Direct Oxidation of Hibiscus cannabinus Stalks to Vanillin Using CeO2 Nanostructure Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalyst
2.2. Extraction of Lignin
2.3. Catalyst Screening
2.4. Catalyst Testing
2.5. Optimization of Reaction Conditions for Direct Oxidation of Kenaf Stalks to Vanillin
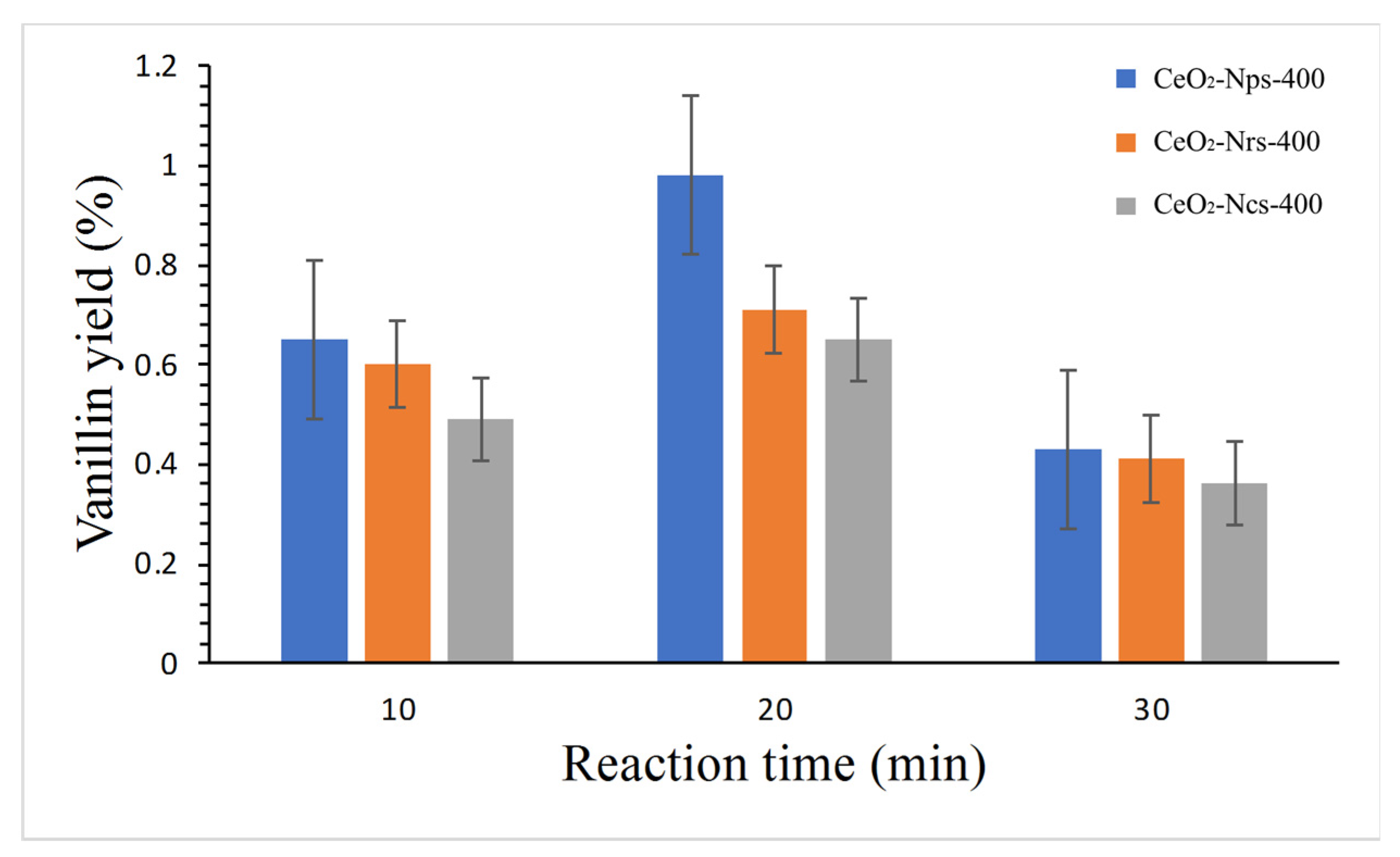
2.5.1. Effect of Reaction Duration
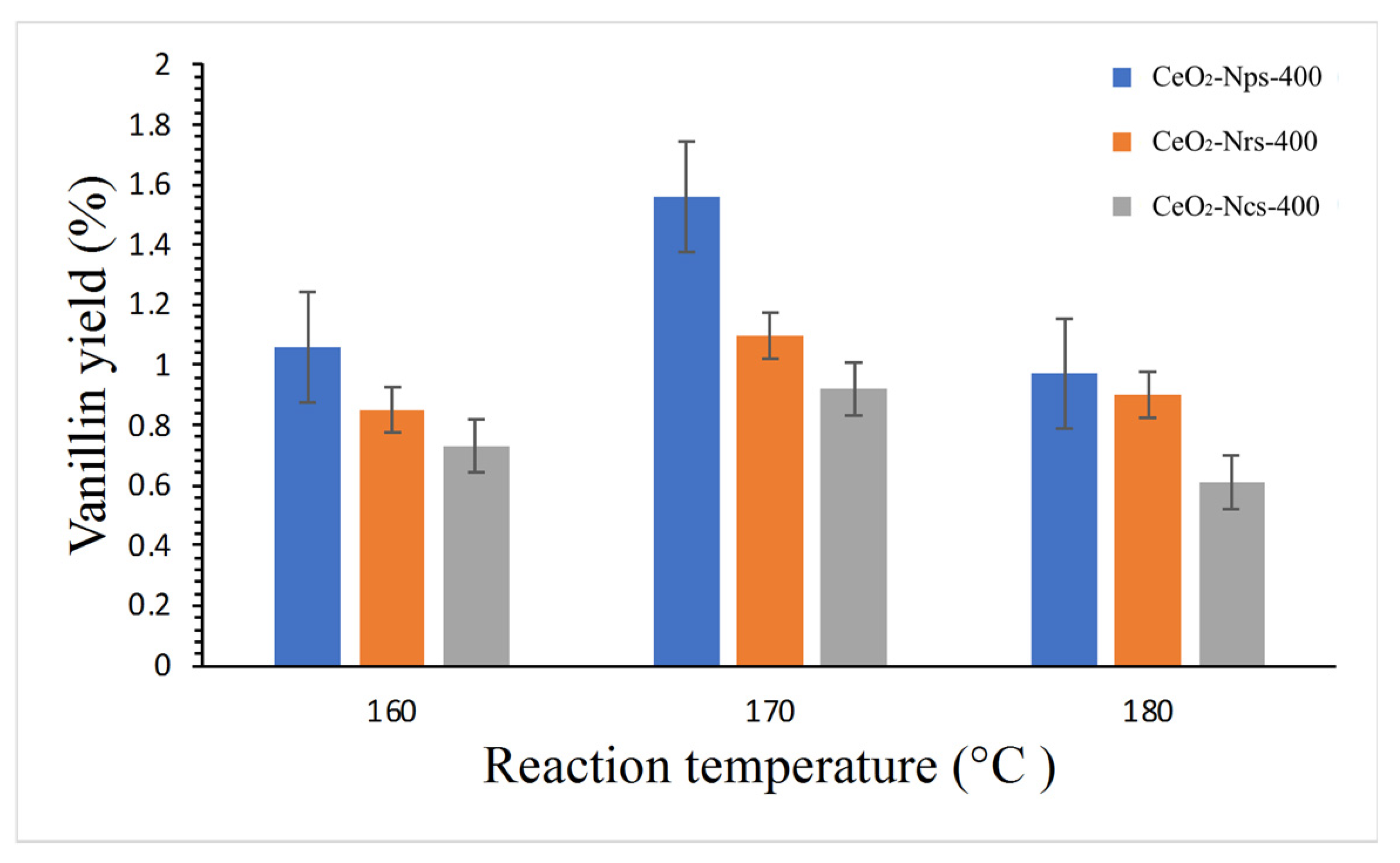
2.5.2. Effect of Reaction Temperature
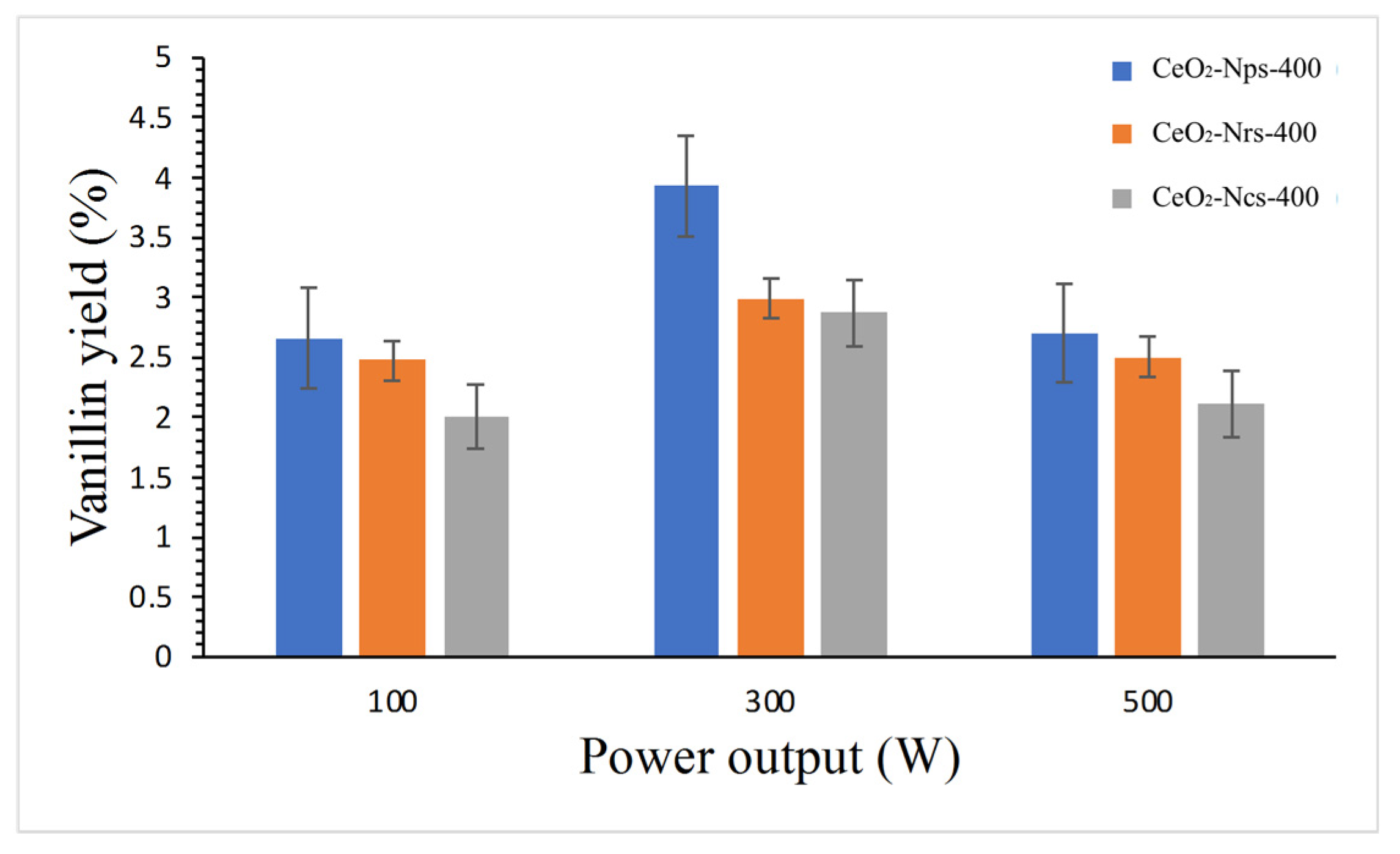
2.5.3. Effect of Power Output
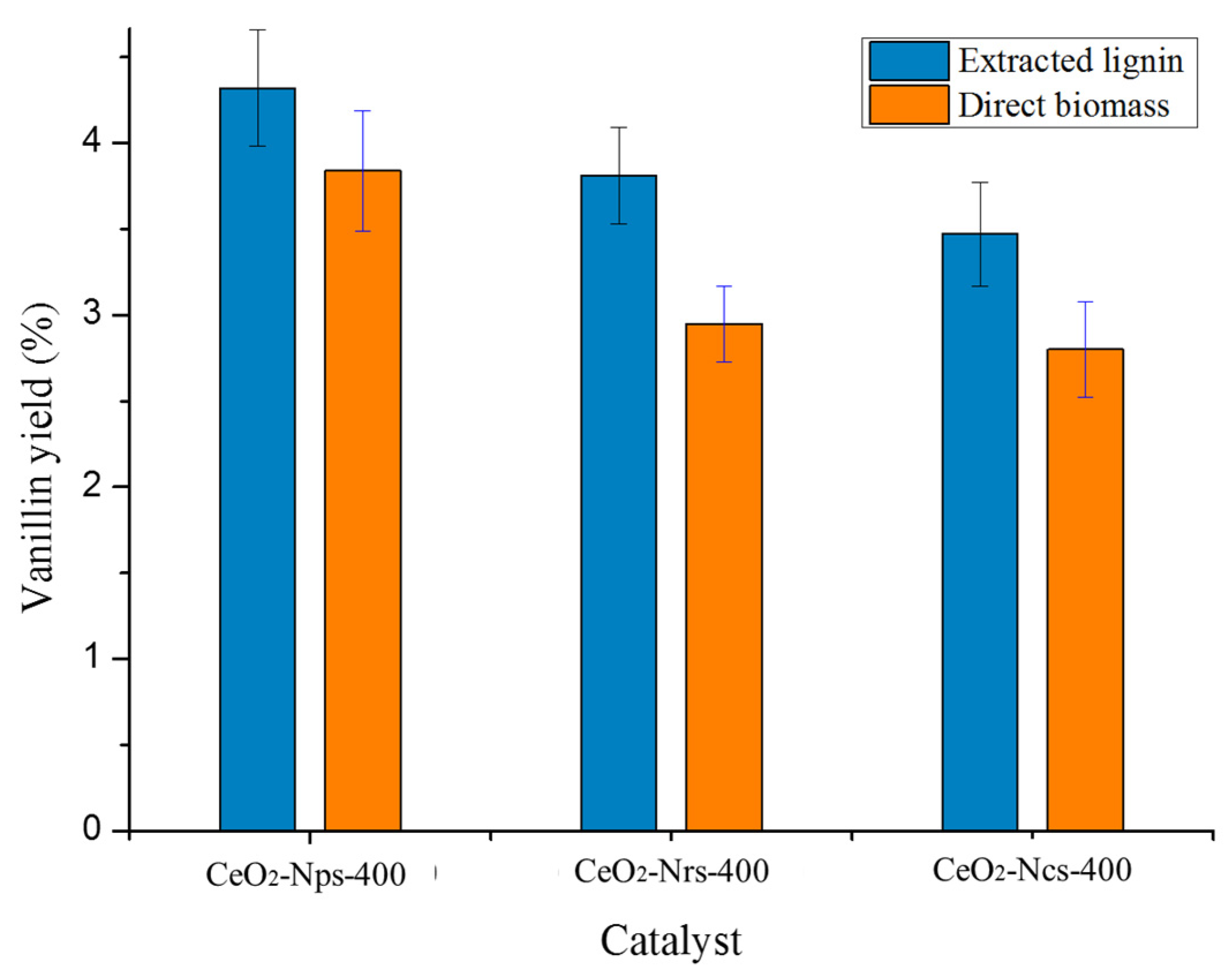
2.5.4. Comparison of Direct Oxidation of Kenaf with Extraction of Lignin
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Extraction of Lignin and Characterization
3.4. Catalytic Oxidation of Raw Kenaf
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bajwa, D.; Pourhashem, G.; Ullah, A.; Bajwa, S. A Concise Review of Current Lignin Production, Applications, Products and Their Environmental Impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Venus, J.; Dufossé, L. Editorial: Recent Advances in Microbial Biotechnology for the Food Industry. Front. Microbiol. 2021, 12, 746636. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra Rao, S.; Ravishankar, G. Vanilla flavour: Production by Conventional and Biotechnological Routes. J. Sci. Food Agric. 2010, 80, 289–304. [Google Scholar] [CrossRef]
- Gu, F.; Xu, F.; Tan, L.; Wu, H.; Chu, Z.; Wang, Q. Optimization of Enzymatic Process for Vanillin Extraction Using Response Surface Methodology. Molecules 2012, 17, 8753–8761. [Google Scholar] [CrossRef]
- Al-Naqeb, G.; Ismail, M.; Bagalkotkar, G.; Adamu, H.A. Vanillin Rich Fraction Regulates LDLR and HMGCR Gene Expression in HepG2 Cells. Food Res. Int. 2010, 43, 2437–2443. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin Production from Lignin and Its Use as a Renewable Chemical. ACS Sustain. Chem. Eng. 2015, 4, 35–46. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, W.; Ren, S.; Liu, F.; Zhao, C.; Liao, H.; Xu, Z.; Huang, J.; Li, Q.; Tu, Y.; et al. Hemicelluloses Negatively Affect Lignocellulose Crystallinity for High Biomass Digestibility under NaOH and H2SO4 Pretreatments in Miscanthus. Biotechnol. Biofuels 2012, 5, 58. [Google Scholar] [CrossRef]
- Sangnak, S.; Neramittagapong, A.; Neramittagapong, S.; Theerakulpisut, S.; Sriprom, P. Optimization of Vanillin Production from Lignin Using Catalytic Depolymerization over a CuO/Al2O3 Catalyst. Appl. Environ. Res. 2023, 45, 1. [Google Scholar]
- Singh, K.; Mehra, S.; Kumar, A. Metal-based Ionic Liquids: Effective Catalysts in Aqueous Media for the Selective Production of Vanillin from Alkali Lignin at Room Temperature. Green Chem. 2022, 24, 9629–9642. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiong, W.; Jin, Y.; Wang, P.; Wei, W.; Ma, J.; Zhang, X. Catalytic Aerobic Oxidation of Lignin-based Vanillyl Alcohol under Base–free Conditions over an Efficient and Reusable LaFeO3 Perovskite for Vanillin Production. Green Chem. 2023, 25, 1179–1190. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.; Liu, Y.; Ruan, X.; Tsang, D.C.; Hunt, A.J.; Ok, Y.S.; Song, H.; Zhang, S. Lignin Valorization for the Production of Renewable Chemicals: State-of-the-art review and future prospects. Bioresource 2018, 269, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Achinivu, E. Protic Ionic Liquids for Lignin Extraction—A Lignin Characterization Study. Int. J. Mol. Sci. 2018, 19, 428. [Google Scholar] [CrossRef]
- Qu, C.; Kaneko, M.; Kashimura, K.; Tanaka, K.; Ozawa, S.; Watanabe, T. Direct Production of Vanillin from Wood Particles by Copper Oxide–Peroxide Reaction Promoted by Electric and Magnetic Fields of Microwaves. ACS Sustain. Chem. Eng. 2017, 5, 11551–11557. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Li, Y.; Schwank, J.W. A Review on Oxygen Storage Capacity of CeO2-based Materials: Influence Factors, Measurement Techniques, and Applications in Reactions Related to Catalytic Automotive Emissions Control. Catal. Today 2019, 327, 90–115. [Google Scholar] [CrossRef]
- Meng, F.; Wang, L.; Cui, J. Controllable Synthesis and Optical Properties of Nano- CeO2 via a Facile Hydrothermal Route. J. Alloys Compd. 2013, 556, 102–108. [Google Scholar] [CrossRef]
- Godlaveeti, S.K.; Jangiti, S.; Bharatiya, D.; Somala, A.R.; Sana, S.S.; Nagireddy, R.R. Electrochemical Performance Comparison Study of Ternary RGO/ß-Ni(OH)2-CeO2 Nanocomposite Prepared from Hydrothermal and Solid-State Methods for Supercapacitors. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 01501. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Zarinkamar, M.; Firoozabadi, T.P. Synthesis of Cerium Oxide (CeO2) nanoparticles using simple CO-precipitation method. Rev. Mex. Física 2016, 62, 496–499. [Google Scholar]
- Gharagozlou, M. Study on the Influence of Annealing Temperature and Ferrite Content on the Structural and Magnetic Properties of x (NiFe2O4)/(100 − x) SiO2 Nanocomposites. J. Alloys Compd. 2010, 495, 217–223. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, V.; Alexander, P.; Olivier, J.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Bose, P.; Mandal, S.; Naskar, M.K. Effect of Anion Type on the Synthesis of Mesoporous Nanostructured MgO, and Its Excellent Adsorption Capacity for the Removal of Toxic Heavy Metal Ions from Water. RSC Adv. 2016, 6, 6038–6047. [Google Scholar] [CrossRef]
- Efika, C.E.; Wu, C.; Williams, P.T. Syngas Production from Pyrolysis–catalytic Steam Reforming of Waste Biomass in a Continuous Screw Kiln Reactor. J. Anal. Appl. Pyrolysis 2012, 95, 87–94. [Google Scholar] [CrossRef]
- Lykaki, M.; Stefa, S.; Carabineiro, S.A.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Facet-Dependent Reactivity of Fe2O3/CeO2 Nanocomposites: Effect of Ceria Morphology on CO oxidation. Catalysts 2019, 9, 371. [Google Scholar] [CrossRef]
- Skrbek, K.; Bartůněk, V.; Lojka, M.; Sedmidubský, D.; Jankovský, O. Synthesis and Characterization of the Properties of Ceria Nanoparticles with Tunable Particle Size for the Decomposition of Chlorinated Pesticides. Appl. Sci. 2020, 10, 5224. [Google Scholar] [CrossRef]
- Lin, Z.; He, M.; Liu, Y.; Meng, M.; Cao, Z.; Huang, S.; Chen, C.; Deng, H. Effect of Calcination Temperature on the Structural and Formaldehyde Removal Activity of Mn/Fe2O3 Catalysts. Res. Chem. Intermed. 2021, 47, 3245–3261. [Google Scholar] [CrossRef]
- Tong, X.; Luo, T.; Meng, X.; Wu, H.; Li, J.; Liu, X.; Zhan, Z. Shape-Dependent Activity of Ceria for Hydrogen Electro-Oxidation in Reduced-Temperature Solid Oxide Fuel Cells. Small 2015, 11, 5581–5588. [Google Scholar] [CrossRef]
- Mohamad Aini, N.A.; Othman, N.; Hussin, M.H.; Sahakaro, K.; Hayeemasae, N. Effect of Extraction Methods on the Molecular Structure and Thermal Stability of Kenaf (Hibiscus cannabinus core) Biomass as an Alternative Bio-filler for Rubber Composites. Int. J. Biol. Macromol 2020, 154, 1255–1264. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, W.; Zhang, Y.; Wang, H.; Zou, F.; Yu, K.; Han, G. A Novel Process of Nanocellulose Extraction from Kenaf Bast. Mater. Res. 2018, 5, 085032. [Google Scholar] [CrossRef]
- Yuting, Z.; Yuhe, L.; Wei, L.; Jing, L.; Xiangbo, S.; Lungang, C.; Chenguang, W.; Bert, F.S.; Longlong, M. Complementing Vanillin and Cellulose Production by Oxidation of Lignocellulose with Stirring Control. ACS Sustain. Chem. Eng. 2020, 8, 2361–2374. [Google Scholar]
- Khalil, H.P.A.; Ismail, H.; Rozman, H.D.; Ahmad, M.N. The Effect of Acetylation on Interfacial Shear Strength between Plant Fiber and Various Matrices. Eur. Polym. J. 2001, 37, 1037–1045. [Google Scholar] [CrossRef]
- Rodrigues, J.; Faix, O.; Pereira, H. Determination of Lignin Content of Eucalyptus globulus Wood using FTIR Spectroscopy. Holzforsch 1998, 52, 46–50. [Google Scholar] [CrossRef]
- Hassan, N.S.; Badri, K.H. Lignin Recovery from Ahydrolysis and Glycerolysis of Oil Palm Fiber. In Proceedings of the 2014 Universiti Kebangsaan Malaysia, Faculty of Science and Technology 2014 Postgraduate Colloquium, Selangor, Malaysia, 9–11 April 2014. [Google Scholar]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal Degradation of Softwood Lignin and Hardwood Lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Mohd Warid, M.N.; Ariffin, H.; Hassan, M.A.; Shirai, Y. Optimization of Superheated Steam Treatment to Improve Surface Modification of Oil Palm Biomass Fiber. BioResources 2016, 11, 5780–5796. [Google Scholar] [CrossRef]
- Huang, W. Crystal Plane-dependent Surface Reactivity and Catalytic Property of Oxide Catalysts Studied with Oxide Nanocrystal Model Catalysts. Top. Catal. 2013, 56, 1363–1376. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, C.; Li, Y.; Ma, Z.; Lv, X. Effect of Calcination Temperature on the Structure and Catalytic Performance of the Cu-MCM-41 Catalysts for the Synthesis of Dimethyl Carbonate. Química Nova 2018, 41, 1156–1161. [Google Scholar] [CrossRef]
- Jin, M.H.; Oh, D.; Park, J.H.; Lee, C.B.; Lee, S.W.; Park, J.S.; Lee, K.Y.; Lee, D.W. Mesoporous Silica Supported Pd-MnOx Catalysts with Excellent Catalytic Activity in Room Temperature Formic aAcid Decomposition. Sci. Rep. 2016, 6, 33502. [Google Scholar] [CrossRef]
- Hailstone, R.K.; DiFrancesco, A.G.; Leong, J.G.; Allston, T.D.; Reed, K.J. A study of Lattice Expansion in CeO2 Nanoparticles by Transmission Electron Microscopy. J. Phys. Chem. C 2009, 113, 15155–15159. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, L.; Xiang, Y.; Xia, D. Effect of Calcination Temperature on Structural Properties and Catalytic Performance of Novel Amorphous NiP/Hβ Catalyst for n-Hexane Isomerization. Catalysts 2020, 10, 811. [Google Scholar] [CrossRef]
- Khairul Anuar, N.A.S.I.; Ramli, A.; Jun Wei, L. Synthesis of Ce/MgO Catalysts for Direct Oxidation of Hibiscus cannabinus Stalks to Vanillin. Catalysts 2021, 11, 1449. [Google Scholar] [CrossRef]
- Nicholas, J.W.; Melinda, J.M.; Arjan, N. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar]
- Xiang, Q.; Lee, Y.Y. Production of Oxychemicals from Precipitated Hardwood Lignin. Appl. Biochem. Biotechnol. 2001, 91–93, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Tarabanko, V.E.; Tarabanko, N. Catalytic Oxidation of Lignins into the Aromatic Aldehydes: General Process Trends and Development Prospects. Int. J. Mol. Sci. 2017, 18, 2421. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.C.R.; da Silva, E.A.B.; Rodrigues, A.E. Lignin as Source of Fine Chemicals: Vanillin and Syringaldehyde. In Biomass Conversion; Springer: Berlin/Heidelberg, Germany, 2012; pp. 381–420. [Google Scholar]
- Jiang, L.; Guo, H.; Li, C.; Zhou, P.; Zhang, Z. Selective cleavage of lignin and lignin model compounds without external hydrogen, catalyzed by heterogeneous nickel catalysts. Chem. Sci. 2019, 10, 4458–4468. [Google Scholar] [CrossRef] [PubMed]
- Fengel, D.; Wegener, G. Wood—Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Munich, Germany, 1989; pp. 13–15. [Google Scholar]
- Ouyang, X.; Ruan, T.; Qiu, X. Effect of Solvent on Hydrothermal Oxidation Depolymerization of Lignin for the Production of Monophenolic Compounds. Fuel Process. Technol. 2016, 144, 181–185. [Google Scholar] [CrossRef]
- Gu, X.; Kanghua, C.; Ming, H.; Shi, Y.; Li, Z. La-Modified SBA-15/H2O2 Systems for the Microwave Assisted Oxidation of Organosolv Beech Wood Lignin. Maderas. Cienc. Y Tecnol. 2012, 14, 31–41. [Google Scholar] [CrossRef]
- Luo, J.; Melissa, P.; Zhao, W.; Wang, Z.; Zhu, Y. Selective Lignin Oxidation Towards Vanillin in Phenol Media. ChemistrySelect 2016, 1, 4596–4601. [Google Scholar] [CrossRef]
- Kovacevic, M.; Mojet, B.L.; van Ommen, J.G.; Lefferts, L. Effects of Morphology of Cerium Oxide Catalysts for Reverse Water Gas Shift Reaction. Catal. Lett. 2016, 146, 770–777. [Google Scholar] [CrossRef]
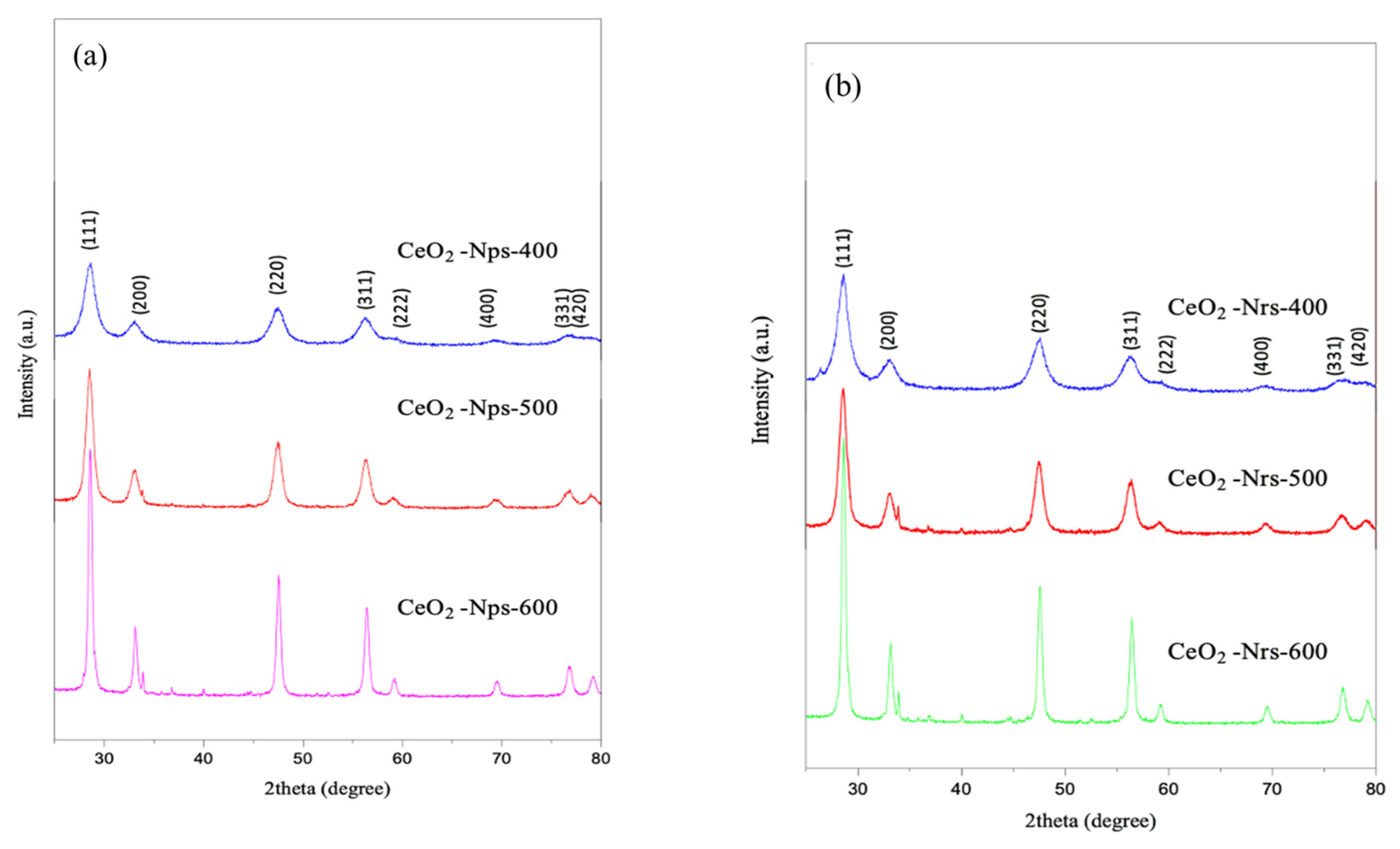


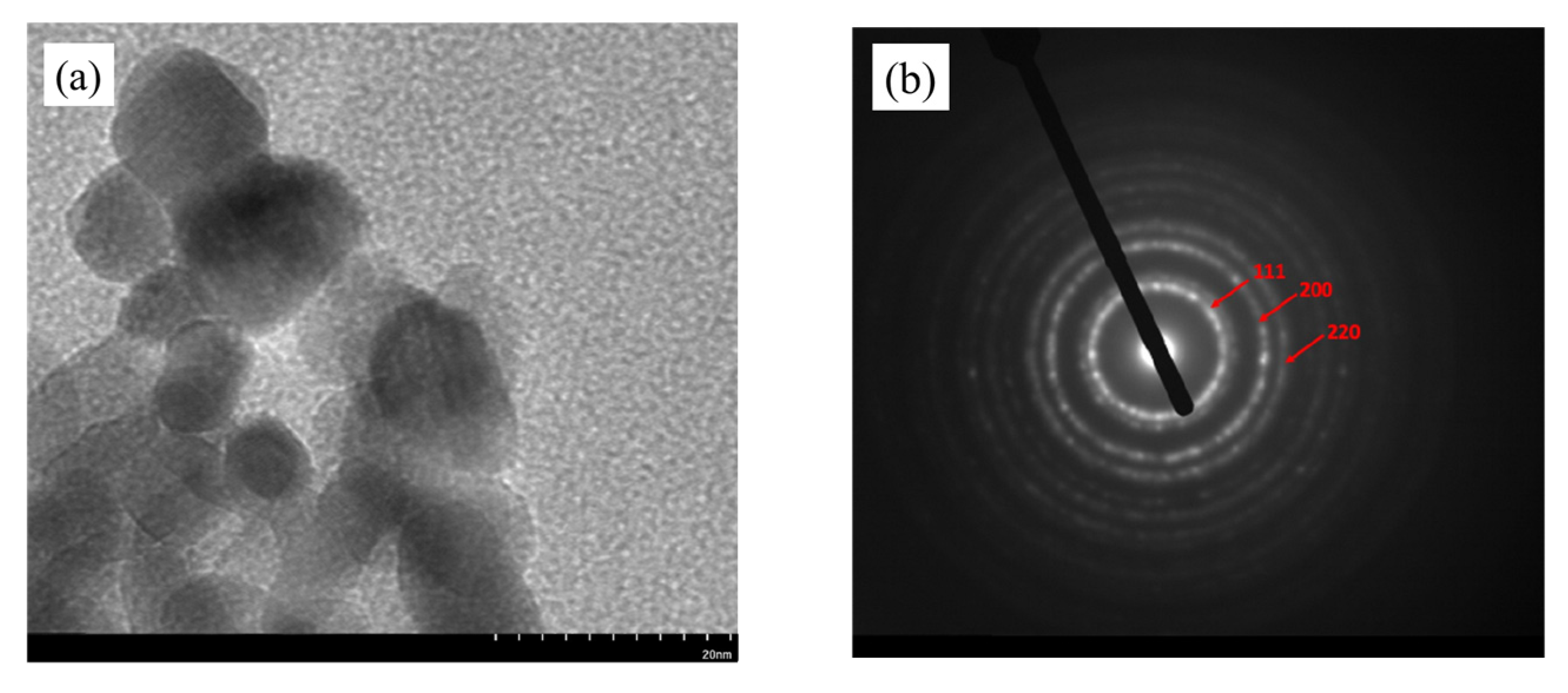
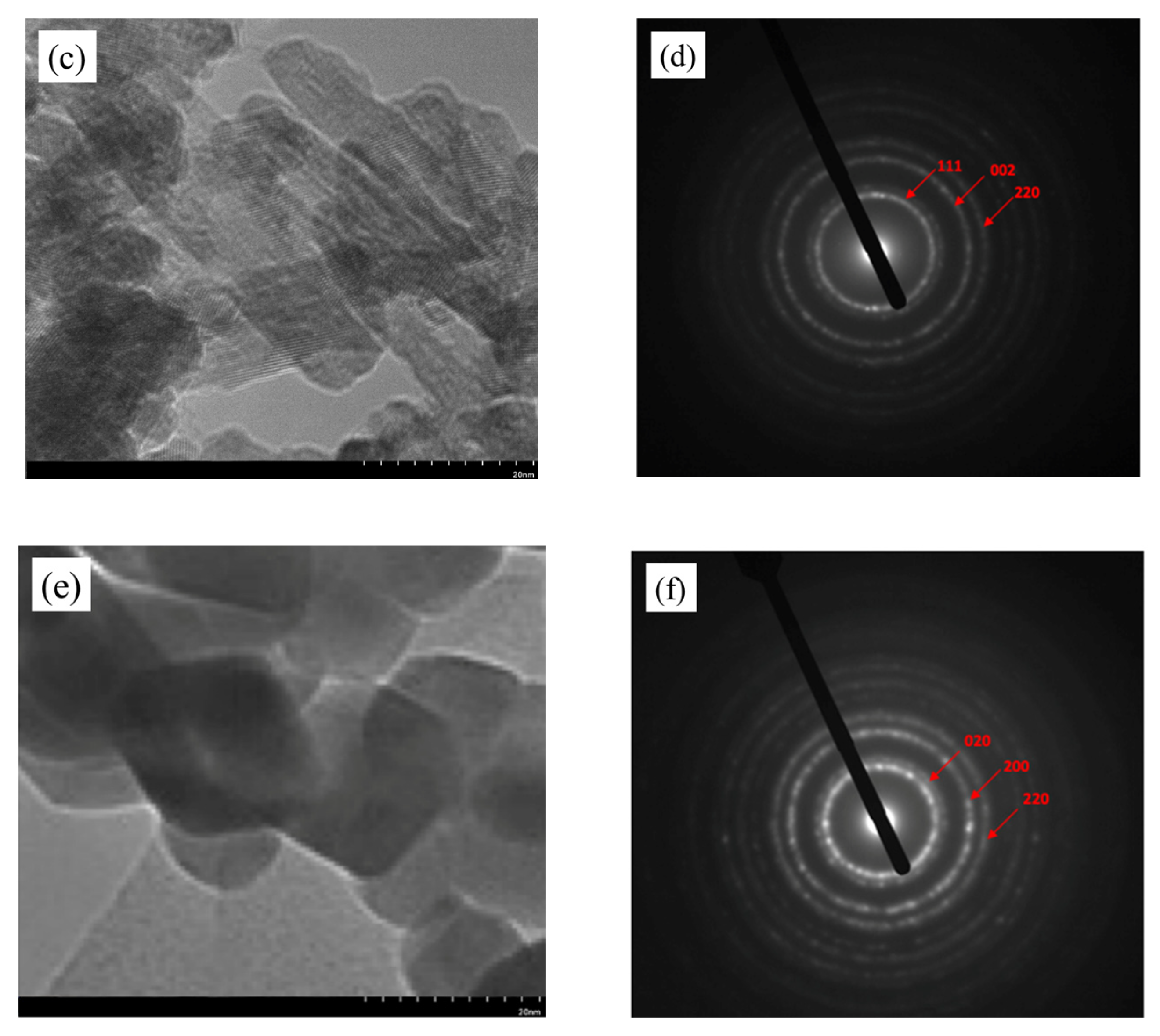
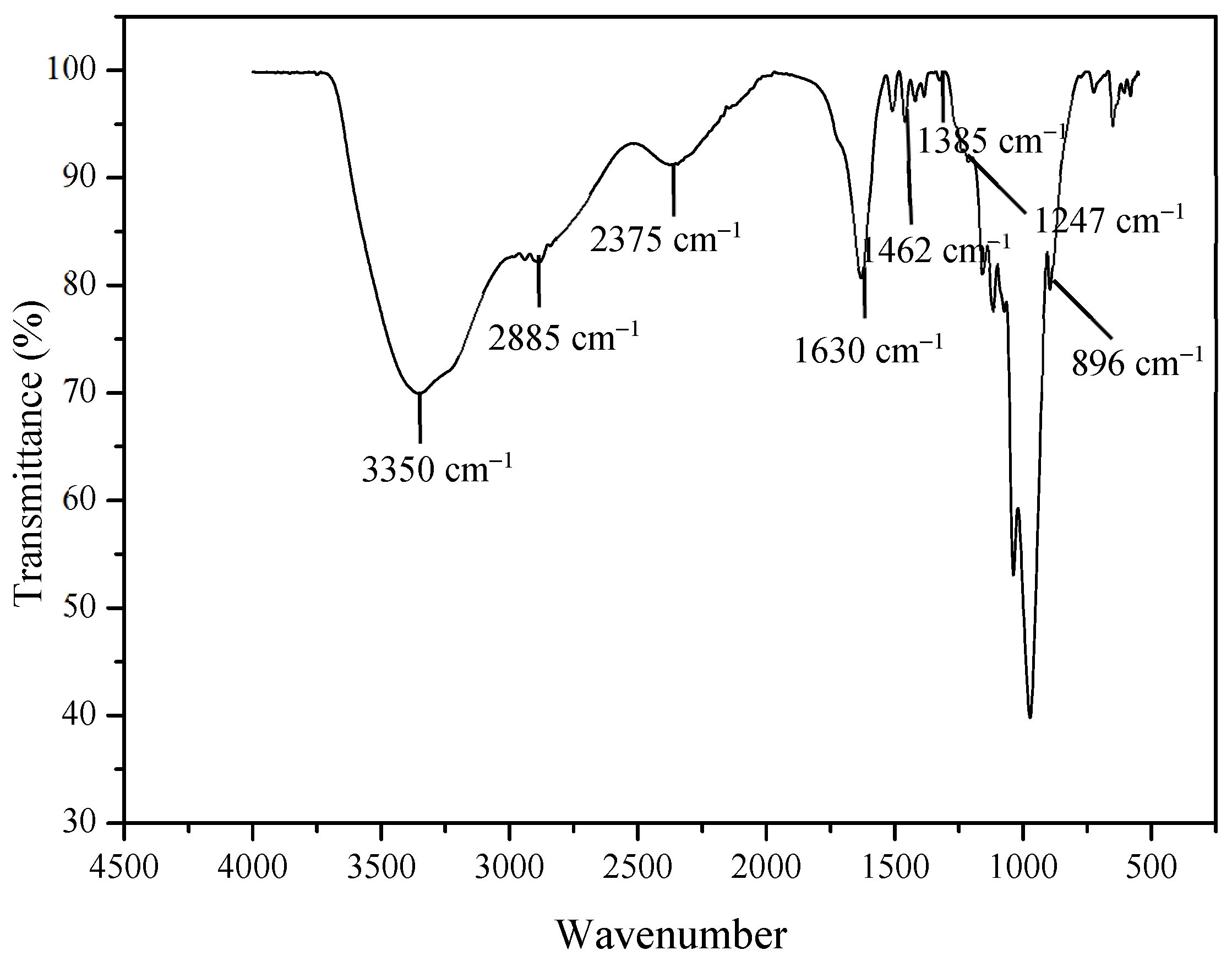
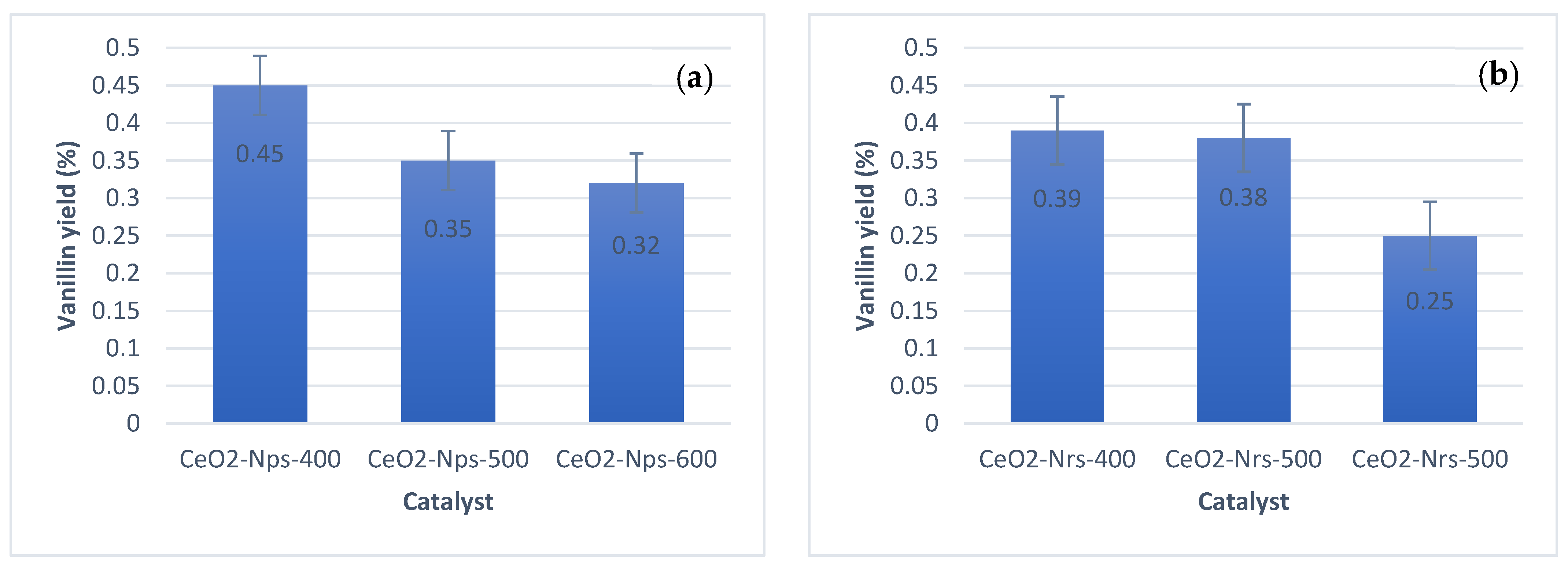
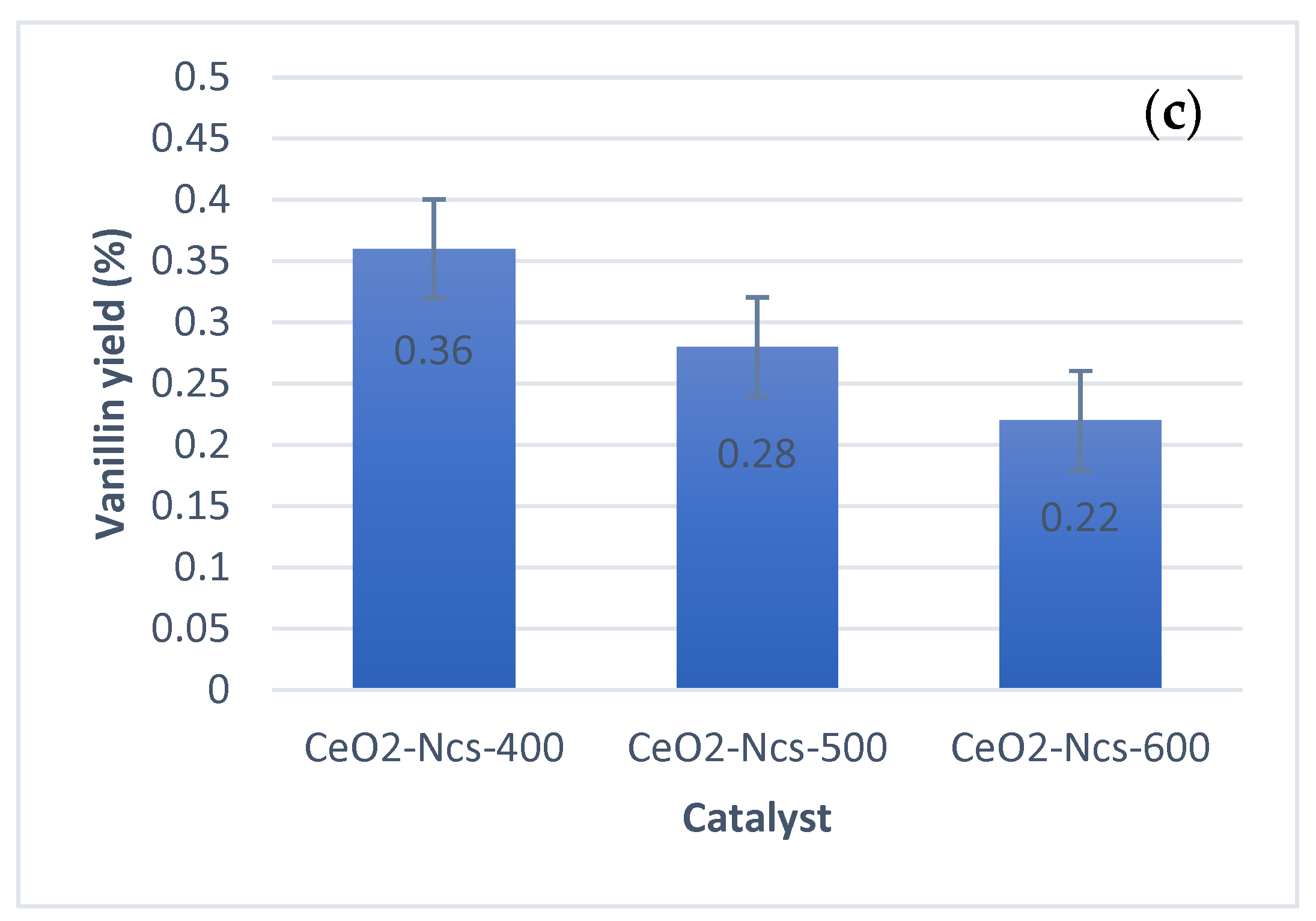
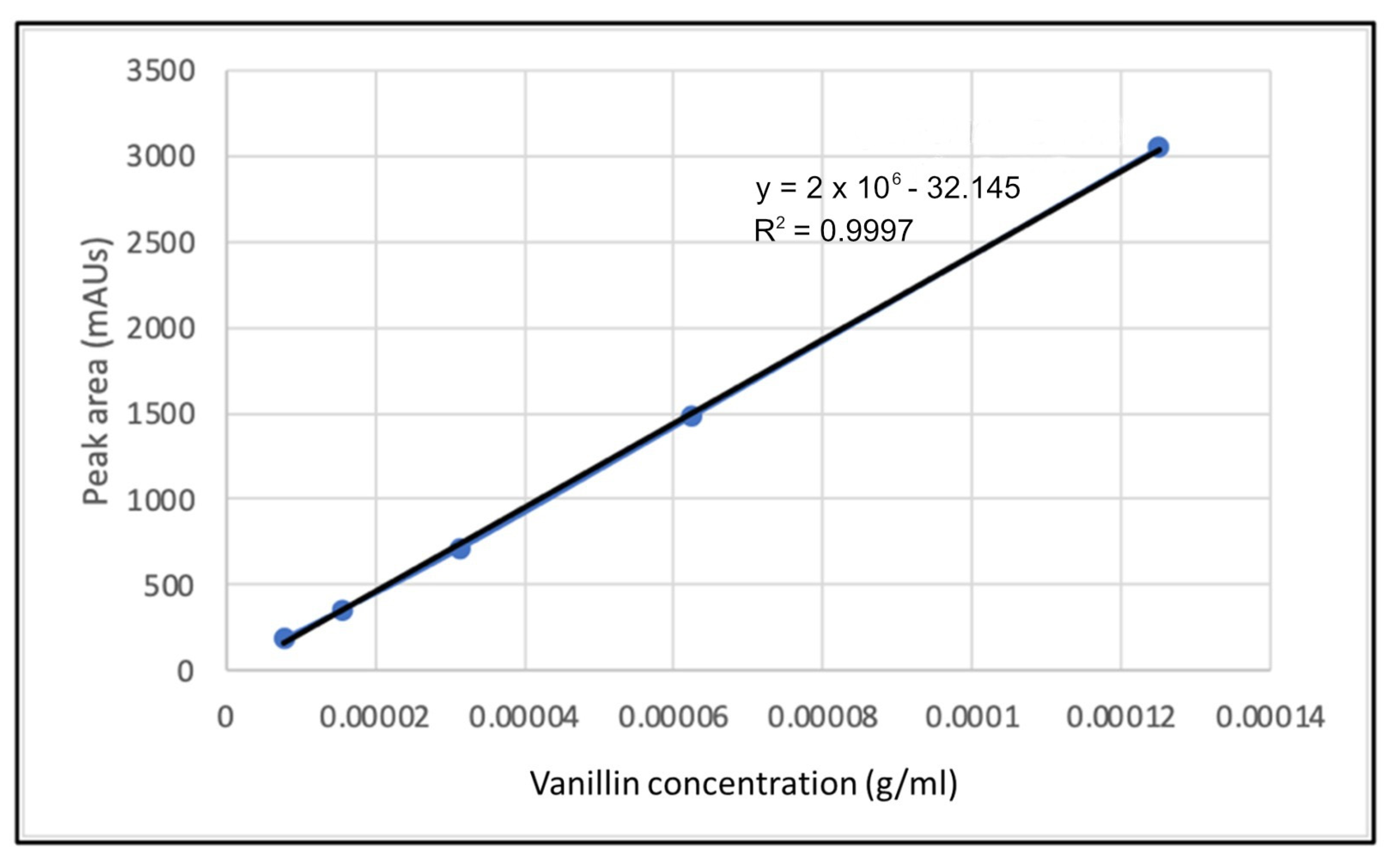
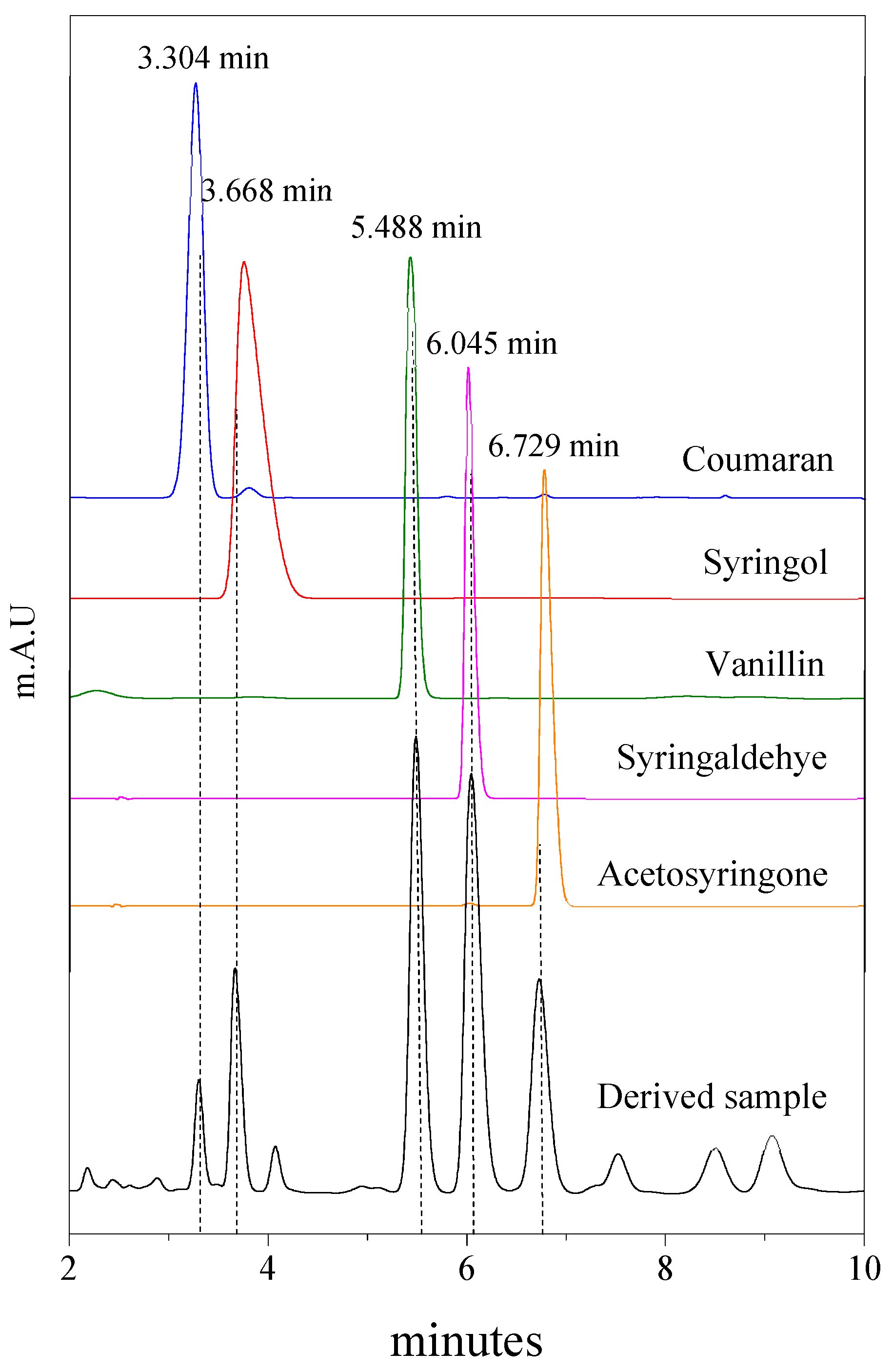
| Catalyst | Crystallite Size at (111) Plane (nm) |
|---|---|
| CeO2-Nps-400 | 11.3 |
| CeO2-Nps-500 | 21.5 |
| CeO2-Nps-600 | 23.5 |
| CeO2-Nrs-400 | 15.0 |
| CeO2-Nrs-500 | 24.0 |
| CeO2-Nrs-600 | 24.9 |
| CeO2-Ncs-400 | 28.7 |
| CeO2-Ncs-500 | 30.8 |
| CeO2-Ncs-600 | 34.0 |
| Catalyst | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | Crystallite Size (nm) |
|---|---|---|---|---|
| CeO2-Nps-400 | 66.1 | 0.275 | 27.5 | 11.3 |
| CeO2-Nps-500 | 47.5 | 0.216 | 14.0 | 21.5 |
| CeO2-Nps-600 | 20.2 | 0.183 | 13.7 | 23.5 |
| CeO2-Nrs-400 | 57.8 | 0.145 | 24.0 | 15.0 |
| CeO2-Nrs-500 | 43.9 | 0.129 | 20.9 | 24.0 |
| CeO2-Nrs-600 | 23.0 | 0.126 | 10.8 | 24.9 |
| CeO2-Ncs-400 | 22.5 | 0.134 | 17.1 | 28.7 |
| CeO2-Ncs-500 | 12.6 | 0.071 | 8.4 | 30.8 |
| CeO2-Ncs-600 | 11.1 | 0.045 | 6.0 | 34.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramli, A.; Khairul Anuar, N.A.S.I.; Bakhtiar, N.A.A.; Mohamad Yunus, N.; Mohamed, A.R. Direct Oxidation of Hibiscus cannabinus Stalks to Vanillin Using CeO2 Nanostructure Catalysts. Molecules 2023, 28, 4963. https://doi.org/10.3390/molecules28134963
Ramli A, Khairul Anuar NASI, Bakhtiar NAA, Mohamad Yunus N, Mohamed AR. Direct Oxidation of Hibiscus cannabinus Stalks to Vanillin Using CeO2 Nanostructure Catalysts. Molecules. 2023; 28(13):4963. https://doi.org/10.3390/molecules28134963
Chicago/Turabian StyleRamli, Anita, Nur Akila Syakida Idayu Khairul Anuar, Nur Aielia Amira Bakhtiar, Normawati Mohamad Yunus, and Alina Rahayu Mohamed. 2023. "Direct Oxidation of Hibiscus cannabinus Stalks to Vanillin Using CeO2 Nanostructure Catalysts" Molecules 28, no. 13: 4963. https://doi.org/10.3390/molecules28134963







