicular haemodynamics, echotexture and testosterone production in Ossimi rams during the breeding season. Reprod. Domest. Anim. 2021, 56, 1456–1463. [Google Scholar] [CrossRef]
- Heidarizadi, S.; Rashidi, Z.; Jalili, C.; Gholami, M. Overview of biological effects of melatonin on testis: A review. Andrologia 2022, 54, e14597. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, C.-Q.; Zeng, L.; Cheng, L.; Ma, L.; Zhang, M.; Zhang, Y.-Z. Melatonin regulates the cross-talk between autophagy and apoptosis by SIRT3 in testicular Leydig cells. Biochem. Biophys. Res. Commun. 2021, 555, 182–189. [Google Scholar] [CrossRef]
- Qingyu, Z. Melatonin inhibits testosterone synthesis in Roosters Leydig cells by regulating lipolysis of lipid droplets. Theriogenology 2022, 189, 118–126. [Google Scholar]
- Yang, W.-C.; Tang, K.-Q.; Fu, C.-Z.; Riaz, H.; Zhang, Q.; Zan, L.-S. Melatonin regulates the development and function of bovine Sertoli cells via its receptors MT1 and MT2. Anim. Reprod. Sci. 2014, 147, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Luboshitzky, R.; Shen-Orr, Z.; Nave, R.; Lavi, S.; Lavie, P. Melatonin administration alters semen quality in healthy men. J. Androl. 2002, 23, 572–578. [Google Scholar] [PubMed]
- Koksal, M.; Oğuz, E.; Baba, F.; Eren, M.A.; Ciftci, H.; Demir, M.E.; Kurcer, Z.; Take, G.; Aral, F.; Ocak, A.R.; et al. Effects of melatonin on testis histology, oxidative stress and spermatogenesis after experimental testis ischemia-reperfusion in rats. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 582–588. [Google Scholar] [PubMed]
- Du Plessis, S.S.; Hagenaar, K.; Lampiao, F. The in vitro effects of melatonin on human sperm function and its scavenging activities on NO and ROS. Andrologia 2010, 42, 112–116. [Google Scholar] [CrossRef]
- Casey, T.M.; Plaut, K. Lactation Biology Symposium: Circadian clocks as mediators of the homeorhetic response to lactation. J. Anim. Sci. 2012, 90, 744–754. [Google Scholar] [CrossRef]
- Turek, F.W.; McMillan, J.P.; Menaker, M. Melatonin: Effects on the circadian locomotor rhythm of sparrows. Science 1976, 194, 1441–1443. [Google Scholar] [CrossRef]
- Poeggeler, B.; Reiter, R.J.; Tan, D.X.; Chen, L.D.; Manchester, L.C. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: A hypothesis. J. Pineal Res. 1993, 14, 151–168. [Google Scholar] [CrossRef]
- Harasimowicz, J.; Marques, K.L.; Silva, A.F.T.; Costa, R.C.B.; Prior, J.A.V.; Rodrigues, S.S.M.; Santos, J.L.M. Chemiluminometric evaluation of melatonin and selected melatonin precursors’ interaction with reactive oxygen and nitrogen species. Anal. Biochem. 2012, 420, 1–6. [Google Scholar] [CrossRef]
- Gilad, E.; Cuzzocrea, S.; Zingarelli, B.; Salzman, A.L.; Szabó, C. Melatonin is a scavenger of peroxynitrite. Life Sci. 1997, 60, PL169–PL174. [Google Scholar] [CrossRef]
- Limson, J.; Nyokong, T.; Daya, S. The interaction of melatonin and its precursors with aluminium, cadmium, copper, iron, lead, and zinc: An adsorptive voltammetric study. J. Pineal Res. 1998, 24, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.; Limson, J.; Nyokong, T.; Daya, S. Melatonin protects against copper-mediated free radical damage. J. Pineal Res. 2002, 32, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bratic, A.; Larsson, N.-G. The role of mitochondria in aging. J. Clin. Invest. 2013, 123, 951–957. [Google Scholar] [CrossRef] [Green Version]
- Poljsak, B. Strategies for reducing or preventing the generation of oxidative stress. Oxid. Med. Cell. Longev. 2011, 2011, 194586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef] [Green Version]
- Gombert, M.; Codoñer-Franch, P. Melatonin in Early Nutrition: Long-Term Effects on Cardiovascular System. Int. J. Mol. Sci. 2021, 22, 6809. [Google Scholar] [CrossRef]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Mirhosseini, N.; Reiter, R.J.; Azami, A.; Asemi, Z. Melatonin, a calpain inhibitor in the central nervous system: Current status and future perspectives. J. Cell. Physiol. 2019, 234, 1001–1007. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, J.; Reiter, R.J.; Ma, X. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: A therapeutic target to reduce intestinal inflammation. Med. Res. Rev. 2020, 40, 606–632. [Google Scholar] [CrossRef]
- Gilat, M.; Coeytaux Jackson, A.; Marshall, N.S.; Hammond, D.; Mullins, A.E.; Hall, J.M.; Fang, B.A.M.; Yee, B.J.; Wong, K.K.H.; Grunstein, R.R.; et al. Melatonin for rapid eye movement sleep behavior disorder in Parkinson’s disease: A randomised controlled trial. Mov. Disord. 2020, 35, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Yang, C.; Wang, J.; Huang, X.; Yu, H.; Li, S.; Li, S.; Zhang, Z.; Liu, J.; Yang, X.; et al. Melatonin ameliorates cognitive deficits through improving mitophagy in a mouse model of Alzheimer’s disease. J. Pineal Res. 2021, 71, e12774. [Google Scholar] [CrossRef]
- Ali, T.; Rahman, S.U.; Hao, Q.; Li, W.; Liu, Z.; Ali Shah, F.; Murtaza, I.; Zhang, Z.; Yang, X.; Liu, G.; et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J. Pineal Res. 2020, 69, e12667. [Google Scholar] [CrossRef]
- Ramos, E.; Patiño, P.; Reiter, R.J.; Gil-Martín, E.; Marco-Contelles, J.; Parada, E.; de Los Rios, C.; Romero, A.; Egea, J. Ischemic brain injury: New insights on the protective role of melatonin. Free Radic. Biol. Med. 2017, 104, 32–53. [Google Scholar] [CrossRef]
- Paulino, L.R.F.M.; Barroso, P.A.A.; Silva, B.R.; Barroso, L.G.; Barbalho, E.C.; Bezerra, F.T.G.; Souza, A.L.P.; Monte, A.P.O.; Silva, A.W.B.; Matos, M.H.T.; et al. Immunolocalization of melatonin receptors in bovine ovarian follicles and in vitro effects of melatonin on growth, viability and gene expression in secondary follicles. Domest. Anim. Endocrinol. 2022, 81, 106750. [Google Scholar] [CrossRef] [PubMed]
- Messman, R.D.; Contreras-Correa, Z.E.; Paz, H.A.; Lemley, C.O. Melatonin-induced changes in the bovine vaginal microbiota during maternal nutrient restriction. J. Anim. Sci. 2021, 99, skab098. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Schutz, L.F.; Morrell, B.C.; Perego, M.C.; Spicer, L.J. Effect of melatonin on bovine theca cells in vitro. Reprod. Fertil. Dev. 2018, 30, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-X.; Lee, S.; Taweechaipaisankul, A.; Kim, G.A.; Lee, B.C. Melatonin regulates lipid metabolism in porcine oocytes. J. Pineal Res. 2017, 62, e12388. [Google Scholar] [CrossRef]
- Niu, Y.-J.; Zhou, W.; Nie, Z.-W.; Shin, K.-T.; Cui, X.-S. Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos. J. Pineal Res. 2020, 68, e12627. [Google Scholar] [CrossRef]
- Martinez, C.A.; Cuello, C.; Parrilla, I.; Maside, C.; Ramis, G.; Cambra, J.M.; Vazquez, J.M.; Rodriguez-Martinez, H.; Gil, M.A.; Martinez, E.A. Exogenous Melatonin in the Culture Medium Does Not Affect the Development of In Vivo-Derived Pig Embryos but Substantially Improves the Quality of In Vitro-Produced Embryos. Antioxidants 2022, 11, 1177. [Google Scholar] [CrossRef] [PubMed]
- Pezo, F.; Zambrano, F.; Uribe, P.; Moya, C.; de Andrade, A.F.C.; Risopatron, J.; Yeste, M.; Burgos, R.A.; Sánchez, R. Oxidative and nitrosative stress in frozen-thawed pig spermatozoa. I: Protective effect of melatonin and butylhydroxytoluene on sperm function. Res. Vet. Sci. 2021, 136, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Jiang, X.; Zhang, C.; Li, B.; Tu, W.; Lei, H.; Yao, W.; Xia, D. Melatonin mediates via melatonin receptor 1 in a temperature-dependent manner regulating ATP metabolism and antioxidative enzyme activity of boar spermatozoa in vitro. Theriogenology 2022, 188, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.-B.; Xiao, L.-F.; Duan, H.-W.; Li, Z.-S.; Jiang, Y.-T.; Yang, S.-S.; Hu, J.-J.; Zhang, Y.; Zhao, X.-X. Melatonin protects against lipopolysaccharide-induced epididymitis in sheep epididymal epithelial cells in vitro. Immunol. Lett. 2019, 214, 45–51. [Google Scholar] [CrossRef]
- Barberino, R.S.; Macedo, T.J.S.; Lins, T.L.B.G.; Menezes, V.G.; Silva, R.L.S.; Monte, A.P.O.; Palheta, R.C.; Smitz, J.E.J.; Matos, M.H.T. Immunolocalization of melatonin receptor type 1 in the sheep ovary and involvement of the PI3K/Akt/FOXO3a signaling pathway in the effects of melatonin on survival and in vitro activation of primordial follicles. Mol. Reprod. Dev. 2022, 89, 485–497. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, A.; Li, G.; Wu, H.; Deng, S.; Yang, H.; Ma, W.; Lv, D.; Fu, Y.; Ji, P.; et al. Melatonin promotes the development of sheep transgenic cloned embryos by protecting donor and recipient cells. Cell Cycle 2022, 21, 1360–1375. [Google Scholar] [CrossRef]
- Ma, J.; Yang, H.; Liu, L.; Wan, Y.; Wang, F. Melatonin alleviated oxidative stress induced by energy restriction on sheep Leydig cells through Sirt1/Sod2 pathway. Theriogenology 2021, 173, 83–92. [Google Scholar] [CrossRef]
- Trotta, R.J.; Lemley, C.O.; Vonnahme, K.A.; Swanson, K.C. Effects of nutrient restriction and melatonin supplementation from mid-to-late gestation on maternal and fetal small intestinal carbohydrase activities in sheep. Domest. Anim. Endocrinol. 2021, 74, 106555. [Google Scholar] [CrossRef]
- Fang, Y.; Zhao, C.; Xiang, H.; Jia, G.; Zhong, R. Melatonin improves cryopreservation of ram sperm by inhibiting mitochondrial permeability transition pore opening. Reprod. Domest. Anim. 2020, 55, 1240–1249. [Google Scholar] [CrossRef]
- Succu, S.; Berlinguer, F.; Pasciu, V.; Satta, V.; Leoni, G.G.; Naitana, S. Melatonin protects ram spermatozoa from cryopreservation injuries in a dose-dependent manner. J. Pineal Res. 2011, 50, 310–318. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhang, K.; Zhou, Y.; Zhao, J.; Wang, J.; Lu, W. Role of Melatonin in Bovine Reproductive Biotechnology. Molecules 2023, 28, 4940. https://doi.org/10.3390/molecules28134940
Li Z, Zhang K, Zhou Y, Zhao J, Wang J, Lu W. Role of Melatonin in Bovine Reproductive Biotechnology. Molecules. 2023; 28(13):4940. https://doi.org/10.3390/molecules28134940
Chicago/Turabian StyleLi, Zhiqiang, Kaiyan Zhang, Yuming Zhou, Jing Zhao, Jun Wang, and Wenfa Lu. 2023. "Role of Melatonin in Bovine Reproductive Biotechnology" Molecules 28, no. 13: 4940. https://doi.org/10.3390/molecules28134940




