Antiangiogenic, Anti-Inflammatory and Antioxidant Properties of Bidens tripartite Herb, Galium verum Herb and Rumex hydrolapathum Root
Abstract
:1. Introduction
2. Results
2.1. GC-MS Analysis of the Extracts
2.2. Antioxidant Activity and Polyphenolic Content
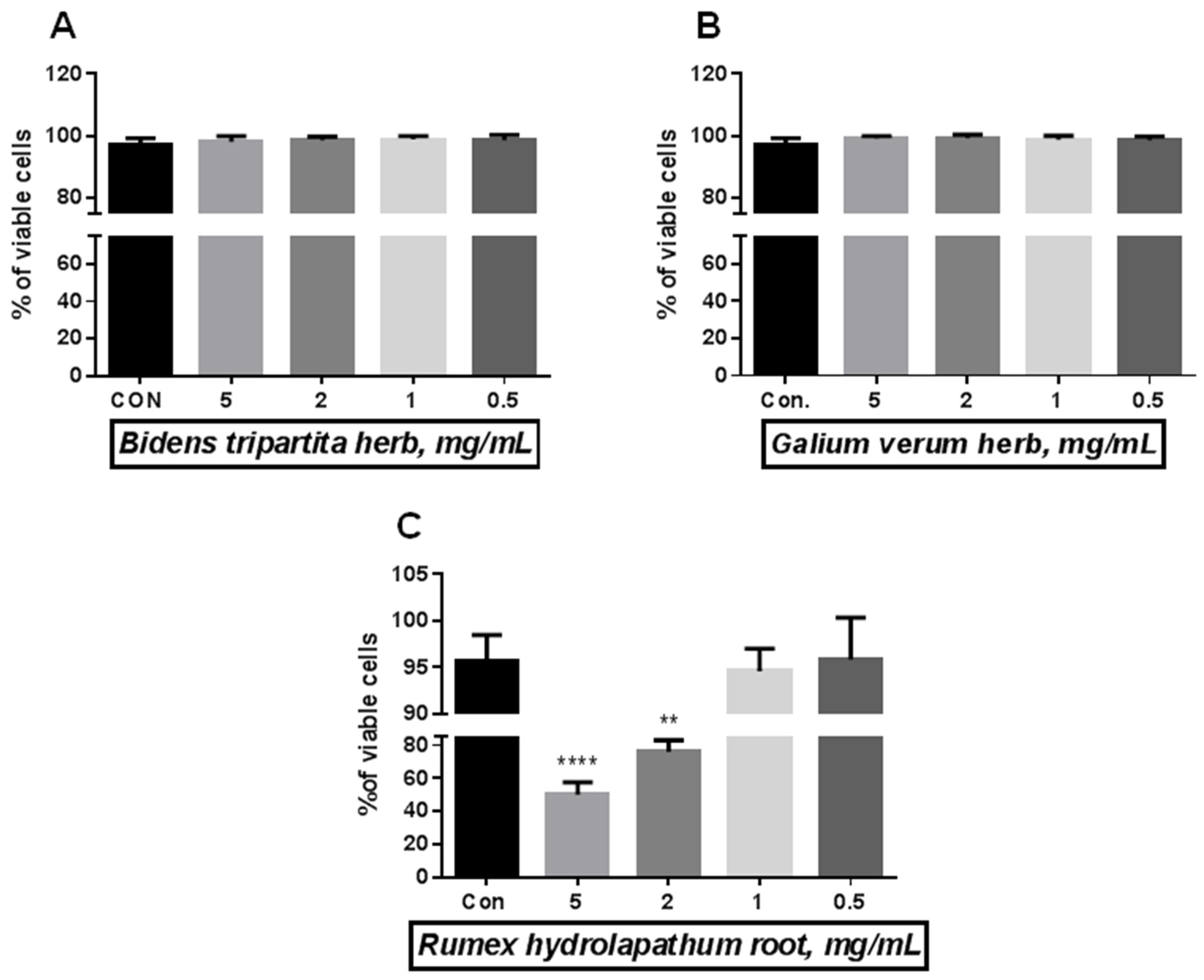
2.3. Effect of BH, GVH, and RR on Endothelial Cells Viability
2.4. Cell Proliferation, Migration and Invasiveness
2.5. Production of Angiogenic/Angiostatic Factors
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Extracts Preparation
4.4. GC-MS Analysis of the Extracts
4.5. Antioxidant Activity and Total Polyphenolic Content
4.5.1. 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) Analysis
4.5.2. The Ferric Reducing Antioxidant Power (FRAP) Analysis
4.5.3. Determination of Total Phenolics Content (TPC)
4.6. Bioactivity Assay—In Vitro Experiments
4.6.1. Cell Culture
4.6.2. Experimental Design
4.6.3. Cell Viability
4.6.4. Proliferation Assay
4.6.5. Migration/Invasion Assay
4.6.6. Cytokine Measurements
4.7. Statistical Analysis
5. Conclusions
- I.
- BTH, GVH, and RHR can modify angiogenesis at various levels. BTH has the most significant antiangiogenic properties.
- II.
- BTH and GVH have the most potent anti-inflammatory properties.
- III.
- The RHR has the highest antioxidant activity. Its antioxidant potency correlated with the polyphenols content.
- IV.
- The modifying influence of examined extracts can be promising in disorders with pathogenesis related to free radicals formation, inflammation, and angiogenesis. BTH is the best choice among the three tested extracts with its antiangiogenic and anti-inflammatory properties.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lu, K.; Bhat, M.; Basu, S. Plants and their active compounds: Natural molecules to target angiogenesis. Angiogenesis 2016, 19, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.Y.; Kwon, S.M. Angiogenesis and its therapeutic opportunities. Mediat. Inflamm. 2013, 2013, 127170. [Google Scholar] [CrossRef] [Green Version]
- Fam, N.P.; Verma, S.; Kutryk, M.; Stewart, D.J. Clinician guide to angiogenesis. Circulation 2003, 108, 2613–2618. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Chemical Constituents and Pharmacological Importance of Bidens tripartitus—A Review. J. Pharm. Sci. Res. 2015, 5, 257–263. [Google Scholar]
- Orhan, N.; Icoz, U.G.; Altun, L.; Aslan, M. Anti-hyperglycaemic and antioxidant effects of Bidens tripartita and quantitative analysis on its active principles. Iran J. Basic Med. Sci. 2016, 19, 1114–1124. [Google Scholar]
- Uysal, S.; Ugurlu, A.; Zengin, G.; Baloglu, M.C.; Altunoglu, Y.C.; Mollica, A.; Custodio, L.; Neng, N.R.; Nogueira, J.M.F.; Mahomoodally, M.F. Novel in vitro and in silico insights of the multi-biological activities and chemical composition of Bidens tripartita L. Food Chem. Toxicol. 2018, 111, 525–536. [Google Scholar] [CrossRef]
- Wolniak, M.; Tomczykowa, M.; Tomczyk, M.; Gudej, J.; Wawer, I. Antioxidant activity of extracts and flavonoids from Bidens tripartita. Acta Pol. Pharm. 2007, 64, 441–447. [Google Scholar]
- Al-Snafi, A.E. Galium verum—A review. Indo Am. J. Pharm. Sci. 2018, 5, 2142–2149. [Google Scholar]
- Schmidt, M.; Polednik, C.; Roller, J.; Hagen, R. Galium verum aqueous extract strongly inhibits the motility of head and neck cancer cell lines and protects mucosal keratinocytes against toxic DNA damage. Oncol. Rep. 2014, 32, 1296–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M.; Scholz, C.J.; Gavril, G.L.; Otto, C.; Polednik, C.; Roller, J.; Hagen, R. Effect of Galium verum aqueous extract on growth, motility and gene expression in drug-sensitive and -resistant laryngeal carcinoma cell lines. Int. J. Oncol. 2014, 44, 745–760. [Google Scholar] [CrossRef] [Green Version]
- Demirezer, O.; Kuruuzum, A.; Bergere, I.; Schiewe, H.J.; Zeeck, A. Five naphthalene glycosides from the roots of Rumex patientia. Phytochemistry 2001, 56, 399–402. [Google Scholar] [CrossRef]
- Dong, J.M.; Ma, Y.L.; Zhang, Z.Y.; Li, R.; Zhu, Y.L.; Ma, L. Effects and related mechanism of flavone from Galium verum L. on peroxide induced oxidative injury in human umbilical vein endothelial cells. Zhonghua Xin Xue Guan Bing Za Zhi 2016, 44, 610–615. [Google Scholar]
- Lakić, N.; Mimica-Dukić, N.; Isak, J.; Božin, B. Antioxidant properties of Galium verum L. (Rubiaceae) extracts. Cent. Eur. J. Biol. 2010, 5, 331–337. [Google Scholar] [CrossRef]
- Layali, I.; Ebrahimzadeh, M.A.; Joulaei, M. Antioxidant properties of Galium verum. J. Life Sci. Pharm. Res. 2016, 6, L31–L37. [Google Scholar]
- Shinkovenko, I.L.; Kashpur, N.V.; Ilina, T.V.; Kovalyova, A.M.; Goryacha, O.V.; Koshovyi, O.M.; Kryvoruchko, O.V.; Komissarenko, A.M. The immunomodulatory activity of ethanolic extracts from Galium verum L. herb. Ceska Slov. Farm. 2018, 67, 101–106. [Google Scholar]
- Vasas, A.; Orban-Gyapai, O.; Hohmann, J. The Genus Rumex: Review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 175, 198–228. [Google Scholar] [CrossRef]
- Shim, K.S.; Lee, B.; Ma, J.Y. Water extract of Rumex crispus prevents bone loss by inhibiting osteoclastogenesis and inducing osteoblast mineralization. BMC Complement. AlternMed. 2017, 17, 483. [Google Scholar] [CrossRef] [Green Version]
- Orban-Gyapai, O.; Liktor-Busa, E.; Kusz, N.; Stefko, D.; Urban, E.; Hohmann, J.; Vasas, A. Antibacterial screening of Rumex species native to the Carpathian Basin and bioactivity-guided isolation of compounds from Rumex aquaticus. Fitoterapia 2017, 118, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.J.; Kim, H.Y.; Kim, H.M. Molecular mechanisms of anti-inflammatory effect of chrysophanol, an active component of AST2017-01 on atopic dermatitis in vitro models. Int. Immunopharmacol. 2018, 54, 238–244. [Google Scholar] [CrossRef]
- Parr, C.; Ali, A.Y. Boswellia frereana suppresses HGF-mediated breast cancer cell invasion and migration through inhibition of c-Met signalling. J. Transl. Med. 2018, 16, 281. [Google Scholar] [CrossRef] [Green Version]
- Feduraev, P.; Chupakhina, G.; Maslennikov, P.; Tacenko, N.; Skrypnik, L. Variation in Phenolic Compounds Content and Antioxidant Activity of Different Plant Organs from Rumex crispus L. and Rumex obtusifolius L. at Different Growth Stages. Antioxidants 2019, 8, 237. [Google Scholar] [CrossRef] [Green Version]
- Minh, T.N.; Van, T.M.; Andriana, Y.; Vinh, L.T.; Hau, D.V.; Duyen, D.H.; Guzman-Gelani, C. Antioxidant, Xanthine Oxidase, alpha-Amylase and alpha-Glucosidase Inhibitory Activities of Bioactive Compounds from Rumex crispus L. Root. Molecules 2019, 24, 3899. [Google Scholar] [CrossRef] [Green Version]
- Shiwani, S.; Singh, N.K.; Wang, M.H. Carbohydrase inhibition and anti-cancerous and free radical scavenging properties along with DNA and protein protection ability of methanolic root extracts of Rumex crispus. Nutr. Res. Pract. 2012, 6, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Han, N.R.; Moon, P.D.; Yoo, M.S.; Ryu, K.J.; Kim, H.M.; Jeong, H.J. Regulatory effects of chrysophanol, a bioactive compound of AST2017-01 in a mouse model of 2,4-dinitrofluorobenzene-induced atopic dermatitis. Int. Immunopharmacol. 2018, 62, 220–226. [Google Scholar] [CrossRef]
- Demirezer, L.Ö. Concentrations of Anthraquinone Glycosides of Rumex crispus during Different Vegetation Stages. 49. Zeitschrift Naturforschung 1994, 49, 404–406. [Google Scholar] [CrossRef]
- Ilyina, T.V.; Goryacha, O.V.; Kovaleva, A.M.; Koshovyi, O.M.; Shinkovenko, I.L. A comparative study of morphological features and flavonoid composition of galium L. genus species. Der Pharm. Lett. 2016, 8, 316–320. [Google Scholar]
- Oproshanskaya, T.V. Fatty Acids from Bidens tripartita Herb. Chem. Nat. Compd. 2015, 51, 944–945. [Google Scholar] [CrossRef]
- Chen, Q.; Li, P.; Li, P.; Xu, Y.; Li, Y.; Tang, B. Isoquercitrin inhibits the progression of pancreatic cancer in vivo and in vitro by regulating opioid receptors and the mitogen-activated protein kinase signalling pathway. Oncol. Rep. 2015, 33, 840–848. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Tang, B.; Tang, K.; Dong, X.; Deng, J.; Liao, L.; Liao, Z.; Yang, H.; He, S. Isoquercitrin inhibits the progression of liver cancer in vivo and in vitro via the MAPK signalling pathway. Oncol. Rep. 2014, 3, 2377–2384. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.W.; Chiang, Y.M.; Chuang, H.C.; Lo, C.P.; Yang, K.Y.; Wang, S.Y.; Shyur, L.F. A novel polyacetylene significantly inhibits angiogenesis and promotes apoptosis in human endothelial cells through activation of the CDK inhibitors and caspase-7. Planta Med. 2007, 73, 655–661. [Google Scholar] [CrossRef]
- Yagasaki, K.; Miura, Y.; Okauchi, R.; Furuse, T. Inhibitory effects of chlorogenic acid and its related compounds on the invasion of hepatoma cells in culture. Cytotechnology 2000, 33, 229–235. [Google Scholar] [CrossRef]
- Lajter, I.; Zupko, I.; Molnar, J.; Jakab, G.; Balogh, L.; Vasas, A.; Hohmann, J. Antiproliferative activity of polygonaceae species from the Carpathian Basin against human cancer cell lines. Phytother. Res. 2013, 27, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.J.; Choi, J.H.; Koo, B.S.; Ryu, S.Y.; Han, Y.H.; Lee, S.I.; Lee, D.U. Antimutagenicity and cytotoxicity of the constituents from the aerial parts of Rumex acetosa. Biol. Pharm. Bull. 2005, 28, 2158–2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berse, B.; Brown, L.F.; van de Water, L.; Dvorak, H.F.; Senger, D.R. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol. Biol. Cell 1992, 3, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Koon, C.M.; Woo, K.S.; Leung, P.C.; Fung, K.P. Salviae Miltiorrhizae Radix and Puerariae Lobatae Radix herbal formula mediates anti-atherosclerosis by modulating key atherogenic events both in vascular smooth muscle cells and endothelial cells. J. Ethnopharmacol. 2011, 138, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Jung, K.H.; Lee, H.S.; Son, M.K.; Yan, H.H.; Kang, N.S.; Lee, J.; Hong, S.S. SB365, Pulsatilla saponin D, targets c-Met and exerts antiangiogenic and antitumor activities. Carcinogenesis 2013, 34, 2156–2169. [Google Scholar] [CrossRef] [Green Version]
- Nakahara, H.; Song, J.; Sugimoto, M.; Hagihara, K.; Kishimoto, T.; Yoshizaki, K.; Nishimoto, N. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003, 48, 1521–1529. [Google Scholar] [CrossRef]
- Gopinathan, G.; Milagre, C.; Pearce, O.M.; Reynolds, L.E.; Hodivala-Dilke, K.; Leinster, D.A.; Zhong, H.; Hollingsworth, R.E.; Thompson, R.; Whiteford, J.R.; et al. Interleukin-6 Stimulates Defective Angiogenesis. Cancer Res. 2015, 75, 3098–3107. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef] [Green Version]
- Le, J.; Lu, W.; Xiong, X.; Wu, Z.; Chen, W. Anti-Inflammatory Constituents from Bidens frondosa. Molecules 2015, 20, 18496–18510. [Google Scholar] [CrossRef] [Green Version]
- Fu, W.J.; Tang, J.J.; Wang, H.; Wei, H.Y.; Cai, S.M.; Zeng, Z.H.; Chen, H.; Chen, Z.Q. In vivo and in vitro anti-sepsis effects of physcion 8-O-beta-glucopyranoside extracted from Rumex japonicus. Chin. J. Nat. Med. 2017, 15, 534–539. [Google Scholar]
- Park, E.S.; Song, G.H.; Kim, S.H.; Lee, S.M.; Kim, Y.G.; Lim, Y.L.; Kang, S.A.; Park, K.Y. Rumex crispus and Cordyceps militaris Mixture Ameliorates Production of Pro-Inflammatory Cytokines Induced by Lipopolysaccharide in C57BL/6 Mice Splenocytes. Prev. Nutr. Food Sci. 2018, 23, 374–381. [Google Scholar] [CrossRef]
- Zhang, C.; Li, K.; Yang, Z.; Wang, Y.; Si, H. The Effect of the Aqueous Extract of Bidens pilosa L. on Androgen Deficiency Dry Eye in Rats. Cell Physiol. Biochem. 2016, 39, 266–277. [Google Scholar] [CrossRef]
- Jin, U.H.; Lee, J.Y.; Kang, S.K.; Kim, J.K.; Park, W.H.; Kim, J.G.; Moon, S.K.; Kim, C.H. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: Isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005, 77, 2760–2769. [Google Scholar] [CrossRef]
- Xue, C.L.; Liu, H.G.; Li, B.Y.; He, S.H.; Yue, Q.F. Physcion 8-O-beta-glucopyranoside exhibits anti-growth and anti-metastatic activities in ovarian cancer by downregulating miR-25. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5101–5112. [Google Scholar]
- Yang, Y.; Yan, Y.M.; Wei, W.; Luo, J.; Zhang, L.S.; Zhou, X.J.; Wang, P.C.; Yang, Y.X.; Cheng, Y.X. Anthraquinone derivatives from Rumex plants and endophytic Aspergillus fumigatus and their effects on diabetic nephropathy. Bioorg. Med. Chem. Lett. 2013, 23, 3905–3909. [Google Scholar] [CrossRef] [Green Version]
- Deryugina, E.I.; Quigley, J.P. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015, 44–46, 94–112. [Google Scholar] [CrossRef]
- Ceylan, S.; Cetin, S.; Camadan, Y.; Saral, O.; Ozsen, O.; Tutus, A. Antibacterial and antioxidant activities of traditional medicinal plants from the Erzurum region of Turkey. Ir. J. Med. Sci. 2019, 188, 1303–1309. [Google Scholar] [CrossRef]
- Lone, I.A.; Kaur, G.; Athar, M.; Alam, M.S. Protective effect of Rumex patientia (English Spinach) roots on ferric nitrilotriacetate (Fe-NTA) induced hepatic oxidative stress and tumor promotion response. Food Chem. Toxicol. 2007, 45, 1821–1829. [Google Scholar] [CrossRef]
- Zhang, Y.; Lan, M.; Lu, J.P.; Li, J.F.; Zhang, K.Y.; Zhi, H.; Zhang, H.; Sun, J.M. Antioxidant, Anti-inflammatory and Cytotoxic Activities of Polyphenols Extracted from Chroogomphus rutilus. Chem. Biodivers 2020, 17, e1900479. [Google Scholar] [CrossRef] [PubMed]
- Kikowska, M.A.; Chmielewska, M.; Włodarczyk, A.; Studzińska-Sroka, E.; Żuchowski, J.; Stochmal, A.; Kotwicka, M.; Thiem, B. Effect of pentacyclic triterpenoids-rich callus extract of Chaenomeles japonica (Thunb.) Lindl. ex Spach on viability, morphology, and proliferation of normal human skin fibroblasts. Molecules 2018, 23, 3009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiveron, A.P.; Melo, P.S.; Bergamaschi, K.B.; Vieira, T.M.F.S.; Regitano-d’Arce, M.A.B.; Alencar, S.M. Antioxidant activity of Brazilian vegetables and its relation with phenolic composition. Int. J. Mol. Sci. 2012, 13, 8943–8957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Studzinska-Sroka, E.; Dudek-Makuch, M.; Chanaj-Kaczmarek, J.; Czepulis, N.; Korybalska, K.; Rutkowski, R.; Luczak, J.; Grabowska, K.; Bylka, W.; Witowski, J. Anti-inflammatory Activity and Phytochemical Profile of Galinsoga Parviflora Cav. Molecules 2018, 23, 2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgell, C.J.; McDonald, C.C.; Graham, J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. USA 1983, 80, 3734–3737. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Witowski, J.; Korybalska, K.; Wisniewska, J.; Breborowicz, A.; Gahl, G.M.; Frei, U.; Passlick-Deetjen, J.; Jorres, A. Effect of glucose degradation products on human peritoneal mesothelial cell function. J. Am. Soc. Nephrol. 2000, 11, 729–739. [Google Scholar] [CrossRef]



| Plants, Extract | Rt (Min.) | Compounds | % Of Total | Formula |
|---|---|---|---|---|
| BTH extract | 11.512 | 1-tert-butyl-3-(1-methylcyclohexyl)-2-aziridinone | 13.71 | C13H23NO |
| 12.238 | ascaridole epoxide | 1.13 | C10H16O3 | |
| 17.318 | 1,2,3,4-tetrahydro-3-O-methyl-papaveroline | 22.14 | C17H19NO4 | |
| 18.190 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol | 4.26 | C20H40O | |
| 18.236 | (1a,2ß,4ß)- 4-(1,1-dimethylethyl)-dimethyl ester 1,2-cyclopentanedicarboxylic acid | 0.27 | C13H22O4 | |
| 18.253 | 3-hydroxy-dodecanoic acid | 0.36 | C12H24O3 | |
| 18.272 | (3ß,5a)-2-methylene-cholestan-3-ol | 0.45 | C28H48O | |
| 18.442 | (Z)-2-(9-octadecenyloxy)-ethanol | 0.73 | C20H40O2 | |
| 18.784 | hanphyllin | 5.99 | C15H20O3 | |
| 18.922 | santonin | 1.34 | C15H18O3 | |
| 19.510 | n-hexadecanoic acid (syn. palmitic acid) | 17.99 | C16H32O2 | |
| 19.906 | (Z,Z)-9,12-octadecadienoic acid | 29.60 | C18H32O2 | |
| 22.918 | 2-[4-methyl-6-(2,6,6-trimethylcyclohex-1-enyl)hexa-1,3,5-trienyl]cyclohex-1-en-1-carboxaldehyde | 2.02 | C23H32O | |
| Total | 100.00 | |||
| GVH extract | 11.696 | 7-ethyl-4-decen-6-one | 45.95 | C12H22O |
| 12.226 | ascaridole epoxide | 4.69 | C10H16O3 | |
| 16.664 | 4-hydroxy-benzenepropanoic acid (syn. p-hydroxyhydrocinnamic acid) | 41.40 | C9H10O3 | |
| 18.803 | (E,Z,Z)-2,4,7-tridecatrienal | 4.24 | C13H20O | |
| 19.499 | estra-1,3,5(10)-trien-17ß-ol | 3.72 | C18H24O | |
| Total | 100.00 | |||
| RHR extract | 8.879 | 2,2′,6,6′-tetramethyl-4,4′-biscyclohexanone | 2.27 | C16H26O2 |
| 9.873 | 2-propyl-tetrahydropyran-3-ol | 5.75 | C8H16O2 | |
| 11.129 | 6-acetyl-ß-d-mannose | 0.24 | C8H14O7 | |
| 11.485 | 4-methyl-4-hepten-3-one | 22.73 | C8H14O | |
| 14.792 | 2-myristynoyl pantetheine | 21.88 | C25H44N2O5S | |
| 19.472 | estra-1,3,5(10)-trien-17ß-ol | 0.31 | C18H24O | |
| 19.484 | oleic acid | 0.41 | C18H34O2 | |
| 19.531 | androst-5-en-4-one | 0.48 | C19H28O | |
| 21.088 | (Z,Z,Z)- 9,12,15-octadecatrienoic acid, 2,3-dihydroxypropyl ester | 0.97 | C21H36O4 | |
| 21.163 | (Z,Z)-9,12-octadecadienoic acid | 3.18 | C18H32O2 | |
| 21.366 | cis-5,8,11,14,17-eicosapentaenoic acid | 0.31 | C20H30O2 | |
| 22.940 | 3-methyl-1,8,9-anthracenetriol | 2.92 | C15H12O3 | |
| 23.230 | 1,8-dihydroxy-3-methyl-9,10-anthracenedione (syn. chrysophanol) | 32.04 | C15H10O4 | |
| 25.605 | 5,10-dihydroxy-2-methoxy-7-methyl-1,4-anthracenedione | 6.50 | C16H12O5 | |
| Total | 100.00 |
| Examined Extract/Standard | Total Phenolic Content TPC (mg GAE/g Raw Materials) | Antioxidant Activity | |
|---|---|---|---|
| DPPH IC50 (mg/mL) | FRAP IC0.5 (mg/mL) | ||
| Bidens tripartita herb (BTH) | 32.70 ± 2.47 | 1.59 | 0.26 |
| Galium verum herb (GVH) | 58.47 ± 4.40 | 0.87 | 0.14 |
| Rumex hydrolapathum root (RHR) | 327.79 ± 1.76 | 0.07 | 0.02 |
| Vitaminum C | Not determined | 0.0077 | 0.0041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoniak, K.; Studzińska-Sroka, E.; Szymański, M.; Dudek-Makuch, M.; Cielecka-Piontek, J.; Korybalska, K. Antiangiogenic, Anti-Inflammatory and Antioxidant Properties of Bidens tripartite Herb, Galium verum Herb and Rumex hydrolapathum Root. Molecules 2023, 28, 4966. https://doi.org/10.3390/molecules28134966
Antoniak K, Studzińska-Sroka E, Szymański M, Dudek-Makuch M, Cielecka-Piontek J, Korybalska K. Antiangiogenic, Anti-Inflammatory and Antioxidant Properties of Bidens tripartite Herb, Galium verum Herb and Rumex hydrolapathum Root. Molecules. 2023; 28(13):4966. https://doi.org/10.3390/molecules28134966
Chicago/Turabian StyleAntoniak, Katarzyna, Elżbieta Studzińska-Sroka, Marcin Szymański, Marlena Dudek-Makuch, Judyta Cielecka-Piontek, and Katarzyna Korybalska. 2023. "Antiangiogenic, Anti-Inflammatory and Antioxidant Properties of Bidens tripartite Herb, Galium verum Herb and Rumex hydrolapathum Root" Molecules 28, no. 13: 4966. https://doi.org/10.3390/molecules28134966
APA StyleAntoniak, K., Studzińska-Sroka, E., Szymański, M., Dudek-Makuch, M., Cielecka-Piontek, J., & Korybalska, K. (2023). Antiangiogenic, Anti-Inflammatory and Antioxidant Properties of Bidens tripartite Herb, Galium verum Herb and Rumex hydrolapathum Root. Molecules, 28(13), 4966. https://doi.org/10.3390/molecules28134966








