Effect of Substituent Groups on the Strength of Intramolecular Hydrogen Bonds in 2,4-Dihydroxybenzophenone UV Absorbers
Abstract
1. Introduction
2. Results and Discussion
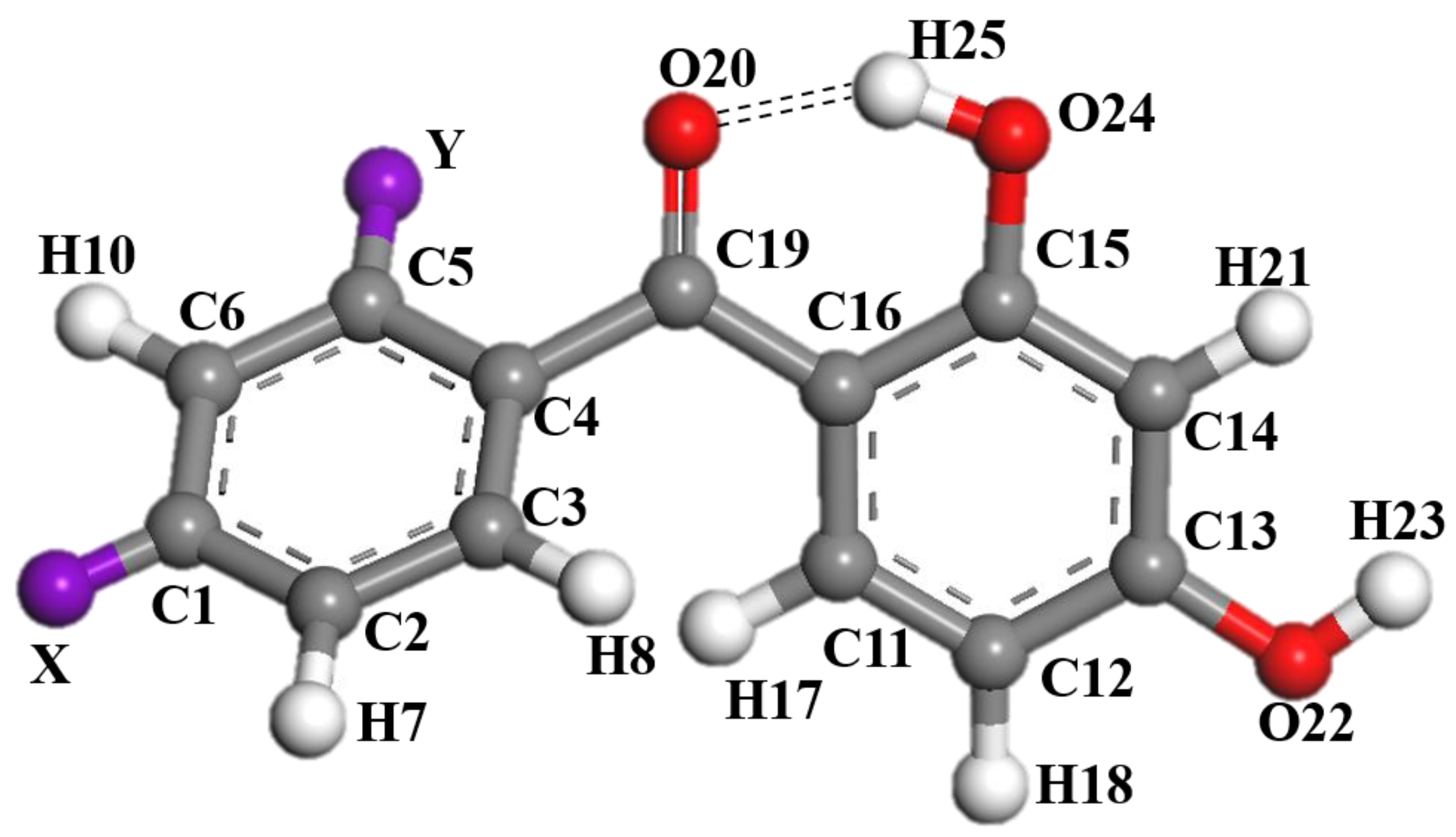
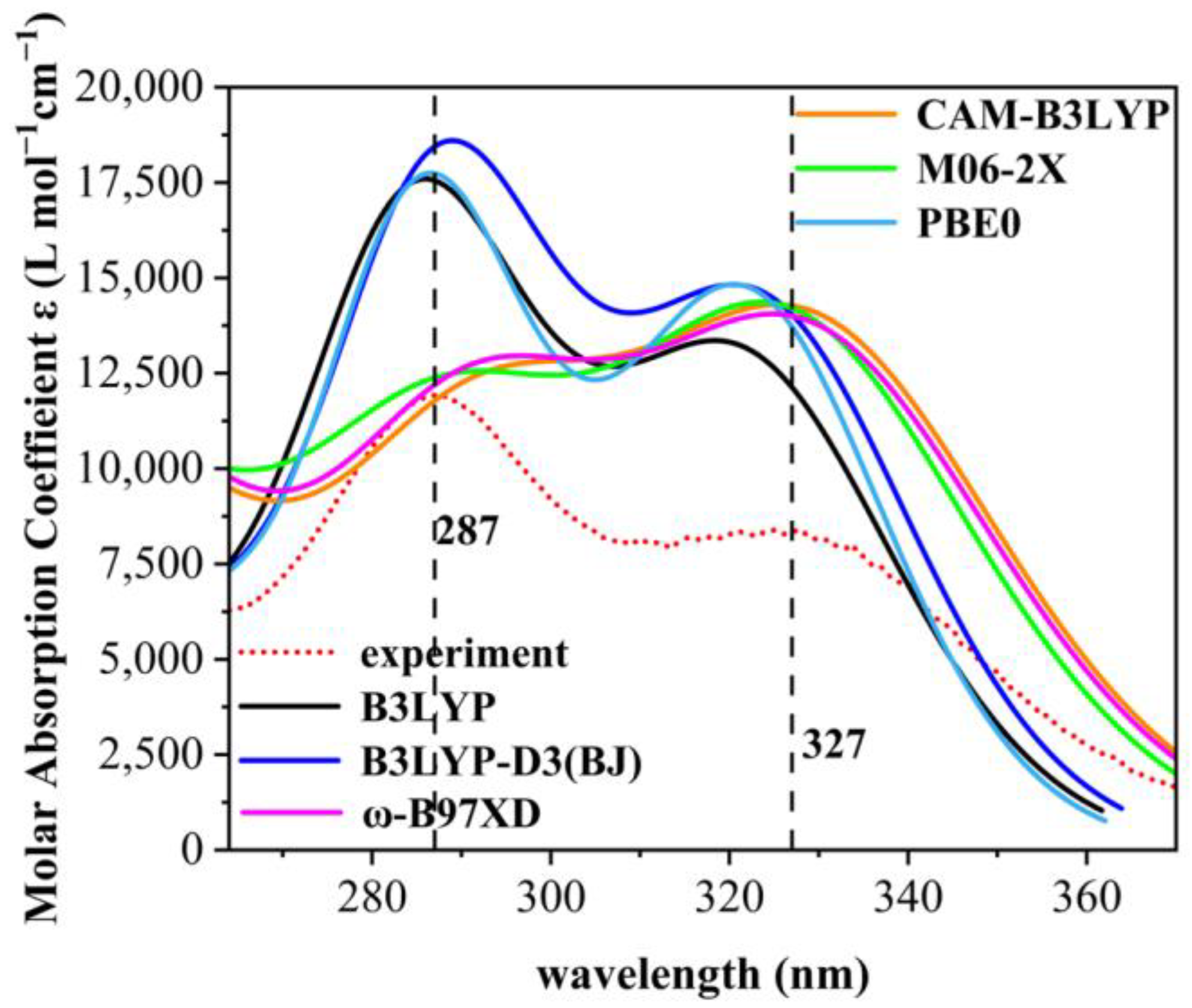
2.1. UV Absorption Performance of 2,4-Dihydroxybenzophenone Absorbers
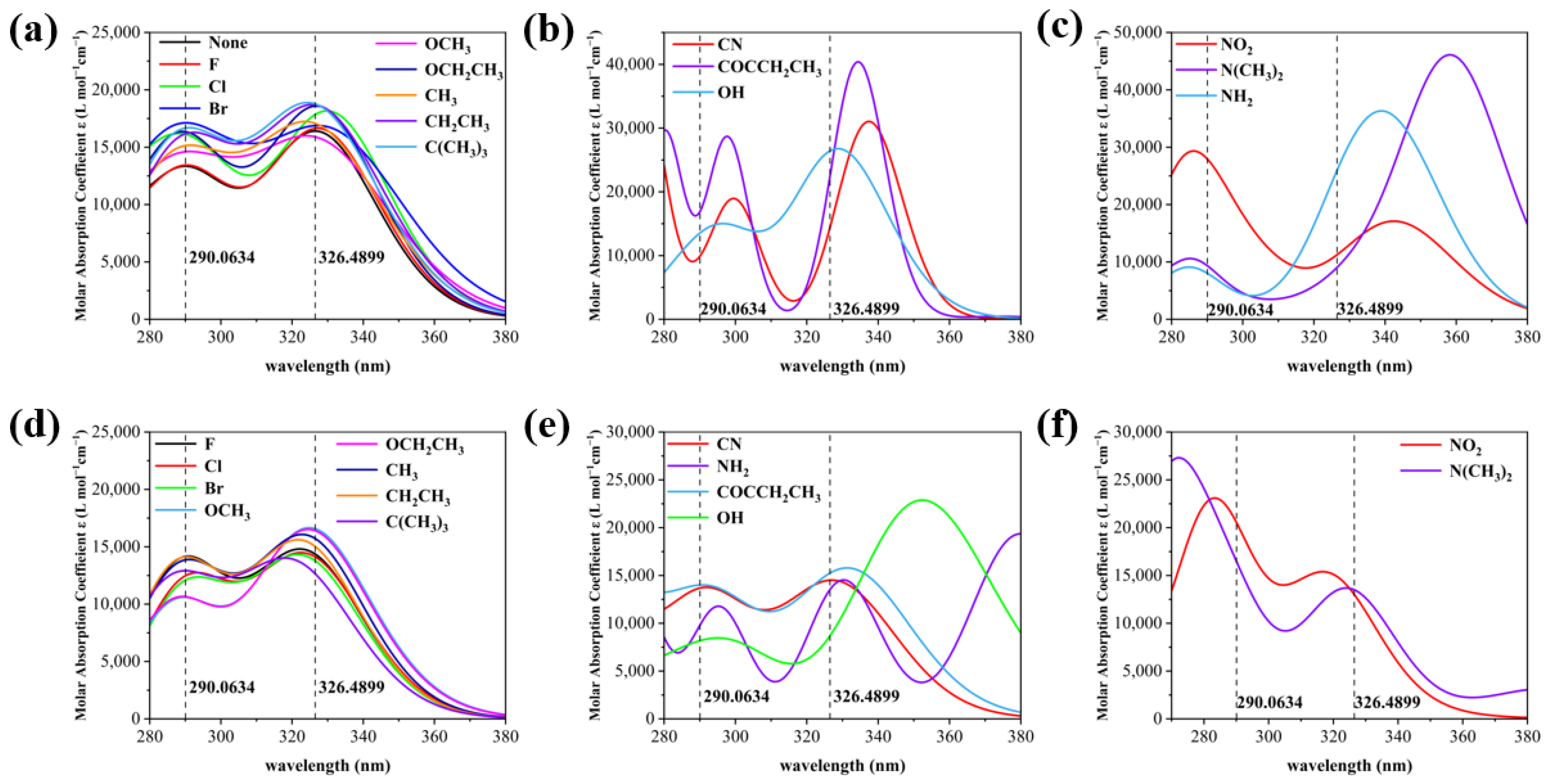
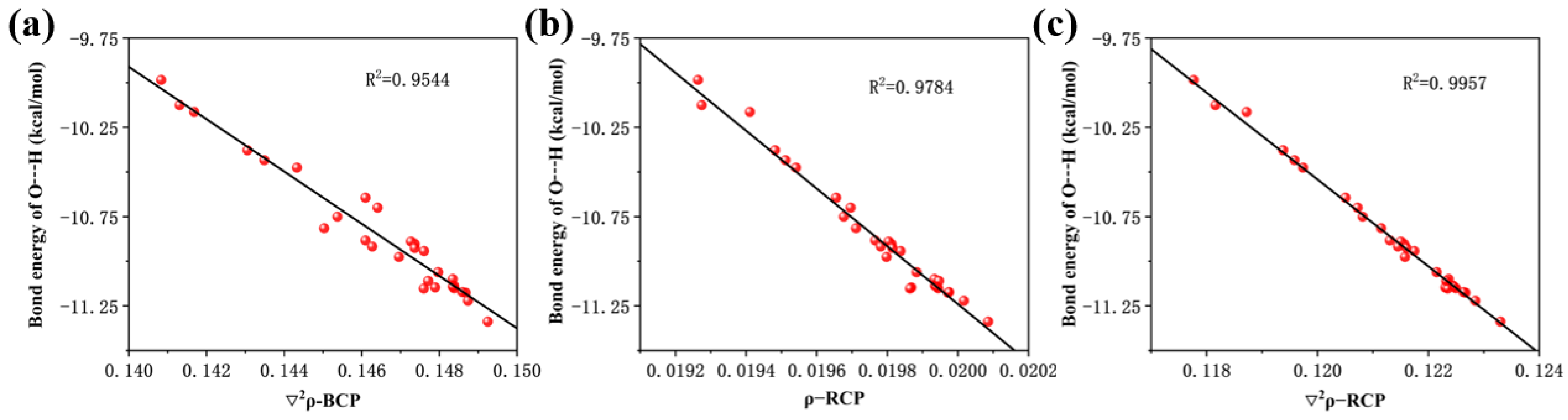
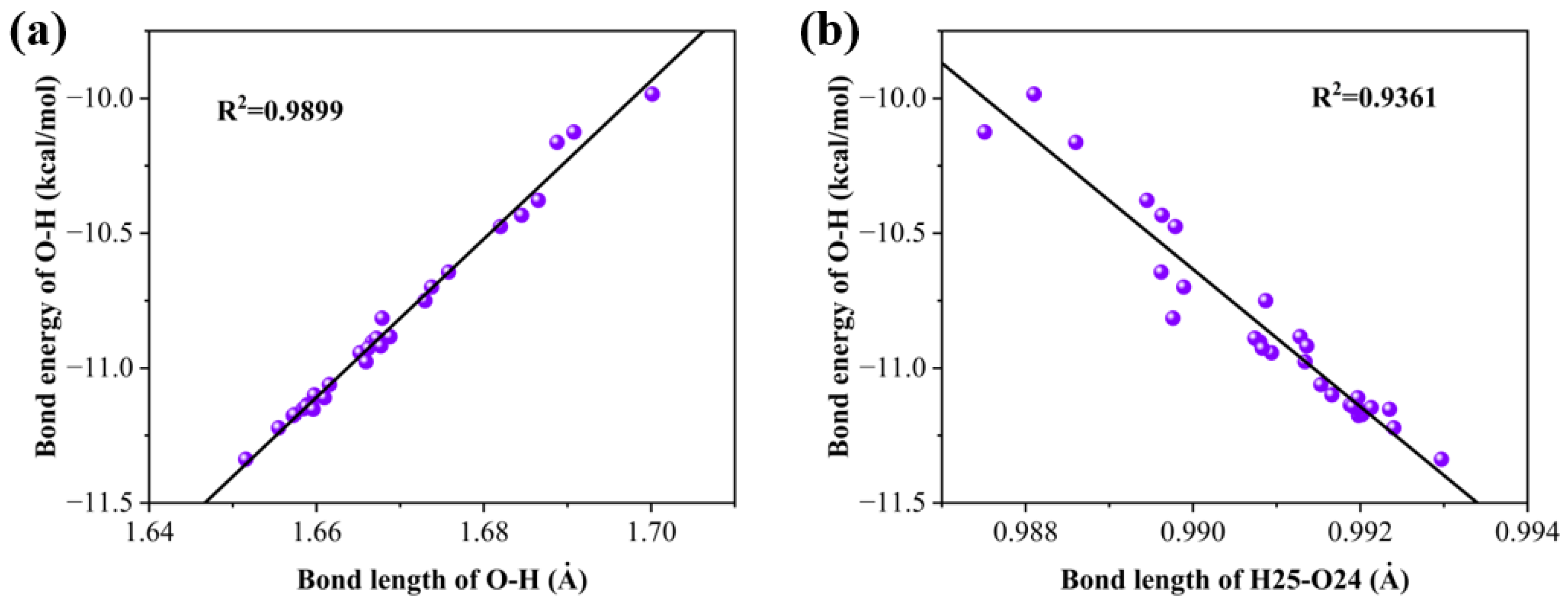
2.2. Effect of Substituent Groups on the Strength of Intramolecular Hydrogen Bonds in 2,4-Dihydroxybenzophenone Absorbers
2.3. Electrostatic Potential and Charge Analysis of 2,4-Dihydroxybenzophenone Molecules
0.24986q(C16) + 0.54071q(C19) + 0.40726
3. Computational Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xiang, S.; Zhang, N.; Fan, X. From fiber to fabric: Progress towards photovoltaic energy textile. Adv. Fiber Mater. 2021, 3, 76–106. [Google Scholar] [CrossRef]
- Riaz, S.; Jabbar, A.; Khaskheli, S.; Sagheer, S.; Choudhary, M.I. Anthraquinone based anti-UV acid-azo dyes; a study of their synthesis, fastness, and UV-protection properties. J. Mol. Struct. 2023, 1272, 134219. [Google Scholar] [CrossRef]
- Kibria, G.; Repon, M.R.; Hossain, M.F.; Islam, T.; Jalil, M.A.; Aljabri, M.D.; Rahman, M.M. UV-blocking cotton fabric design for comfortable summer wears: Factors, durability and nanomaterials. Cellulose 2022, 29, 7555–7585. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Bick, M.; Chen, J. Smart textiles for electricity generation. Chem. Rev. 2020, 120, 3668–3720. [Google Scholar] [CrossRef]
- Agumba, D.O.; Kumar, B.; Panicker, P.S.; Kim, J. Biobased-interlayer glass composite with improved mechanical properties and ultraviolet radiation shielding. Opt. Mater. 2022, 133, 112898. [Google Scholar] [CrossRef]
- Perfetti-Bolaño, A.; Muñoz, K.; Kolok, A.S.; Araneda, A.; Barra, R.O. Analysis of the contribution of locally derived wastewater to the occurrence of Pharmaceuticals and Personal Care Products in Antarctic coastal waters. Sci. Total Environ. 2022, 851, 158116. [Google Scholar] [CrossRef]
- Ahmed, E.; Maamoun, D.; Abdelrahman, M.S.; Hassan, T.M.; Khattab, T.A. Imparting cotton textiles glow-in-the-dark property along with other functional properties: Photochromism, flame-retardant, water-repellency, and antimicrobial activity. Cellulose 2023, 30, 4041–4055. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, W.; Ma, T.; Zhang, C.; Kuang, J.; Wang, R. Anti-UV and hydrophobic dual-functional coating fabrication for flame retardant polyester fabrics by surface-initiated PET RAFT technique. Eur. Polym. J. 2022, 173, 111275. [Google Scholar] [CrossRef]
- Mondal, S. Nanomaterials for UV protective textiles. J. Ind. Text. 2022, 51, 5592S–5621S. [Google Scholar] [CrossRef]
- Sankaran, A.; Kamboj, A.; Samant, L.; Jose, S. Synthetic and natural UV protective agents for textile finishing. In Innovative and Emerging Technologies for Textile Dyeing and Finishing; Rather, L.J., Haji, A., Shabbir, M., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 301–324. [Google Scholar]
- Yu, S.; Du, J.; Zheng, Y.; Yan, L. Synthesis and characterization of carboxymethyl chitosan containing functional ultraviolet absorber substituent. J. Appl. Polym. Sci. 2007, 106, 4098–4103. [Google Scholar] [CrossRef]
- Cao, W.Q.; Ma, X.P.; Lv, B.L. Synthesis of Water-soluble Ultraviolet Absorbent 2, 4-Dihydroxy-5-sulfo-benzophenone. Adv. Fine Petrochem. 2008, 9, 35–38. [Google Scholar]
- Tong, X.Y. Synthesis of UV absorber UV-531. Zhejiang Chem. Ind. 2003, 20, 21–22. [Google Scholar]
- Gantz, G.M.; Sumner, W.G. Stable ultraviolet light absorbers. Text. Res. J. 1957, 27, 244–251. [Google Scholar] [CrossRef]
- Rieker, J.; Lemmert-Schmitt, E.; Goeller, G.; Roessler, M.; Stueber, G.J.; Schettler, H.; Hoier, H. Ultraviolet stabilizers of the 2-(hydroxyphenyl) benzotriazole class: Influence of substituents on structure and spectra. J. Phys. Chem. 1992, 96, 10225–10234. [Google Scholar] [CrossRef]
- Woessner, G.; Goeller, G.; Rieker, J.; Hoier, H.; Stezowski, J.J.; Daltrozzo, E.; Kramer, H.E. Ultraviolet stabilizers of the 2-hydroxyphenylbenzotriazole class-influence of the solvent on the absorption spectra and photochemical deactivation mechanism. J. Phys. Chem. 1985, 89, 3629–3636. [Google Scholar] [CrossRef]
- Jeffrey, G.A.; Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Wu, F.; Tan, S.Q.; Fang, Z.J.; Deng, J.Y.; He, Z.J.; Huang, C.Y.; Yi, B. Substituent Effects on the Ultraviolet Absorption Properties of 2,4-Dihydroxy Dibenzophenone. Molecules 2022, 27, 8169. [Google Scholar] [CrossRef] [PubMed]
- Zahedi-Tabrizi, M.; Tayyari, S.F.; Badalkhani-Khamseh, F.; Ghomi, R.; Afshar-Qahremani, F. Molecular structure and intramolecular hydrogen bonding in 2-hydroxybenzophenones: A theoretical study. J. Chem. Sci. 2016, 126, 919–929. [Google Scholar] [CrossRef]
- Zahedi-Tabrizi, M.; Badalkhani-Khamseh, F.; Ghomi, R. Effect of Chlorine substitution on the strength of intramolecular hydrogen bond and vibrational spectrum of Orthohydroxybenzophenone. Clin. Biochem. 2011, 13, S316. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Walker, M.; Harvey, A.J.; Sen, A.; Dessent, C.E. Performance of M06, M06-2X, and M06-HF density functionals for conformationally flexible anionic clusters: M06 functionals perform better than B3LYP for a model system with dispersion and ionic hydrogen-bonding interactions. J. Phys. Chem. A 2013, 117, 12590–12600. [Google Scholar] [CrossRef]
- Altürk, S.; Avcı, D.; Tamer, Ö.; Atalay, Y. Comparison of different hybrid DFT methods on structural, spectroscopic, electronic and NLO parameters for a potential NLO material. Comput. Theor. Chem. 2017, 1100, 34–45. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring nature and predicting strength of hydrogen bonds: A correlation analysis between atoms-in-molecules descriptors, binding energies, and energy components of symmetry-adapted perturbation theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Krishnan, R.B.J.S.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Bader, R.F. Atoms in molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.R. Natural bond orbitals and extensions of localized bonding concepts. Chem. Educ. Res. Pract. 2001, 2, 91–104. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]


| Substituent | ∇2ρ-BCP (a.u.) | ρ-RCP (a.u.) | ∇2ρ-RCP (a.u.) | EO···H (kcal/mol) | dO···H (Å) | dH25–O24 (Å) |
|---|---|---|---|---|---|---|
| None | 0.1480 | 0.0199 | 0.1221 | −11.0613 | 1.6615 | 0.9915 |
| X-F | 0.1476 | 0.0198 | 0.1217 | −10.9434 | 1.6652 | 0.9909 |
| X-Cl | 0.1474 | 0.0198 | 0.1216 | −10.9032 | 1.6667 | 0.9908 |
| X-Br | 0.1473 | 0.0198 | 0.1215 | −10.8890 | 1.6672 | 0.9907 |
| X-OCH3 | 0.1487 | 0.0200 | 0.1227 | −11.1760 | 1.6572 | 0.9920 |
| X-OCH2CH3 | 0.1486 | 0.0200 | 0.1226 | −11.1724 | 1.6574 | 0.9920 |
| X-CH3 | 0.1484 | 0.0199 | 0.1224 | −11.1363 | 1.6589 | 0.9919 |
| X-CH2CH3 | 0.1484 | 0.0199 | 0.1225 | −11.1515 | 1.6584 | 0.9920 |
| X-C(CH3)3 | 0.1483 | 0.0199 | 0.1225 | −11.1418 | 1.6588 | 0.9919 |
| X-CN | 0.1464 | 0.0197 | 0.1207 | −10.6994 | 1.6738 | 0.9899 |
| X-NH2 | 0.1487 | 0.0200 | 0.1228 | −11.2216 | 1.6555 | 0.9924 |
| X-NO2 | 0.1461 | 0.0197 | 0.1205 | −10.6443 | 1.6758 | 0.9896 |
| X-N(CH3)2 | 0.1492 | 0.0201 | 0.1233 | −11.3382 | 1.6515 | 0.9930 |
| X-COCCH2CH3 | 0.1474 | 0.0198 | 0.1216 | −10.9266 | 1.6662 | 0.9908 |
| X-OH | 0.1483 | 0.0199 | 0.1224 | −11.0994 | 1.6597 | 0.9917 |
| Y-F | 0.1454 | 0.0197 | 0.1208 | −10.7501 | 1.6730 | 0.9909 |
| Y-Cl | 0.1435 | 0.0195 | 0.1196 | −10.4334 | 1.6845 | 0.9896 |
| Y-Br | 0.1431 | 0.0195 | 0.1194 | −10.3781 | 1.6865 | 0.9895 |
| Y-OCH3 | 0.1461 | 0.0198 | 0.1213 | −10.8832 | 1.6688 | 0.9913 |
| Y-OCH2CH3 | 0.1463 | 0.0198 | 0.1215 | −10.9179 | 1.6677 | 0.9914 |
| Y-CH3 | 0.1479 | 0.0199 | 0.1223 | −11.1465 | 1.6595 | 0.9921 |
| Y-CH2CH3 | 0.1476 | 0.0199 | 0.1223 | −11.1529 | 1.6596 | 0.9924 |
| Y-C(CH3)3 | 0.1470 | 0.0198 | 0.1216 | −10.9763 | 1.6659 | 0.9913 |
| Y-CN | 0.1443 | 0.0195 | 0.1197 | −10.4752 | 1.6820 | 0.9898 |
| Y-NH2 | 0.1450 | 0.0197 | 0.1211 | −10.8148 | 1.6679 | 0.9898 |
| Y-NO2 | 0.1408 | 0.0193 | 0.1178 | −9.9842 | 1.7001 | 0.9881 |
| Y-N(CH3)2 | 0.1477 | 0.0199 | 0.1223 | −11.1098 | 1.6609 | 0.9920 |
| Y-COCCH2CH3 | 0.1417 | 0.0194 | 0.1187 | −10.1631 | 1.6888 | 0.9886 |
| Y-OH | 0.1413 | 0.0193 | 0.1182 | −10.1251 | 1.6908 | 0.9875 |
| Substituent | q(H25) | q(O20) | q(O24) | q(C15) | q(C16) | q(C19) | Δ(q(O20) − q(O24)) |
|---|---|---|---|---|---|---|---|
| None | 0.5033 | −0.6230 | −0.6682 | 0.4073 | −0.2499 | 0.5407 | 0.0463 |
| X-F | 0.5036 | −0.6245 | −0.6676 | 0.4076 | −0.2500 | 0.5390 | 0.0437 |
| X-Cl | 0.5037 | −0.6226 | −0.6675 | 0.4081 | −0.2503 | 0.5380 | 0.0450 |
| X-Br | 0.5037 | −0.6221 | −0.6717 | 0.4081 | −0.2503 | 0.5379 | 0.0454 |
| X-OCH3 | 0.5032 | −0.6314 | −0.6720 | 0.4054 | −0.2518 | 0.5489 | 0.0404 |
| X-OCH2CH3 | 0.5032 | −0.6318 | −0.6704 | 0.4051 | −0.2515 | 0.5490 | 0.0402 |
| X-CH3 | 0.5033 | −0.6262 | −0.6706 | 0.4064 | −0.2484 | 0.5408 | 0.0442 |
| X-CH2CH3 | 0.5031 | −0.6253 | −0.6708 | 0.4064 | −0.2533 | 0.5503 | 0.0453 |
| X-C(CH3)3 | 0.5032 | −0.6252 | −0.6645 | 0.4045 | −0.2445 | 0.5495 | 0.0456 |
| X-CN | 0.5039 | −0.6155 | −0.6735 | 0.4105 | −0.2574 | 0.5428 | 0.0490 |
| X-NH2 | 0.5033 | −0.6373 | −0.6634 | 0.4034 | −0.2443 | 0.5379 | 0.0362 |
| X-NO2 | 0.5041 | −0.6141 | −0.6749 | 0.4111 | −0.2531 | 0.5325 | 0.0493 |
| X-N(CH3)2 | 0.5031 | −0.6394 | −0.6675 | 0.4027 | −0.2475 | 0.5462 | 0.0355 |
| X-COCCH2CH3 | 0.5033 | −0.6197 | −0.6710 | 0.4089 | −0.2562 | 0.5464 | 0.0478 |
| X-OH | 0.5034 | −0.6317 | −0.6658 | 0.4055 | −0.2473 | 0.5393 | 0.0393 |
| Y-F | 0.5034 | −0.5997 | −0.6651 | 0.4103 | −0.2538 | 0.5452 | 0.0661 |
| Y-Cl | 0.5033 | −0.6057 | −0.6647 | 0.4117 | −0.2569 | 0.5507 | 0.0593 |
| Y-Br | 0.5033 | −0.6038 | −0.6695 | 0.4115 | −0.2575 | 0.5525 | 0.0609 |
| Y-OCH3 | 0.5029 | −0.6156 | −0.6701 | 0.4092 | −0.2502 | 0.5561 | 0.0539 |
| Y-OCH2CH3 | 0.5027 | −0.6161 | −0.6683 | 0.4089 | −0.2502 | 0.5576 | 0.0539 |
| Y-CH3 | 0.5028 | −0.6210 | −0.6682 | 0.4091 | −0.2585 | 0.5495 | 0.0473 |
| Y-CH2CH3 | 0.5027 | −0.6204 | −0.6664 | 0.4095 | −0.2604 | 0.5518 | 0.0478 |
| Y-C(CH3)3 | 0.5027 | −0.6213 | −0.6609 | 0.4080 | −0.2638 | 0.5524 | 0.0450 |
| Y-CN | 0.5049 | −0.5951 | −0.6722 | 0.4145 | −0.2582 | 0.5364 | 0.0658 |
| Y-NH2 | 0.5043 | −0.6724 | −0.6627 | 0.4039 | −0.2438 | 0.5473 | −0.0002 |
| Y-NO2 | 0.5042 | −0.6018 | −0.6708 | 0.4138 | −0.2567 | 0.5517 | 0.0610 |
| Y-N(CH3)2 | 0.5024 | −0.6287 | −0.6634 | 0.4055 | −0.2613 | 0.5475 | 0.0422 |
| Y-COCCH2CH3 | 0.5043 | −0.6037 | −0.6685 | 0.4102 | −0.2590 | 0.5321 | 0.0596 |
| Y-OH | 0.5063 | −0.6902 | −0.6682 | 0.4061 | −0.2421 | 0.5447 | −0.0217 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.; Zhang, X.; Wu, F.; Huang, B.; Au, C.; Yi, B. Effect of Substituent Groups on the Strength of Intramolecular Hydrogen Bonds in 2,4-Dihydroxybenzophenone UV Absorbers. Molecules 2023, 28, 5017. https://doi.org/10.3390/molecules28135017
Fang Z, Zhang X, Wu F, Huang B, Au C, Yi B. Effect of Substituent Groups on the Strength of Intramolecular Hydrogen Bonds in 2,4-Dihydroxybenzophenone UV Absorbers. Molecules. 2023; 28(13):5017. https://doi.org/10.3390/molecules28135017
Chicago/Turabian StyleFang, Zhengjun, Xinhua Zhang, Feng Wu, Baoyu Huang, Chaktong Au, and Bing Yi. 2023. "Effect of Substituent Groups on the Strength of Intramolecular Hydrogen Bonds in 2,4-Dihydroxybenzophenone UV Absorbers" Molecules 28, no. 13: 5017. https://doi.org/10.3390/molecules28135017
APA StyleFang, Z., Zhang, X., Wu, F., Huang, B., Au, C., & Yi, B. (2023). Effect of Substituent Groups on the Strength of Intramolecular Hydrogen Bonds in 2,4-Dihydroxybenzophenone UV Absorbers. Molecules, 28(13), 5017. https://doi.org/10.3390/molecules28135017






